ABSTRACT
An experimental method is developed for the purpose of simulating plutonium aerosol source terms with conventional metals in laboratory. In this method, metal samples are aerosolized by high explosive detonation in a containment vessel. Aerosols having aerodynamic diameter (AD) less than 10 µm are then collected by a cascade impactor and analyzed by atomic absorption spectroscopy. Two sets of experiments were conducted. In the first set, five candidate metal samples (Ag, W, Sn, Ce, and V) were tested. It is found that the cumulative mass distribution of silver under certain conditions was in good agreement with that of plutonium from the Operation Roller Coaster-Double Track experiment. Thus, silver is chosen as a surrogate to simulate the plutonium aerosol source term. In the second set, silver aerosol source term was studied in detail with different test configurations. The results demonstrate that the peak of the mass-size distribution of silver is in the AD range 1.1–3.3 µm. The amount and fraction of relatively small silver aerosols decrease significantly with time due to coagulation and deposition. Interestingly, the amount of silver in aerosols could be expressed as a quadratic function of the peak detonation pressure.
© 2016 American Association for Aerosol Research
EDITOR:
1. Introduction
Plutonium (Pu) is a radioactive and toxic metal with its chemical property highly active. As the most important isotope of plutonium, 239Pu is a key fissile component in nuclear devices. The most likely and dangerous entry route for plutonium is inhalation (Fetter and Von Hippel Citation1990; Voelz and Buican Citation2000). Once inhaled, plutonium is able to remain in the lungs or lymph system, or be absorbed by the blood and transported to the liver or bones, depending on its size and increasing the probability of cancer (Stewart et al. Citation1965; Fetter and Von Hippel Citation1990; Voelz and Buican Citation2000; Liu et al. Citation2011). It is commonly assumed that particles having aerodynamic diameter (AD) 10 µm and less are respirable (Mishima and Pinkston Citation1994). These particles can partially or completely penetrate into the gas-exchange region of the lungs (World Health Organization Citation1999). The maximum allowed burden of plutonium in lungs is 0.3 µg and the prompt fatality amount of plutonium in lungs is 4.3 mg (Condit Citation1993). In the case of explosion or combustion by accident, plutonium from nuclear devices could be liquefied and violently oxidized. Meanwhile, the reaction product (PuO2) is dispersed into the atmosphere in the form of aerosols (Haschke and Martz Citation1998; Martz and Haschke Citation1998). For example, several accidents happened in 1960s in which high explosive in nuclear warheads exploded and contaminated the environment with plutonium (Fetter and Von Hippel Citation1990). Therefore, evaluation of the plutonium aerosol source term (the total mass and size distribution of aerosols) is of main importance for accident prevention, risk assessment, and emergency response.
A series of studies on the plutonium aerosol source term and its dispersal (Andersen Citation1964; Fuller Citation1965; Stewart et al. Citation1965; Titu, Citation1965; Ettinger et al. Citation1972; Elder et al. Citation1974; Craig et al. Citation1976; Haschke Citation1992; Stephens Citation1995; Haschke and Martz Citation1998) have been conducted by the United States, including field and laboratory experiments. The most famous field experiment, called Operation Roller Coaster (ORC), was developed by the United States and the United Kingdom in 1963 (Fuller Citation1965; Stewart et al. Citation1965; Titus Citation1965) to simulate accidental chemical explosion involving nuclear material. Systematic and detailed data on the source term and the dispersal of plutonium aerosols under the conditions of chemical explosions were obtained. After ORC, all the field experiments that may cause plutonium dispersal into the atmosphere were stopped. Thus, ORC provides the most important explosive source term and dispersal distribution data of plutonium aerosols for future numerical simulation investigations (Dewar et al. Citation1982; Boughton and DeLaurentis Citation1992; Homann and Wilson Citation1995; Steele et al. Citation1998). Different numerical models such as DIFOUT (Luna and Church Citation1969), HOTSPOT (Homann and Wilson Citation1995), MACCS2 (Steele et al. Citation1998), ERAD (Boughton and DeLaurentis Citation1992), etc., have been developed by Los Alamos National Laboratory (LANL), Lawrence Livermore National Laboratory (LLNL), and Sandia National Laboratory (SNL) for the simulation of plutonium aerosol dispersal. The former three models are based on the Gaussian plume model and the last one is a three-dimensional model calculated by Monte Carlo method. There are other approaches relevant to the study of plutonium aerosols and its dispersal, such as the evaluation of the deposition (Bunzl and Kracke Citation1988; Johansen et al. Citation2014) and resuspension (Shinn et al. Citation1986; Shinn Citation2002) of plutonium in nuclear test (or accident) sites, the transport of plutonium from nuclear test (or accident) sites (Wendel et al. Citation2013; Shinonaga et al. Citation2014), the examination of the plutonium uptake into wildlife (Johansen et al. Citation2013, Citation2014).
