Abstract
Single-particle mass spectrometry (SPMS) has been widely used for characterizing the chemical mixing state of ambient aerosol particles. However, processes occurring during particle ablation and ionization can influence the mass spectra produced by these instruments. These effects remain poorly characterized for complex atmospheric particles. During the 2005 Study of Organic Aerosols in Riverside (SOAR), a thermodenuder was used to evaporate the more volatile aerosol species in sequential temperature steps up to 230°C; the residual aerosol particles were sampled by an aerosol mass spectrometer (AMS) and a single-particle aerosol time-of-flight mass spectrometer (ATOFMS). Removal of the secondary species (e.g., ammonium nitrate/sulfate) through heating permitted assessment of the change in ionization patterns as the composition changed for a given particle type. It was observed that a coating of secondary species can reduce the ionization efficiency by changing the degree of laser absorption or particle ablation, which significantly impacted the measured ion peak areas. Nonvolatile aerosol components were used as pseudo-internal standards (or “reference components”) to correct for this LDI effect. Such corrected ATOFMS ion peak areas correlated well with the AMS measurements of the same species up to 142°C. This work demonstrates the potential to accurately relate SPMS peak areas to the mass of specific aerosol components.
Copyright 2014 American Association for Aerosol Research
INTRODUCTION
Single-particle mass spectrometry (SPMS) is used to measure the mixing state (chemical associations) of individual aerosol particles in the atmosphere (Murphy et al. Citation2006; Pratt and Prather Citation2012). Real-time SPMS instruments typically utilize an ultraviolet (UV) laser to desorb and ionize both refractory and nonrefractory aerosol material from individual particles in a single step. Subsequent analysis of the ionized components by time-of-flight mass spectrometry yields a full mass spectrum per particle. However, there are several known ionization effects that can complicate the mass spectral interpretation, including shot-to-shot variability due to laser inhomogeneities (Wenzel and Prather Citation2004; Zelenyuk et al. Citation2009) and matrix effects caused by reactions within the laser plume (Gross et al. Citation2000; Reilly et al. Citation2000).
Many aspects of the ablation process during LDI have been probed in laboratory studies. The effects of laser wavelength and energy on particle ablation have been reported for a number of studies using laboratory-generated aerosol particles (Carson et al. Citation1997; Thomson et al. Citation1997; Wade et al. Citation2008; Zelenyuk et al. Citation2009). Molecular dynamics of particle disintegration during picosecond laser pulses found that aerosol morphology can significantly influence particle ablation (Schoolcraft et al. Citation2000; Schoolcraft et al. Citation2001). Additional experimental work has demonstrated that particle morphology can also influence the resulting mass spectral ion peaks and intensities (Cai et al. Citation2006; Zelenyuk et al. Citation2008). Further, particles greater than 1000 nm in diameter are unlikely to be fully vaporized by one laser pulse (Zauscher Citation2012), suggesting that the mass spectra of large, inhomogeneous particles may be influenced by the orientation of the particle relative to the laser beam.
Several studies have characterized the ionization mechanism in SPMS instruments. Reinard and Johnston (Citation2008) assessed the mass spectral ion patterns derived from simple mixtures of common aerosol components. They determined that the desorption/ionization process likely proceeds first via desorption of neutral species, with subsequent photoionization producing cations and electrons; formation of anions occurs through electron capture by components with high electron affinities (Reinard and Johnston Citation2008). Reilly et al. (Citation2000) observed extensive charge transfer due to ion plume reactions. In these reactions, the component with the lowest ionization potential (IP) is favored, thereby suppressing the components with higher IPs in positive ion mass spectra (Gross et al. Citation2000; Reilly et al. Citation2000). The resulting mass spectral ion intensities are, therefore, dependent on the chemical components present in the particle (the matrix). As a result of these matrix effects, a trace particle constituent with a low IP can dominate the resulting spectrum. Similar processes occur for negative ions, where the species with the highest electron affinity are detected most easily and with higher ion peak areas (Reinard and Johnston Citation2008).
Several studies have shown that one can account for matrix effects by determining relative sensitivity factors to reconstruct the actual particle composition from mass spectral ion peak areas (Ge et al. Citation1998; Gross et al. Citation2000). Other researchers have successfully quantified SPMS data of ambient particles using co-located quantitative measurements (Bhave et al. Citation2002; Dall’Osto et al. Citation2006; Jeong et al. Citation2011) or laboratory calibrations (Pratt et al. Citation2009a). However, it is not clear if and how the LDI process and resulting mass spectra change as particles undergo significant processing (aging) during their atmospheric lifetime. Particles may acquire low-volatility and semi-volatile species, such as ammonium sulfate/nitrate and many organics, via condensation, coagulation, heterogeneous reactions, and cloud processing (Seinfeld and Pandis Citation2006). Although laboratory studies are essential for the systematic characterization of the ion formation mechanism in SPMS instruments, atmospheric particles display vastly more complex composition and morphologies than those that can be produced in the laboratory. Therefore, it is important to attempt to characterize ionization effects within ambient particles to better account for changes in particle composition via atmospheric aging and to help improve the quantitative capabilities of SPMS instruments. In this work, we applied SPMS measurements of thermally conditioned ambient aerosols in the highly polluted urban environment of Riverside, CA—a receptor site for emissions from Los Angeles and extensive agricultural operations (Russell and Cass Citation1986)—to systematically examine the effect on ion abundances that occurred after the removal of secondary species.