The plutonium contamination caused by high explosive detonation is one of the most interested scenarios of nuclear accident. Whereas explosive experiments on plutonium are currently infeasible for safety and political reasons. Finding an alternative material to simulate the plutonium aerosol source term experimentally is thus desirable. For example, cerium (cerium oxide) and sodium chloride were used as surrogates for plutonium (oxide) in different studies (Fernandez and Burghoffer Citation1995; Zamoryanskaya and Burakov Citation2000; Olson et al. Citation2007; Kim et al. Citation2008). In addition, a deep understanding of different characteristics of aerosols and conditions influencing them is needed (Simones et al. Citation2011; Lemma et al. 2014; Simones and Loyalka Citation2015). Silver was used in explosive tests substituted for plutonium (Sagartz Citation1995; Hao et al. Citation2009). In 1995, M. Sagartz from SNL simulated the release of plutonium aerosol in a nuclear accident by imploding a silver spherical shell with PBX-9404 (Sagartz Citation1995). The impact of experimental configuration and explosive chemical energy on the release fraction was investigated. In 2009, Hao et al. conducted an experimental study on the explosive silver aerosol source term focusing on the size distribution and the coagulation (Hao et al. Citation2009). In these works, the reason of using silver as a surrogate was, however, not explicitly clarified, and the source term of silver aerosol was not compared with that of plutonium. The characteristics of silver aerosols were also investigated in other approaches (Ku and Maynard Citation2005; Simones et al. Citation2011; Harra et al. Citation2012). To our knowledge, the basis on which we could use silver as a surrogate for plutonium as well as the explosive silver aerosol source term with different test configurations is not extensively investigated.
In this article, an experimental method is developed to investigate the explosive aerosol source term in laboratory using a confinement vessel and a non-viable cascade impactor. Two sets of experiments were conducted. In the first set, the aerosol source terms of five candidate metals (Ag, W, Sn, Ce, and V) were tested and compared with that of plutonium obtained from ORC-DT (Double Tracks) experiment. The results show that the only metal among them that can be used to simulate the plutonium aerosol source term is silver, whose cumulative mass distribution (CMD) of aerosols is in good agreement with that of plutonium (Luna et al. Citation1971) under certain conditions. In this work, the CMD is defined as
, where
is the mass of metal associated with aerosol particles having AD
µm and
is the mass of metal in the respirable aerosols with AD ≤ 10 µm. In the second set, different aspects of the silver aerosol source term, including the evolution characteristic of the size–mass distribution, the phenomenon of coagulation and sedimentation, and the impact of detonation pressure, were studied in detail. Interestingly, it is found that the amount of silver in aerosols can be expressed as a quadratic function of the detonation pressure. The simulation of plutonium aerosol source term by a conventional metal is realized in this work. The source term data of our experiments would be significant for accident prevention, emergency response, etc., on the other hand, it provides input parameters for numerical simulations.
2. Experimental method
2.1. Candidate metals
In this article, silver (Ag), tungsten (W), tin (Sn), cerium (Ce), and vanadium (V) are chosen as the candidate metals for the aerosol tests to simulate the plutonium aerosol source term mainly due to their physical properties. The basic physical properties (Lide Citation2005) of the candidate metals as well as plutonium are presented in . These metals share some similar physical properties with plutonium, which are summarized as follows. Like plutonium, silver and tin have low melting point and high boiling points. Tungsten and plutonium have similar densities. As a widely used surrogate of plutonium (Fernandez and Burghoffer Citation1995; Zamoryanskaya and Burakov Citation2000; Kim et al. Citation2008), cerium has low melting point, high boiling point, high number of allotropes, low-symmetry crystal structure, and change in volume with phase transitions like plutonium. The boiling point of vanadium is close to that of plutonium.
Table 1. Basic physical properties of Pu and the candidate metals.
The oxidization properties of these metals are summarized as follows. Plutonium slowly oxidizes at room temperature and readily oxidizes with elevated temperature and humid air. It spontaneously ignites at about 150°C when divided under 1 mm diameter. The chemical properties of silver is inactive, it does not oxidize in the air under normal circumstances, and oxidizes in air at 200°C. Tungsten is stable in dry and humid air at moderate temperature. It starts to oxidize at about 400°C and the oxidation rate increases rapidly above 700°C. Tin is stable under normal circumstances, it oxidizes when heated in air. Cerium oxidizes very readily at room temperature, especially in moist air and ignites at 160°C. Vanadium is stable in air under normal temperature and oxidizes readily above 660°C. It is seen that the candidate metals all oxidizes at high temperature, of which cerium has similar chemical properties with plutonium.
2.2. Instrumentation
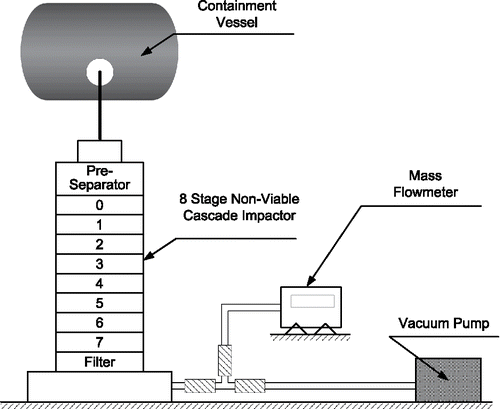
The experimental set-up consists of a highly sealed explosion containment vessel (2.5 kg TNT equivalent), an aerosol collecting system, and a pumping system as depicted in . The main body of the containment vessel is a cylindrical shell (horizontally placed) with two ellipsoidal heads. The inner diameter and length of the cylindrical shell are 2.000 m and 1.600 m, respectively. The volume of the vessel is 7.300 m3. The pumping system is composed of a vacuum pump, angle valves, and bellows, which is used to evacuate the containment vessel. The height of the pumping hole is 0.44 m higher than the lowest inner surface of the vessel. The collecting system includes an eight-stage non-viable Andersen cascade impactor (Model TE-20-800, USA) and a mass flow meter.
Aerosol particles are separated and collected by a preseparator, eight collection plates, and a final filter as they pass through the cascade impactor. First, aerosol particles with AD > 10 µm are trapped in the preseparator. Then, smaller aerosol particles are sampled in the following AD range: stage (0) 9–10 µm, stage (1) 5.8–9 µm, stage (2) 4.7–5.8 µm, stage (3) 3.3–4.7 µm, stage (4) 2.1–3.3 µm, stage (5) 1.1–2.1 µm, stage (6) 0.7–1.1 µm, stage (7) 0.4–0.7 µm, where (0–7) is the number of the corresponding collection plate. At last, aerosol particles with AD < 0.4 µm are collected in the final filter. In each stage, particles of corresponding AD range are inertially impacted onto the collection plate and collected on a collection substrate. Detailed information of the cascade impactor could be seen from “Model 20–800 Ambient Cascade Impactor (Non-viable) OPERATIONS MANUAL.” In our experiments, quartz filters (Tisch International, Inc., USA) are used as collection substrates for better performance of metal aerosol deposition (Kulkarni et al. Citation2011). To ensure the separation accuracy of the impactor, the flow rate is 28.3 ± 1.5 L/min with the help of the mass flow meter and the adjustable valves of the pumping system.
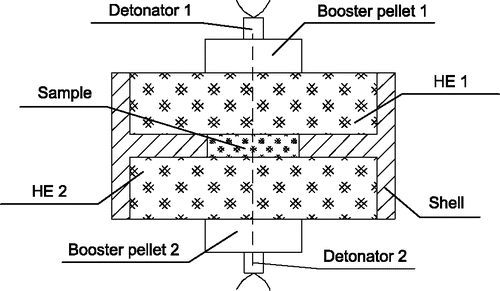
The candidate metal samples are explosively loaded in a device as shown in , where the sample (disk or powder) is placed in the center of two facing cylindrical disks of high explosive. This is a simplified non-weapon model as used in the plutonium source term study in ORC-DT field experiments. The size of the high explosive disk is typically Φ100 mm × 20 mm. The device is held by a plexiglass shell of 5 mm thickness. Two BL-21 detonators with their booster pellets (PETN, Φ32 mm × 11 mm) are on the outer surface of the device to initiate the explosives (). Detonations at two points and at one point (with detonator 2 and booster pellet 2 removed) can both be realized with the device. In our experiments, the high explosive RHT-901 is used for the detonation of all the five metals. Other explosives such as HMX, TNT, and RTB-904 are used for further investigations of silver. The main explosive performances (Zhou and Dong Citation1989) are listed in , including the detonation heat, specific gas volume (volume of gas produced per mass of explosive), detonation velocity, and detonation pressure. Several steps have been taken to reduce the impurities of the explosive products. For example, the inner wall of the vessel is cleaned before tests, the detonating device is suspended by cotton ropes (rather than wooden holder) and the detonator cover is made of plexiglass (rather than aluminum).
Table 2. Main properties of the explosives used in our experiments.
2.3. Experimental procedure
Two sets of experiments were conducted to determine the appropriate metal for the simulation of plutonium aerosol and further investigate the characteristics of silver aerosol source term, respectively. The procedures of the two sets of experiments are introduced in the following subsections.
2.3.1. Set 1
In the first set, six tests were carried out in which five metal samples were explosively loaded by RHT-901, and silver was additionally loaded by HMX. The explosives were two-point detonated in all the six tests. The detailed test parameters are shown in . In each test, the first sampling was implemented 1.5 min after detonation followed by one or two later samplings. The sampling instants of the tests are listed in . Each sampling duration was 10 min in which 283 liters (approximately 3.877% of the volume of the containment vessel) of gases were pumped out.
Table 3. Test parameters of the first set of experiments (the mass of explosive is for two disks in a test).
Table 4. Sampling instants in the first set of experiments.
For each impactor stage, the amount of metal in aerosols was determined by atomic absorption spectroscopy after electrochemical digestion. The cumulative mass distribution, the mass–size distribution, and the total amount of the metals were then obtained. The total amount of metal in aerosols (AD ≤ 10 µm) in the containment vessel was calculated based on the first sampling result. It is assumed in the calculations that the spatial distribution of detonation products in the vessel is uniform since the samples were detonated in the center of the vessel. The cumulative mass distribution of silver (test 1, 2nd sampling) was found to be in good agreement with the results from the ORC-DT experiment (Section 3). Thus, the silver aerosol was further investigated in the second set of experiments. In addition, the solid residues in the contaminate vessel after test 1 were examined by scanning electron microscope (SEM).
2.3.2. Set 2
Seven explosive tests on silver were conducted in the second set of experiments. In these tests, all samples are 45 g silver disks (99.99% purity). The detailed test parameters are shown in . The first three tests were carried out mainly to evaluate the sampling error of the impactor where RHT-901 and two-point detonation are adopted. In test 4–7, the samples were one-point or two-point detonated by RHT-901, TNT, or RTB-904. The following four tests aim to study the amount and size distribution of the silver in aerosols with different test configurations. The peak detonation pressures with different detonation types and explosives were theoretically calculated and shown in . The relationship of the silver amount in aerosols and the peak detonation pressure is discussed in Section 3.2.3. Three samplings were implemented in test 3 and one sampling in the other tests. The sampling instants are listed in and the sampling duration (volume of the pumped gases each sampling) is the same with that in set 1.