EXPERIMENTAL
Measurements of thermally conditioned aerosol particles were conducted during the summer Study of Organic Aerosols in Riverside (SOAR-1) in Riverside, CA; ambient particles measured during SOAR-1 have been previously described in detail (Docherty et al. Citation2011; Qin et al. Citation2012). The data represent measurements conducted on 12 August 2005 only (0:00–24:00 PST), as a representative summer day in Riverside. A thermodenuder (TD) was used to evaporate semi-volatile components from ambient aerosol particles; it consists of a heated region followed by a charcoal denuder to prevent vapor re-condensation (Huffman et al. Citation2008). The heated section steps through seven temperatures from 54–230°C (Huffman et al. Citation2008), thereby removing different particle constituents as a function of their volatility. Sampling switched between unheated and heated channels every 10 min; a full cycle was completed in 160 min and thus each TD temperature () was sampled several (∼8×) times on 12 August. The aerosol residuals were measured simultaneously by an aerosol time-of-flight mass spectrometer (ATOFMS) and a compact time-of-flight aerosol mass spectrometer (C-ToF-AMS [Drewnick et al. Citation2005]).
Single-particle analysis was performed using the ground-based prototype of the aircraft ATOFMS (Pratt et al. Citation2009b). Briefly, aerosols enter via an aerodynamic lens inlet and are accelerated to size-dependent terminal velocities. Each particle traverses and scatters light from two 532-nm continuous-wave lasers, providing the particle velocity. The particle velocity is then converted to vacuum aerodynamic diameter via calibration with polystyrene latex spheres of known diameters. Individual particles were desorbed and ionized at 266 nm using a Q-switched Nd:YAG laser prior to analysis by a dual-polarity reflectron time-of-flight mass spectrometer; the laser power was ∼0.9–1.0 mJ throughout the study.
The ATOFMS measured particles in the 100–1000 nm size range (with peak detection between ∼250–500 nm [Pratt et al. Citation2009b]), whereas the AMS measured particles in the 50–600 nm size range with nearly 100% efficiency (Drewnick et al. Citation2005). The AMS data were analyzed similarly to previous TD-AMS studies (Huffman et al. Citation2009a,Citationb). For ATOFMS data analysis, all sampling periods at the same temperature were merged to bolster particle-number statistics. ATOFMS data analysis was performed by importing single-particle mass spectra into Matlab (The MathWorks, Inc.) using the YAADA toolkit (Allen Citation2001). Particles were clustered on the basis of similarities in mass spectral peak identities and intensities using the ART-2a adaptive resonance theory method (Song et al. Citation1999) with a vigilance factor of 0.8, learning rate of 0.05, and 20 iterations. ART-2a clusters were identified as specific particle types, or as originating from specific sources, based on the predominant ion peaks or known source signatures.
RESULTS AND DISCUSSION
General Ambient Particle Observations
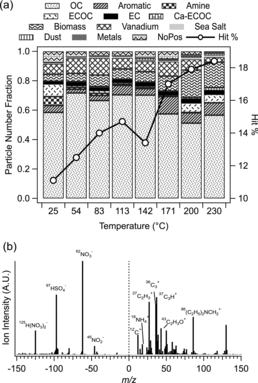
The size-resolved chemical mixing state of particles at ambient temperatures (measured by a nozzle-inlet ATOFMS) throughout SOAR-1 has been summarized by Qin et al. (Citation2012). The relative number fraction of each submicron particle type identified during SOAR-1 is shown in for each TD temperature. The predominant particle type was classified as organic carbon (OC), constituting up to ∼70% of the detected submicron particles by number. The positive ion mass spectral signature of the OC particle type () shows organic fragments (with the most likely peak identifications in parentheses) at m/z +27 (C2H3+), +37 (C3H+), and +43 (C2H3O+) with smaller contributions from elemental carbon markers, Cn+ (n = 1–3) (). Unheated OC particles were internally mixed with ammonium, amines, nitrate, and sulfate (). The abundance of secondary species and oxidized OC reflects the highly aged nature of aerosols in Riverside due to photochemical processing occurring during the summertime leading to secondary inorganic and organic aerosol formation (Docherty et al. Citation2008). Other minor particle classes, also enriched in nitrate and sulfate, included vanadium-rich particles (derived from ships), biomass burning, a mixed elemental carbon (EC)-OC class, EC, aromatic (characterized by markers of polycyclic aromatic hydrocarbons), amine particles (characterized by a dominant m/z +86 ((C2H5)2NCH2+) ion marker), dust, and sea salt (Qin et al. Citation2012).
From , it is clear that the relative number fractions of particle types did not change appreciably with heating during SOAR-1, particularly compared to the dramatic changes observed in the TD-ATOFMS study during the SOAR-2 campaign of fall 2005 (Pratt and Prather Citation2009). One exception was an increase in the biomass burning particles from ∼5% by number at ambient temperature to ∼17% at 230°C (). This increase likely occurred due to evaporation of secondary species, which exposed more of the biomass burning core and caused a reclassification from “OC” to “Biomass.” Despite the minimal changes in the particle-type fractions, the fraction of hit particles (hit efficiency) increased by a factor of 1.7, from ∼11% at ambient temperature to ∼18% at 230°C. (For comparison, the hit efficiency of monodisperse polystyrene latex spheres used as calibration standards was ∼20% during SOAR-1). The hit efficiency—defined as the number of particles that generated a mass spectrum (“hit”) divided by the total number of particles that were sized (hit/hit+missed)—is a function of the inlet transmission efficiency, laser alignment, and the degree of laser absorption at 266 nm by particles within the source region (Kane and Johnston Citation2000). The laser alignment was not adjusted during the measurement period, and thus laser alignment is unlikely to have caused the systematic changes in hit efficiency. In contrast, transmission efficiency through the instrument can be influenced by particle shape. For example, nonspherical particles experience greater beam divergence after exiting the aerodynamic lens (Liu et al. Citation1995; Huffman et al. Citation2005) and subsequent pumping chambers. Particles may become more fractal/irregular as secondary coatings are removed, and thus their transmission efficiencies can become reduced after heating. We further note that larger particles shrinking into the detectable size range are unlikely to account for the increase in hit efficiency, as discussed in the online supplementary information (SI). Therefore, the increase in the fraction of hit particles is likely due to changes in the particle composition, consistent with previous ATOFMS studies of ambient aerosols that have correlated temporal changes in particle hit percentages with changes in aerosol age (Wenzel et al. Citation2003; Dall’Osto et al. Citation2006; Jeong et al. Citation2011). The increase in hit efficiency indicates that the existing particle types became easier to ablate following removal of secondary coatings and water due to heating.
Variations in LDI Efficiency with Particle Age
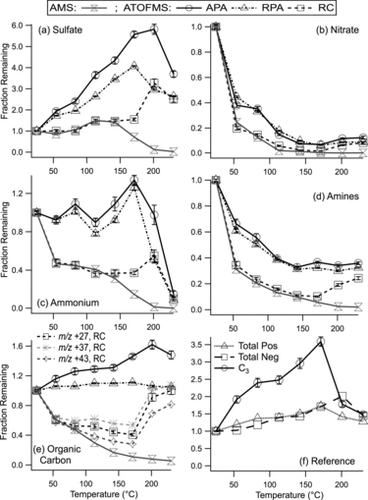
Increasing TD temperature can be regarded as a rough proxy for decreasing particle age given the progressive removal of secondary species with heating. Indeed, particle residuals at high TD temperatures have previously been shown to match the mass spectral signatures of freshly emitted primary particles (Pratt and Prather Citation2009). Therefore, we assessed the changes in ATOFMS ion peak areas following heating in the TD. For both ATOFMS and AMS measurements, heated particles were referenced to the unheated case, yielding the species’ “fraction remaining” as a function of temperature (the “thermogram”). The AMS data were used as an independent and quantitative measure of the nonrefractory mass fraction remaining at each temperature.
We focused on the OC particle type because it was the most abundant (), and therefore provided the best statistics at all temperatures. In addition, the OC particle type likely contained predominantly nonrefractory forms of the secondary inorganic species of interest (e.g., ammonium/ammonium nitrate/sulfate salts) that will evaporate at the operating temperatures of the TD (up to 230°C) (Huffman et al. Citation2008, Citation2009a; Salo et al. Citation2011; Qiu and Zhang Citation2012). The absence of metals in the average OC spectrum () suggests that nonvolatile salts (e.g., sodium nitrate) were negligible within these particles. The OC particle type is therefore an ideal system for comparison with the AMS data because the volatility trends should be similar for these two instruments. We also discuss the vanadium-rich particle type as a representative metal-containing particle class in the SI.