Table 5. Test parameters of the second set of experiments on silver.
Table 6. Sampling instants in the second set of experiments.
As done in the first set of experiment, the amount of silver in aerosols in each impactor stage is obtained by atomic absorption spectroscopy after electrochemical digestion. The size distribution and total amount of silver in each sampling are then obtained.
2.3.3. Temperature and pressure
In this subsection, we briefly estimate the temperature and pressure of the gas during the samplings and their influences on the cascade impactor. In the experiments, the containment vessel was initially filled with air at ambient temperature and pressure (about 20°C and 1 atm). The temperature and pressure of the gas during the sampling could be theoretically and empirically estimated as follows. The detonation heat of the explosives are typically 3–5 MJ/kg, the specific gas volume of the explosives are 800–1000 L/kg, and the mass of explosive are typically 0.54 kg. For rough estimation (ignore the gas produced by explosion and the metal samples), the equilibrium temperature of the air in the vessel after detonation is about 300°C. However, experimental study (Liu et al. Citation2013) shows that the temperature on the inner wall of a vessel can soon drop to the ambient temperature (less than 40°C) in seconds from over 200°C. Considering our samplings were conducted at least 30 s after detonation, the temperature of gas in the impactor during the samplings would be close to the ambient temperature due to the heat exchange with the environment. The pressure of gas in the vessel could be estimated based on the specific gas volume of explosives and vessel's volume (7.3 m3). Assume that the temperature after detonation has reached the ambient temperature, the pressure in the vessel (using 1000 L/kg specific gas volume and 0.54 kg explosive) is about 1.07 atm. In fact, a rubber hose was used for connecting the vacuum pump and no evidence of high temperature and pressure was found by us. During the experiments, the pressure would decease due to the pumped gas and the decrease of temperature. To sum up, it is estimated that the cascade impactor was working at (or close to) the ambient temperature and pressure and the cut-points of the impactor were not significantly affected.
3. Results and analyses
3.1. Determination of the surrogate for plutonium
In the first set of experiments, five candidate metal samples were explosively loaded and the aerosols were sampled at different instants (see for details). In order to find a surrogate metal to simulate the plutonium aerosol source term, the CMDs of the five metals for every sampling were calculated and compared with that of plutonium from ORC-DT (Luna et al. Citation1971).
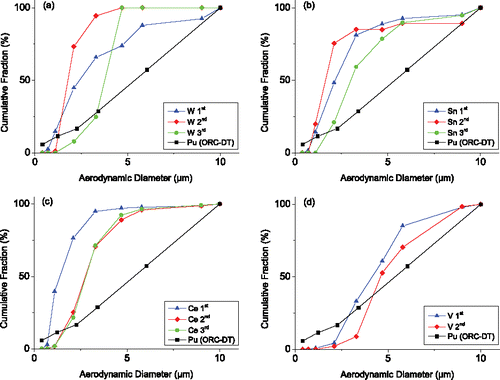
The four CMDs of silver (tests 1 and 6 of set 1, all samplings) are compared with the ORC-DT result in . The corresponding mass–size distribution of silver is shown in . It is seen that the sampling instant and explosive type significantly influence the size distribution of silver. The overall CMD curves of test 6 (loaded by HMX) significantly deviate from the ORC-DT result. In the AD range 2.3–10 µm, the CMD curve (test 6) for the first sampling (after 1.5 min) is lower than the ORC-DT result evidently. Whereas in the AD range 2.1–10 µm, the CMD curve (HMX) for the second sampling (after 106.5 min) is evidently higher than the ORC-DT result. The increase of CMD curve of silver in test 6 can be explained by the deposition of larger particles. shows that in the first sampling of test 6, a large fraction of silver is contained in aerosol particles of 5.8–10 µm AD. According to the Stokes's Law, the terminal settling velocity of particles may be theoretically estimated by , where
is the aerodynamic diameter,
is the gravitational acceleration, and
is the viscosity of air. The maximal settling height (the inner diameter of the vessel) is 2 m. Considering the above conditions, aerosol particles with AD > 5.8 µm would have
> 0.001 m/s and fully deposit in the vessel in about 33 min. Thus, in the second sampling of test 6, the amount of these large particles decreased significantly due to deposition. In contrast, the coagulation effect is weak because of the small amount of smaller particles. For silver aerosols generated by the RHT-901 explosive (test 1), the CMD curve for the second sampling (after 106.5 min) is close to the ORC-DT result in all the AD range. In contrast, the CMD curve (test 1) for the first sampling (after 1.5 min) is higher than the ORC-DT result in most AD range. The evolution of the CMD could also be explained from the mass–size distribution result in . In the first sampling of test 1, the peak of the mass–size distribution curve lies in the AD range 1.1–2.1 µm. Thus, the coagulation of smaller particles played an important role in this test, leading to an increasing fraction of large particles as seen in . Following the above analysis, the initial aerosol particles with AD > 3.2 µm will fully deposit in 106.5 min before the second sampling. However, the amount of silver in aerosol particles with AD 3.3–10 µm did not drop too much as seen in . This implies that the coagulation in test 1 formed larger particles of AD 3.3–10 µm. The coagulation process is caused by Brownian motion, gravitational settling, and gas turbulence (early after detonation), and affected by charge (Williams and Loyalka Citation1991; Simones and Loyalka Citation2015).