The ATOFMS absolute peak area (APA) denotes the peak area of each ion marker averaged over all particles in a given class at each TD temperature; it is proportional to the average absolute number of ions detected at a given m/z. APA has been used in a number of prior SPMS studies (Mansoori et al. Citation1994; Bhave et al. Citation2002; Dall’Osto et al. Citation2006; Pratt et al. Citation2009a). shows the thermograms calculated using APAs for the ion markers of sulfate (m/z −97, HSO4−), nitrate (m/z −62, NO3−), ammonium (m/z +18, NH4+), amines (m/z +86, (C2H5)2NCH2+), and organic carbon (m/z +27, C2H3+ as a representative marker). It is apparent that the APA fractions remaining for all species are significantly higher than the AMS thermograms at all temperatures. In particular, the ion peak area actually increased with temperature for several of these ion markers (sulfate, ammonium, OC). In the AMS measurements, the thermogram only increased for sulfate (by a factor of ∼1.5) up to ∼150°C, which is thought to be due to reduced particle bounce in the AMS vaporizer as discussed by Huffman et al. (Citation2009a). However, the effect was far more pronounced in the ATOFMS measurements: the APA of sulfate increased ∼6-fold. In addition, the ATOFMS ion intensity of the nonvolatile EC marker (m/z +36, C3+) and the average integrated (total) positive and negative ion intensity per particle were all observed to increase with temperature (). These trends are only possible if: (1) the particles had more material/mass available to form ions (e.g., by larger particles shrinking into the detectable size range, thus increasing the average particle size), or (2) the ion yield per particle increased due to changes in laser absorption or ablation/ionization efficiency. The contribution from shrinking supermicron OC particles was likely minor since the AMS and aircraft-ATOFMS have similar aerodynamic lens inlets and the AMS mass fractions of each species predominantly decreased with increasing temperature, as expected with the removal of semivolatile components (). Further, the median size of the OC particles, as measured by ATOFMS, decreased with increasing temperature (Figure S2). Therefore, an increase in overall particle mass can be ruled out, implying that the ion yield increased following heating through changes in ionization efficiency and/or extent of particle ablation. This is supported by the fact that the ion intensity increased for both positive and negative ion markers (e.g., m/z −97, HSO4− and +36, C3+). As a result, the APA method resulted in artificially high fractions remaining, even for semi-volatile components such as nitrate and amines (). Sulfate displayed the largest increase in ion intensity because it is nonvolatile below 150°C for the TD residence time used here (Johnson et al. Citation2004; Qiu and Zhang Citation2012), and therefore the primary factor contributing to the change in APA below 150°C was the increase in sulfate ion yield. That the changes in laser-particle interactions impacted the ion intensities of all species within the particle, rather than just individual components, illustrates that changes in collision-induced matrix effects were likely not the reason for the differences between the ATOFMS and AMS data. This observation is consistent with the increase in ATOFMS hit efficiency with temperature described above because absorption of the laser pulse rather than matrix effects will dictate whether particles are “hit.”
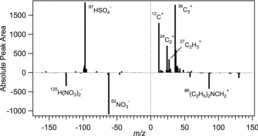
To investigate the reason for the increased ion yield with heating, the mass spectral patterns of the heated and unheated OC particles were compared; a difference plot of the average absolute mass spectrum for OC particles measured at 83°C minus that for unheated OC particles is shown in . Difference plots based on higher temperature mass spectra yielded similar results. It is clear that the carbon cluster peaks (Cn+, n = 1–3) became more prominent with heating, indicating that these particles were likely composed of an EC core, coated with secondary species (organics, ammonium nitrate/sulfate) and is consistent with prior observations of aged soot morphologies in Riverside (Moffet and Prather Citation2009). As the particle coating was stripped away with heating, the more strongly absorbing EC core was exposed. Therefore, a possible explanation for the increase in ion peak area is that the secondary coating (which absorbs the LDI photons less efficiently than soot) inhibited the absorption by scattering the laser radiation. Once those shell components were removed, or at least thinned, the particles absorbed the laser radiation more efficiently, thereby generating more cations, as well as more electrons to generate anions. Using SPMS, Thomson et al. (Citation1997) previously observed that the ionization threshold decreased with increasing absorption coefficient; in other words, more ions were produced from particles composed of more strongly absorbing species, consistent with this hypothesis. An alternative explanation is that the increased peak area was due to changes in the extent of particle ablation rather than absorption. Schoolcraft et al. (Citation2001) observed a decrease in particle disintegration using molecular dynamics simulations of an absorbing particle core coated with a thick, amorphous, and transparent coating. In their simulations, the particle core absorbed the laser pulse; however, the coating stretched around the expanding core and prevented the particle from ablating (Schoolcraft et al. Citation2001). A similar phenomenon may have played a role here, where a thinner secondary coating following heating could have led to ablation of a greater fraction of the particle and therefore more ions formed. Whether laser absorption or degree of particle ablation played a stronger role in this system is unknown, but the net effect in either case was an increase in the total number of ions formed per particle as the secondary coating was removed, which impacted the observed peak area trends.
Normalized, or “relative” peak areas (RPAs) are also commonly used in SPMS studies as a means of accounting for different ion intensities commonly observed among different particle types and other LDI artifacts, including shot-to-shot variability (Gross et al. Citation2000; Jeong et al. Citation2011). RPA refers to absolute peak area of the ion of interest normalized by the total ion intensity of the corresponding polarity at the single-particle level. Compared to the APA method, the RPA approach applied to the ATOFMS data resulted in thermograms that were generally closer to the AMS measurements, but still remained significantly elevated (). In this case, the deviation was caused by the evaporation of semi-volatile species, which influenced the total ion intensity because the amount of material available for ionization was reduced. For example, the total negative ion intensity is roughly the sum of the nitrate and sulfate ion peak areas (). At 142°C, the sulfate ion intensity (APA) was 4.3-fold higher than at ambient temperature (). In contrast, the total negative ion intensity increased by a factor of only 1.3 at 142°C because the nitrate mass had been dramatically reduced to ∼2% of its value at ambient temperature (). Therefore, the total ion intensity is not an independent indicator of the changes in LDI efficiency with particle aging and in this case was not an appropriate normalization factor to correct the peak areas of individual species.