Figure 3. (a) Cumulative mass distributions of silver from set 1 compared with the ORC-DT result. Here 1st (2nd) represents the first (second) sampling. The cumulative mass distribution (RHT-901) for the second sampling is in good agreement with the ORC-DT result. (b) Mass–size distribution of silver in correspondence with (a).
The CMDs of tungsten, tin, cerium, and vanadium (test 2–5 of set 1, all samplings) are shown in , respectively, and compared with the ORC-DT result. The CMDs for the four metals all exhibit evident deviations from the ORC-DT result. More concretely, the CMD curves of cerium, tin, and tungsten (all samplings) are much higher than the ORC-DT result in the middle AD range. The CMDs of vanadium are closer to the ORC-DT result compared with tungsten, tin, and cerium, yet the overall curve are still different from the ORC-DT result.
Figure 4. Cumulative mass distributions of W, Sn, Ce, and V compared with the ORC-DT result. The CMDs of the four metals do not agree with the ORC-DT result.
The above results demonstrate that the CMD of plutonium aerosol could be simulated by silver with certain conditions such as two-point detonation, the RHT-901 explosive, and a later sampling instant. Thus, silver could be chosen from the candidate metals as a surrogate for plutonium to simulate the CMD of plutonium. Another important source term parameter is the respirable release fraction (RRF), which is defined as the product of ARF (aerosol release fraction) and RF (respirable fraction of ARF). Here, the RRF is calculated by , where
is the mass of metal contained in the respirable aerosols (AD ≤ 10 µm) in a test, and
is the total mass of the metal sample. Unfortunately, the RRF of all the five candidate metals are quite different from that of plutonium. shows the RRFs of silver, tungsten, tin, cerium, and vanadium calculated from tests 1–5 of set 1 (loaded by RHT-901) and a typical RRF of plutonium (high explosive detonation) (Stephens Citation1995). The RRFs of the five metals are 1–4 orders of magnitude less than the plutonium RRF, in which the RRF of V is the highest because of its initial powder form. Therefore, the RRF of plutonium could not be simulated directly by the candidate metals in our experiments. Moreover, no correlation between the RRFs of different metals and their melting points is found in our results.
Table 7. Respirable release fraction of the candidate metals (loaded by RHT-901) and plutonium.
The explosives are detonated in air and the detonation temperature is in the order of 1000°C. The fraction of aerosolized metal is low as seen in the manuscript and most metal became large particles. According to the chemical properties of the candidate metals, we infer that all the candidate metals were oxidized or partially oxidized in the detonation process. Besides into the respirable aerosols, most of the metals became larger particles, which may include metal, metal oxide, and other impurities (from the explosive shell, detonator, etc.). These large particles would settle on the ground of the vessel in a short time.
3.2. Characters of silver aerosol source term
After choosing silver as the surrogate metal of plutonium, seven tests of silver were carried out in the second set of experiments and nine samplings were implemented (). The mass–size distribution data of the nine samplings are shown in , where the mass of silver in aerosols collected in each impactor stage and in each sampling are listed. The characteristics of silver aerosol source term are discussed in detail in this subsection based mainly on the result in .
Table 8. Mass-size distribution data from the second set of experiments.
3.2.1. Feasibility of our experimental method
Before discussing the characteristics of silver aerosols, we demonstrate the feasibility of our experiment by checking the error of the atomic absorption spectroscopy (for the measurement of silver mass) and the repeatability of the CMD of silver.
To estimate the error of the measurement of silver mass after electrochemical digestion, four sample silver solutions with known concentrations (5 µg/L and 30 µg/L) were tested by atomic absorption spectroscopy. The measured concentrations and errors of the sample solutions are presented in . The errors of the four measurements are δ1 = +0.42%, δ2 = +1.97%, δ3 = +1.57%, and δ4 = −0.06%, respectively, with an average error δ = 0.975%. The concentration tests show that the amount of silver in aerosols could be measured with a good accuracy.
Table 9. Measurement results of silver concentration in sample solutions.
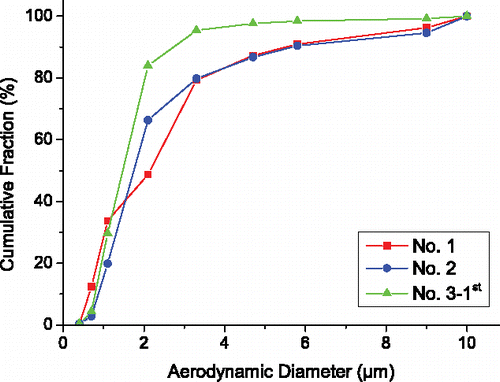
In set 2, the sampling instants of test 1 and 2 are early and that of test 3 (first sampling) are a little later. Other test conditions such as the explosive and detonation type for the three samplings are the same. Thus, the three samplings (tests 1, 2, and test 3–1st) in set 2 can be used to examine the repeatability of our experiment. The CMDs for the three samplings are shown in . It is observed that the CMD curves for test 1 and test 2 are close, especially in the AD range 3.3–10 µm. The slight difference of the two curves in the AD range 0–3.3 µm may originate from the randomness of the explosion processes and the errors in the samplings and measurements. The CMD curve of test 3–1st is higher than that of test 1 and test 2 in the middle AD range. However, the shape of the CMD curve for test 3–1st is similar to the other two curves. The results in demonstrate that the CMD of silver in our tests is repeatable and reliable.
Figure 5. Cumulative mass distributions of silver in aerosol for test 1, test 2, and test 3–1st of set 2. The test configurations of the three tests are the same and their sampling instants are early. The similarity of the three curves demonstrate the CMD of silver in aerosols in our tests is repeatable and reliable.
3.2.2. Size distribution of silver in aerosols
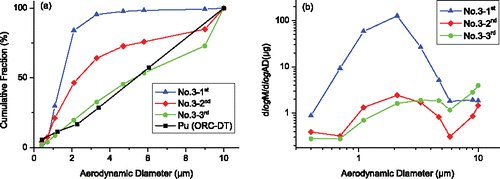
In test 3 of the second set of experiment, silver sample was two-point detonated by RHT-901 (as done in set 1) with three samplings. The results of test 3 are illustrated in and discussed in the following. compares the CMDs of silver (tests 3–1st, 2nd, 3rd) with the plutonium CMD in ORC-DT. It is seen that the CMD for the third sampling of test 3 is close to the ORC-DT result, as the second sampling in . This phenomenon again confirms that silver could be used to simulate the plutonium aerosol source term and, on the other hand, the CMD result is repeatable. The sampling instants for the two CMDs are not far (106.5 and 120 min), which shows that the evolution of CMD becomes somewhat steady about 2 h after detonation. This phenomenon implies that our results could be realized in wider (time) conditions. The result of the first samplings in test 1 of set 1 and in test 1–3 of set 2 are different. The reasons might be the difference of sampling times, the non-uniform spatial distribution of aerosols in early time, and other different conditions between the two sets of experiments. However, the trend of CMD evolution of silver is similar in sets 1 and 2. The mass–size distributions of silver for the three samplings in test 3 are shown in . The peak of the mass–size distribution curve is in the AD range 1.1–2.1 µm, and the silver amount in aerosols with AD ≥ 3.3 µm is low as seen in and . According to the estimation of terminal settling velocity and the geometry of the vessel, the initial aerosol particles with AD < 4.3 µm cannot fully deposit in 1 h. However, it is observed that the amount of silver in aerosols with most size (except in the range 9–10 µm) decreases significantly from the first sampling to the second sampling. Therefore, the coagulation effect of smaller aerosol particles with AD < 4.3 µm is strong in the first hour after detonation, leading to a decreasing fraction of smaller particles. In the next hour, the fraction of smaller aerosol particles keeps decreasing from test 3–2nd to test 3–3rd due to coagulation. However, the amount of silver increased from the second sampling to the third sampling, which seems abnormal. Note that the amounts in the last two samplings are one order less than that of the first sampling. Thus, the increase of silver amount may come from non-ideal conditions in the sampling and analysis process, such as the change of the pumping flow rate, the non-uniform spatial distribution of aerosol concentration in the vessel, and the error in the measurement of silver.
Figure 6. Size distribution of silver in aerosols for test 3 in the second set of experiment. (a) Cumulative mass distribution. (b) Mass-size distribution.
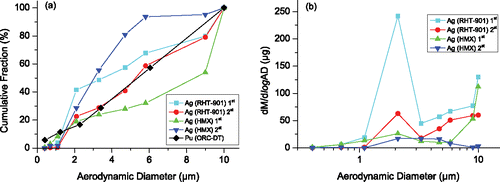
The mass–size distributions of silver in aerosols (first sampling) for all the tests in set 2 are shown in . Although the test conditions are different in these tests, the mass–size distributions of silver share some common features. Under the condition of high explosive loading, the peak of the mass–size distribution of silver is in the AD range 1.1–3.3 µm, which is consistent with the result in Hao et al. (Citation2009). Moreover, it is observed that aerosol particles with the range 0.7–5.8 µm (corresponding to impactor stages (2)–(6)) contain most of the silver in these samplings. The percentages of silver in aerosol particles with this range are calculated and listed in . It is seen that the percentages are all higher than 78%.
Figure 7. Mass-size distributions of silver in aerosol for the first sampling of all tests in set 2.
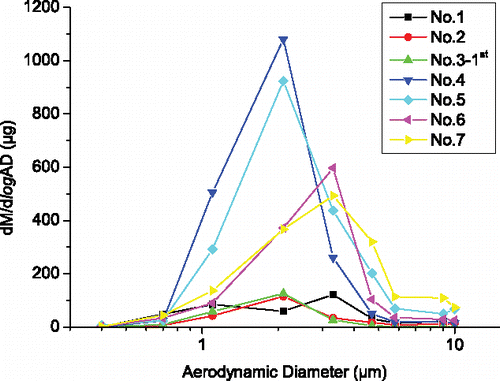
Table 10. Percent of silver in aerosols with AD range 0.7–5.8 μm for the first samplings in set 2.