As an alternative to the APA and RPA methods, a third method is proposed in which a single ion marker serves as a pseudo-internal standard (hereafter called the reference component [RC] method) to normalize all aerosol constituents. This RC normalization was used to account for the changes in LDI efficiency with limited influence from evaporating particle constituents. In this RC approach, the APA of each ion peak of interest was divided by the APA of the RC at the corresponding temperature. We note that this normalization step was effectively performed using the peak area integrated over all particles in the class, which more closely reflects the bulk composition for comparison to the AMS and also evens out particle-to-particle variability in the size of the EC core or particle ablation depth. The RC-normalized peak areas corresponding to the heated particles were then ratioed to those from the unheated particles to generate the thermograms for several secondary ion markers (). Reference components were chosen by the following criteria such that their particle-phase mass could be assumed to stay relatively constant across all temperatures. An appropriate RC should be: (1) nonvolatile and emitted by a primary source so that gas-particle partitioning is not a factor; (2) nonreactive or not react in a way that changes the mass spectral response to that component; (3) a substantial component of the spectrum, without saturating the detector. For the OC particles, we use the elemental carbon marker, m/z +36 (C3+), as the best option because it represents a component that is nonvolatile at the temperatures considered here and is also one of the predominant peaks in the mass spectrum of this particle type (); alternative RCs are compared in the SI. Although some organic compounds may produce carbon–cluster fragments (Cn+) (Silva and Prather Citation2000), a previous ATOFMS study determined that normalizing OC components by EC ion markers (including C3+ as the dominant EC ion marker) produced good agreement with a co-located instrument measuring OC/EC ratios by thermal/optical methods during SOAR-1 (Spencer and Prather Citation2006). Therefore, C3+ can be reasonably assumed to arise predominantly from elemental carbon in this work. We extend the method of Spencer and Prather (Citation2006) and use the EC marker to normalize all ions of interest within the OC particle type. Although the ionization mechanisms of positive and negative ions are largely decoupled (once electrons are generated), we take the peak area of the m/z +36 (C3+) marker as the normalization factor for both positive and negative ions because the absolute ion intensities indicated that the dominant influence on peak areas (aside from evaporation) was due to an increase in the overall ion yield from the particles, affecting both polarities proportionately. No suitable negative ion peak was found to act as an RC for the OC particles because all components exhibited observable reduction in mass within the TD temperature range (see the AMS measurements in ).
Using the RC as a normalization factor produced ATOFMS thermograms in close agreement with those from the AMS for ammonium and sulfate at temperatures up to 142°C (; R2 = 0.982 and 0.917, respectively) and amines and nitrate up to 171°C (; R2 = 0.999 and 0.994, respectively). The differences between the ATOFMS and AMS thermograms for ammonium and sulfate at 171°C may be due to the different transmission capabilities for small particles between the two instruments, but this hypothesis has not been tested. We note that the changes in LDI efficiency described above can explain the apparent differences in amine volatility reported in previous ATOFMS and AMS studies (Huffman et al. Citation2009a; Pratt et al. Citation2009a), as discussed by Docherty et al. (Citation2011). When the ionization processes of ATOFMS are properly taken into account, the amine measurements from the two methods agree quite well (). A seasonal difference in amine volatility (Pratt et al. Citation2009a) is still observed, as shown in the SI. Above 171°C, the marked increase in the fraction remaining of all components via the RC method may be due to a change in particle classification. The change in classification is demonstrated by the increase in the fraction of ECOC particles above 200°C (), and the associated drop in m/z +36 (C3+) ion peak area in OC particles (), because particles with greater EC ion peak intensity were moved to the separate ECOC particle class. When OC and ECOC particles were combined at the two highest TD temperatures, the ATOFMS peak area trends were indeed similar to the AMS (Figure S5). In contrast to the other ion markers (sulfate, nitrate, ammonium, amines), the organic carbon markers were consistently above the AMS data at temperatures greater than 83°C (). The reasons for this discrepancy are not clear; but could indicate either real differences in measured volatility (and therefore differences in measured organic aerosol composition) or perhaps an influence from changing matrix effects in the ATOFMS measurements as components were removed with heating. These possibilities are discussed in the SI. Overall, however, the ability to use a single ion marker as a reference component for these volatility measurements confirms the above hypothesis that changes in particle age (achieved through heating in this work) influenced the ion yield nearly proportionately for all species contained within the measured OC particles. Further, the application of the RC method to ambient temperature data during SOAR-2 demonstrated ∼3–5 fold improvement in the correlation between ATOFMS and AMS data relative to the APA approach for ammonium and nitrate (see the SI). The agreement between the ATOFMS and AMS thermograms with this RC approach demonstrates the potential to achieve quantitative measurements (such as mass concentrations by particle type) from single-particle mass spectrometers without the need to scale data to co-located bulk-phase measurements.