3.2.3. Relationship of silver amount and peak pressure
It is shown in and that the amount of silver in aerosol sampled soon after detonation (first sampling) varies widely due to different test configurations. To investigate the key factors influencing the amount of silver in aerosols, we theoretically calculated the peak detonation pressure in each test of set 2 (shown in ). The calculation procedure is briefly introduced as follows. Plot the Hugoniot p-u curve of the explosive and the p-u curve calculated from the shock EOS (equation of state) of silver. Then the peak detonation pressure by one-point detonation (exists in the center of the upper surface of silver disk) can be read from the point of intersection of the two curves. Using the pressure and velocity of the intersection point as an initial condition of the two-point detonation process, one can plot another p-u curve of silver from its shock EOS, whose zero velocity point gives the peak detonation pressure for the two-point detonation (exists in the center of the silver disk). The dependence of the amount of silver (first sampling) on the peak detonation pressure (determined by the test configuration, e.g., the detonation and explosive type) is illustrated in . Here, the amount of silver for the test configuration in tests 1, 2, 3 (RHT-901, two-point detonation) is averaged over the amounts in the three tests. The amount of silver first increases and then decreases with the peak detonation pressure. Interestingly, the aerosol amount can be approximated by a quadratic function of the peak detonation pressure as shown in . According to the quadratic fit, the maximum silver amount happens when the peak detonation pressure is 68.69 GPa.
Figure 8. Relationship of the silver amount in aerosol (in the first sampling) and the peak pressure in the tests of set 2 with a quadratic fit. The five points correspond to the configurations of test 6, 7, 4, 5, and test 1–3rd, respectively, from left to right.
The resuspension study of plutonium aerosol from soils at Nevada test site (Shinn et al. Citation1986) suggested that the plutonium aerosol concentrations of nuclear test sites were much lower than other non-nuclear test sites. The reason was that the plutonium was contained in tiny glass beads that were not resuspendable. Here, our silver aerosol result is similar to the result of plutonium in the sense that high detonation pressure (exceeding a limit) will reduce the aerosol amount.
3.2.4. SEM results
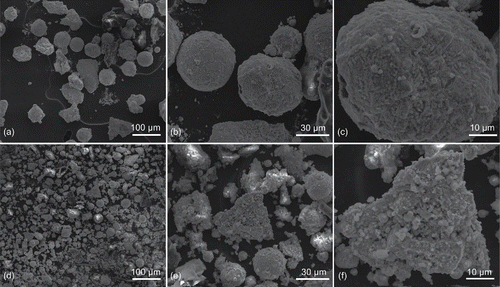
The solid residues in the contaminate vessel loaded by RHT-901 explosive (test 1 of set 1) were examined by scanning electron microscope (SEM) for further investigation of the coagulation and deposition of silver aerosols. The SEM results are representative since the test configuration is the same with that in tests 1–3 of set 2. The residual particles are collected 24 h after detonation and separated by a sieve shaker (MODEL RX-29-10, W.S. TYLER, Mentor, OH, USA). The scanning electron micrographs of particles in the size range 53–63 µm and 0–38 µm are present in with different magnification powers. The large-sized particles (53–63 µm) are mainly composed of single large particles with little very small particles (less than 5 µm) attached to their surfaces (). The morphology of middle-sized and small-sized particles (0–38 µm) are nevertheless more complicated (). Typically, as shown in , numerous very small particles (less than 5 µm) are attached to a middle-sized particle of an irregular shape. The SEM results imply that large-sized particles deposit rapidly on the vessel bottom after detonation and thus have little small particles attached on them. On the other hand, a large number of very small particles coagulate with middle-sized particles and then deposit on the vessel bottom. Meanwhile, there are also depositions of very small particles. The SEM results coincide with the decrease of aerosol particles from the first sampling to the second sampling shown in .
4. Conclusions and discussions
An experimental method is developed for the simulation of plutonium aerosol source term with conventional metals in the circumstance of high explosive detonation. In our experiments, metal samples were aerosolized by high explosive detonation in a contaminate vessel, after which the aerosols were collected by a cascade impactor and analyzed by atomic absorption spectroscopy and SEM. Silver was chosen as the surrogate for plutonium in the first set of experiment due to their similar CMDs under certain conditions. Different characteristics of silver aerosol source term were investigated in detail in the second set of experiment where more detailed source term data were obtained. The results yield the following main conclusions.
Silver could be used as a surrogate for plutonium to simulate the CMD of plutonium under certain conditions (explosive, detonation type, sampling time, and so on).
The RRFs of the investigated candidate metals (Ag, W, Sn, Ce, and V) are generally orders of magnitude less than that of plutonium. Thus, the RRF of plutonium could not be simulated by these metals.
In our test configurations, the peak of the mass-size distribution of silver aerosol lies in the AD range 1.1–3.3 µm. And most silver (AD ≤ 10 µm) is contained in aerosols of 0.7–5.8 µm AD.
Coagulation and deposition significantly affect the size distribution of silver aerosols. In a representative test (test 3 of set 2), the total amount of silver in respirable aerosol particles drops evidently in the first hour after detonation. In addition, the faction of relatively small silver aerosol particles keeps decreasing in 2 h after detonation.
The total amount of silver in aerosol exhibits a quadratic relation with the peak detonation pressure. In our experimental set-up, the maximum silver aerosol amount may occur with peak detonation pressure 68.69 GPa.
The total amount of sampled aerosol exhibits certain fluctuations. For example, the amount of aerosol in test 3–3rd of set 2 is larger than that in test 3–2nd, although being both very small. Moreover, different amount of aerosol was collected in the first samplings in tests 1–3 of set 2. The reason might be the randomness of the explosion processes, the non-ideal conditions in the aerosol collecting system, and the non-uniform spatial distribution of aerosol particles in the contaminate vessel. However, the CMD (reflecting the fraction of aerosols with different sizes) of silver aerosols shows good repeatability as shown in , or by the comparison of the silver result in the first and second set of experiment. The peak detonation pressure leading to the maximum aerosol amount is to be confirmed by future experiments. In addition, whether the decrease of silver aerosol amount with peak detonation pressure has a similar mechanism with that of plutonium will be investigated in the future.