CONCLUSIONS AND IMPLICATIONS
In this work, a thermodenuder was used in-line with ATOFMS to characterize the ionization effects of ambient aerosol particles as semi-volatile coatings were removed. Jeong et al. (Citation2011) previously postulated that an observed decrease in ATOFMS hit efficiency for ambient particles could have been due to accumulation of secondary species with high albedo such as ammonium sulfate. Our results support their hypothesis, as the ATOFMS hit efficiency increased as secondary coatings were removed and exposed a more strongly absorbing core. Further, it was observed that secondary coatings influence ion formation. After such coatings were removed through heating, the particles were more readily ablated, resulting in greater photoionization ion yield. These results highlight that particle aging in the atmosphere can influence the degree of ionization and thus the resulting ion peak areas measured by ATOFMS. These ionization effects may be corrected by utilizing relatively inert aerosol components as a reference for the changes in LDI efficiency; the resulting normalized ion peak areas were clearly proportional to the species’ mass, as demonstrated by the strong agreement with the independent AMS data. The correlation between ATOFMS and AMS via the RC method for nearly all ion markers considered confirms that the deviations in the ATOFMS APAs relative to the AMS results were largely due to ionization effects that impacted all particle constituents. If the APA trends had been the result of changes in competitive charge-transfer or collision-induced matrix effects, then the intensity of each ion marker would have been affected to different extents and the success of the RC-normalization approach would be more variable. Notably, the organic ions were a significant exception to these findings and thus future comparisons between the AMS and ATOFMS should be conducted to better understand each instrument's respective biases. In general, however, normalizing peak areas by an “inert” component of the particle most accurately reproduced the volatility of these secondary species because the RC gave a relatively unbiased measure of how the LDI efficiency changed with heating. These results underscore the importance of better understanding how SPMS measurements of ambient aerosols change as a function of atmospheric processing in order to ultimately achieve quantitative measurements of chemical mixing state. The methods outlined herein provide a significant step toward that goal.
SUPPLEMENTARY MATERIALS
Supplemental data for this article can be accessed on the publisher's website.
LHatch_et_al.__Impacts_of_aerosol_aging__SuppInfo_Final.docx.zip
Download Zip (3.6 MB)ACKNOWLEDGMENTS
Paul Ziemann (UC-Riverside), Ken Docherty, Peter DeCarlo, and Michael Cubison (CU-Boulder), the UC Riverside Air Pollution Research Center, and the entire Prather Group are thanked for support during SOAR. Helpful discussions and manuscript reviews by John Cahill are gratefully acknowledged.
Additional information
Funding
REFERENCES
- Allen, J.O. (2001). YAADA: Software Toolkit to Analyze Single-Particle Mass Spectral Data. Software Reference Manual.
- Bhave, P.V., Allen, J.O., Morrical, B.D., Fergenson, D.P., Cass, G.R., and Prather, K.A. (2002). A Field-Based Approach for Determining ATOFMS Instrument Sensitivities to Ammonium and Nitrate. Environ. Sci. Tech., 36(22):4868–4879.
- Cai, Y., Zelenyuk, A., and Imre, D. (2006). A High Resolution Study of the Effect of Morphology on the Mass Spectra of Single PSL Particles with Na-Containing Layers and Nodules. Aerosol Sci. Technol., 40(12):1111–1122.
- Carson, P.G., Johnston, M.V., and Wexler, A.S. (1997). Real-Time Monitoring of the Surface and Total Composition of Aerosol Particles. Aerosol Sci. Technol., 26(4):291–300.
- Dall’Osto, M., Harrison, R.M., Beddows, D.C. S., Freney, E.J., Heal, M.R., and Donovan, R.J. (2006). Single-Particle Detection Efficiencies of Aerosol Time-of-Flight Mass Spectrometry During the North Atlantic Marine Boundary Layer Experiment. Environ. Sci. Tech., 40(16):5029–5035.
- Docherty, K.S., Aiken, A.C., Huffman, J.A., Ulbrich, I.M., DeCarlo, P.F., Sueper, D., et al. (2011). The 2005 Study of Organic Aerosols at Riverside (SOAR-1): Instrumental Intercomparisons and Fine Particle Composition. Atmos. Chem. Phys., 11(23):12387–12420.
- Docherty, K.S., Stone, E.A., Ulbrich, I.M., DeCarlo, P.F., Snyder, D.C., Schauer, J.J., et al. (2008). Apportionment of Primary and Secondary Organic Aerosols in Southern California During the 2005 Study of Organic Aerosols in Riverside (SOAR-1). Environ. Sci. Tech., 42(20):7655–7662.
- Drewnick, F., Hings, S.S., DeCarlo, P., Jayne, J.T., Gonin, M., Fuhrer, K., et al. (2005). A New Time-of-Flight Aerosol Mass Spectrometer (TOF-AMS) - Instrument Description and First Field Deployment. Aerosol Sci. Technol., 39(7):637–658.
- Ge, Z.Z., Wexler, A.S., and Johnston, M.V. (1998). Laser Desorption/Ionization of Single Ultrafine Multicomponent Aerosols. Environ. Sci. Tech., 32(20):3218–3223.
- Gross, D.S., Galli, M.E., Silva, P.J., and Prather, K.A. (2000). Relative Sensitivity Factors for Alkali Metal and Ammonium Cations in Single Particle Aerosol Time-of-Flight Mass Spectra. Anal. Chem., 72(2):416–422.
- Huffman, J.A., Docherty, K.S., Aiken, A.C., Cubison, M.J., Ulbrich, I.M., DeCarlo, P.F., et al. (2009a). Chemically-Resolved Aerosol Volatility Measurements from Two Megacity Field Studies. Atmos. Chem. Phys., 9(18):7161–7182.
- Huffman, J.A., Docherty, K.S., Mohr, C., Cubison, M.J., Ulbrich, I.M., Ziemann, P.J., et al. (2009b). Chemically-Resolved Volatility Measurements of Organic Aerosol from Different Sources. Environ. Sci. Tech., 43(14):5351–5357.
- Huffman, J.A., Jayne, J.T., Drewnick, F., Aiken, A.C., Onasch, T., Worsnop, D.R., et al. (2005). Design, Modeling, Optimization, and Experimental Tests of a Particle Beam width Probe for the Aerodyne Aerosol Mass Spectrometer. Aerosol Sci. Technol., 39(12):1143–1163.
- Huffman, J.A., Ziemann, P.J., Jayne, J.T., Worsnop, D.R., and Jimenez, J.L. (2008). Development and Characterization of a Fast-Stepping/Scanning Thermodenuder for Chemically-Resolved Aerosol Volatility Measurements. Aerosol Sci. Technol., 42:395–407.
- Jeong, C.H., McGuire, M.L., Godri, K.J., Slowik, J.G., Rehbein, P.J. G., and Evans, G.J. (2011). Quantification of Aerosol Chemical Composition using Continuous Single Particle Measurements. Atmos. Chem. Phys., 11(14):7027–7044.
- Johnson, G., Ristovski, Z., and Morawska, L. (2004). Application of the VH-TDMA Technique to Coastal Ambient Aerosols. Geophys. Res. Lett., 31(16):L16105.
- Kane, D.B., and Johnston, M.V. (2000). Size and Composition Biases on the Detection of Individual Ultrafine Particles by Aerosol Mass Spectrometry. Environ. Sci. Tech., 34(23):4887–4893.
- Liu, P., Ziemann, P.J., Kittelson, D.B., and Mcmurry, P.H. (1995). Generating Particle Beams of Controlled Dimensions and Divergence.1. Theory of Particle Motion in Aerodynamic Lenses and Nozzle Expansions. Aerosol Sci. Technol., 22(3):293–313.
- Mansoori, B.A., Johnston, M.V., and Wexler, A.S. (1994). Quantitation of Ionic Species in Single Microdroplets by Online Laser Desorption/Ionization. Anal. Chem., 66(21):3681–3687.
- Moffet, R.C., and Prather, K.A. (2009). In-Situ Measurements of the Mixing State and Optical Properties of Soot with Implications for Radiative Forcing Estimates. Proc. Nat. Acad. Sci., 106(29):11872–11877.
- Murphy, D.M., Cziczo, D.J., Froyd, K.D., Hudson, P.K., Matthew, B.M., Middlebrook, A.M., et al. (2006). Single-Particle Mass Spectrometry of Tropospheric Aerosol Particles. J. Geophys. Res., 111(D23):D23S32.
- Pratt, K.A., Hatch, L.E., and Prather, K.A. (2009a). Seasonal Volatility Dependence of Ambient Particle Phase Amines. Environ. Sci. Tech., 43(14):5276–5281.
- Pratt, K.A., Mayer, J.E., Holecek, J.C., Moffet, R.C., Sanchez, R.O., Rebotier, T.P., et al. (2009b). Development and Characterization of an Aircraft Aerosol Time-of-Flight Mass Spectrometer. Anal. Chem., 81(5):1792–1800.
- Pratt, K.A., and Prather, K.A. (2009). Real-Time, Single-Particle Volatility, Size, and Chemical Composition Measurements of Aged Urban Aerosols. Environ. Sci. Tech., 43(21):8276–8282.
- Pratt, K.A., and Prather, K.A. (2012). Mass Spectrometry of Atmospheric Aerosols: Recent Developments and Applications. Part II: On-Line Mass Spectrometry Techniques. Mass Spectrom. Rev., 31(1):17–48.
- Qin, X., Pratt, K.A., Shields, L.G., Toner, S.M., and Prather, K.A. (2012). Seasonal Comparison of Single-Particle Chemical Mixing State in Riverside, CA. Atmos. Environ., 59, 587–596.
- Qiu, C., and Zhang, R.Y. (2012). Physiochemical Properties of Alkylaminium Sulfates: Hygroscopicity, Thermostability, and Density. Environ. Sci. Tech., 46(8):4474–4480.
- Reilly, P.T. A., Lazar, A.C., Gieray, R.A., Whitten, W.B., and Ramsey, J.M. (2000). The Elucidation of Charge-Transfer-Induced Matrix Effects in Environmental Aerosols via Real-Time Aerosol Mass Spectral Analysis of Individual Airborne Particles. Aerosol Sci. Technol., 33(1–2):135–152.
- Reinard, M.S., and Johnston, M.V. (2008). Ion Formation Mechanism in Laser Desorption Ionization of Individual Nanoparticles. J. Am. Soc. Mass. Spectrom., 19(3):389–399.
- Russell, A.G., and Cass, G.R. (1986). Verification of a Mathematical-Model for Aerosol Nitrate and Nitric-Acid Formation and Its Use for Control Measure Evaluation. Atmos. Environ., 20(10):2011–2025.
- Salo, K., Westerlund, J., Andersson, P.U., Nielsen, C., D’Anna, B., and Hallquist, M. (2011). Thermal Characterization of Aminium Nitrate Nanoparticles. J. Phys. Chem. A, 115(42):11671–11677.
- Schoolcraft, T.A., Constable, G.S., Jackson, B., Zhigilei, L.V., and Garrison, B.J. (2001). Molecular Dynamics Simulations of Laser Disintegration of Amorphous Aerosol Particles with Spatially Nonuniform Absorption. Nuclear Instrum Methods Phy Res Sec B—Beam Interac Materials Atoms, 180:245–250.
- Schoolcraft, T.A., Constable, G.S., Zhigilei, L.V., and Garrison, B.J. (2000). Molecular Dynamics Simulation of the Laser Disintegration of Aerosol Particles. Anal. Chem., 72(21):5143–5150.
- Seinfeld, J.H., and Pandis, S.N. (2006). Atmospheric Chemistry & Physics: From Air Pollution to Climate Change (2nd. ed.), John Wiley & Sons, Inc., New Jersey.
- Silva, P.J., and Prather, K.A. (2000). Interpretation of Mass Spectra from Organic Compounds in Aerosol Time-of-Flight Mass Spectrometry. Anal. Chem., 72(15):3553–3562.
- Song, X.H., Hopke, P.K., Fergenson, D.P., and Prather, K.A. (1999). Classification of Single Particles Analyzed by ATOFMS using an Artificial Neural Network, ART-2A. Anal. Chem., 71(4):860–865.
- Spencer, M.T., and Prather, K.A. (2006). Using ATOFMS to Determine OC/EC Mass Fractions in Particles. Aerosol Sci. Technol., 40(8):585–594.
- Thomson, D.S., Middlebrook, A.M., and Murphy, D.M. (1997). Thresholds for Laser-Induced Ion Formation from Aerosols in a Vacuum Using Ultraviolet and Vacuum-Ultraviolet Laser Wavelengths. Aerosol Sci. Technol., 26(6):544–559.
- Wade, E.E., Farquar, G.R., Steele, P.T., McJimpsey, E.L., Lebrilla, C.B., and Fergenson, D.P. (2008). Wavelength and Size Dependence in Single Particle Laser Aerosol Mass Spectra. J. Aerosol Sci, 39(8):657–666.
- Wenzel, R.J., Liu, D.Y., Edgerton, E.S., and Prather, K.A. (2003). Aerosol Time-of-Flight Mass Spectrometry During the Atlanta Supersite Experiment: 2. Scaling Procedures. J. Geophys. Res., 108(D7):8427.
- Wenzel, R.J., and Prather, K.A. (2004). Improvements in Ion Signal Reproducibility Obtained using a Homogeneous Laser Beam for on-Line Laser Desorption/Ionization of Single Particles. Rapid Commun. Mass Spectrom., 18(13):1525–1533.
- Zauscher, M.D. (2012). Extending the Physicochemical Characterization of Aerosol Particles in California, Ph.D. Thesis, University of California, San Diego, La Jolla, CA.
- Zelenyuk, A., Yang, J., and Imre, D. (2009). Comparison Between Mass Spectra of Individual Organic Particles Generated by UV Laser Ablation and in the IR/UV Two-Step Mode. Int. J. Mass spectrom., 282(1–2):6–12.
- Zelenyuk, A., Yang, J., Song, C., Zaveri, R.A., and Imre, D. (2008). “Depth-Profiling” and Quantitative Characterization of the Size, Composition, Shape, Density, and Morphology of Fine Particles with SPLAT, a Single-Particle Mass Spectrometer. J. Phys. Chem. A, 112(4):669–677.