Acknowledgments
The authors would like to acknowledge Y. J. Zhang for helpful discussions.
Funding
This work is supported by the National Natural Science Foundation of China under Grant No. 21307119.
References
- Andersen, B. V. (1964). Plutonium Aerosol Particle Size Distributions in Room Air. Health Phys., 10(12):899–907.
- Boughton, B. A., and DeLaurentis, J. M. (1992). Description and Validation of ERAD: An Atmospheric Dispersion Model for High Explosive Detonations (No. SAND–92-2069). Sandia National Labs., Albuquerque, NM, United States.
- Bunzl, K., and Kracke, W. (1988). Cumulative Deposition of 137Cs, 238Pu, 239+240Pu and 241Am from Global Fallout in Soils from Forest, Grassland and Arable Land in Bavaria (FRG). J. Environ. Radioactiv., 8(1):1–14.
- Condit, R. H. (1993). Plutonium Dispersal in Fires: Summary of What is Known (No. UCRL-ID–114164). Lawrence Livermore National Lab., CA, United States.
- Craig, D. K., Cannon, W. C., Filipy, R. E., Powers, G. J., and Dionne, P. J. (1976). SNS Source Term Evaluation Program (No. BNWL–1971). Battelle Pacific Northwest Labs., Richland, Washington, USA.
- Dewart, J. M., Bowen, B. M., and Elder, J. C. (1982). Supplementary Documentation for an Environmental Impact Statement Regarding the Pantex Plant: Dispersion Analysis for Postulated Accidents (No. LA-9445-PNTX-D). Los Alamos National Lab., NM, USA.
- Di Lemma, F. G., Colle, J. Y., Ernstberger, M., Rasmussen, G., Thiele, H., and Konings, R. J. M. (2014). RADES an Experimental Set-up for the Characterization of Aerosol Release from Nuclear and Radioactive Materials. J. Aerosol Sci., 70:36–49.
- Elder, J. C., Gonzales, M., and Ettinger, H. J. (1974). Plutonium Aerosol Size Characteristics. Health Phys., 27(1):45–53.
- Ettinger, H. J., Elder, J. C., and Gonzales, M. (1972). Size Characteristics of Plutonium Aerosols, in Proc. 12th AEC Air Cleaning Conf., USAEC Rept., CONF-720823, M. W. First, ed., USAEC, pp. 740-754. Available at http://rsearch.hitechsvc.com/nuclearsafety/qa/hepa/nureg_12Vol2/session11.pdf.
- Fernandez, Y., and Burghoffer, P. (1995). Radioactive Aerosols Emission in Fires. Aerosol Sci. Technol., 23(2):231–238.
- Fetter, S., and Von Hippel, F. (1990). The Hazard from Plutonium Dispersal by Nuclear-Warhead Accidents. Sci. Global Security, 2(1):21–41.
- Fuller, R. K. (1965). Operation Roller Coaster Project Officer's Report-Project 2.6a: Special Particulate Characteristics (No. POR-2506). U.S. Naval Radiological Defense Laboratory, San Francisco, CA.
- Hao, F., Liu, X., Xiao, C, Xiong, W., Ze, R., Gong, J., and Hu, S. (2009). Size Distributing of Explosion-Generated Particles in a Sealed Container. Nucl. Techniq., 32(5):343–346. (in Chinese)
- Harra, J., Mäkitalo, J., Siikanen, R., Virkki, M., Genty, G., Kobayashi, T., Kauranen, M., and Mäkelä, J. M. (2012). Size-Controlled Aerosol Synthesis of Silver Nanoparticles for Plasmonic Materials. J. Nanopart. Res., 14(6):1–10.
- Haschke, J. M. (1992). Evaluation of Source-Term Data for Plutonium Aerosolization (No. LA-12315-MS). Los Alamos National Lab., NM, United States.
- Haschke, J. M., and Martz, J. C. (1998). Oxidation Kinetics of Plutonium in Air from 500 to 3500°C- Application to Source Terms for Dispersal. J. Alloy Compounds, 266(1):81–89.
- Homann, S., and Wilson, D. V. (1995). HOTSPOT Training Manual: Health Physics Codes for the PC, UCRL-MA-118617. Lawrence Livermore National Laboratory, Livermore, California.
- Johansen, M. P., Child, D. P., Davis, E., Doering, C., Harrison, J. J., Hotchkis, M. A. C., Payne, T. E., Thiruvoth, S., Twining, J. R., and Wood, M. D. (2014). Plutonium in Wildlife and Soils at the Maralinga Legacy Site: Persistence Over Decadal Time Scales. J. Environ. Radioactiv., 131:72–80.
- Johansen, M. P., Kamboj, S., and Kuhne, W. W. (2013). Whole-Organism Concentration Ratios for Plutonium in Wildlife from Past US Nuclear Research Data. J. Environ. Radioactiv., 126:412–419.
- Kim, H. S., Joung, C. Y., Lee, B. H., Oh, J. Y., Koo, Y. H., and Heimgartner, P. (2008). Applicability of CeO2 as a Surrogate for PuO2 in a MOX Fuel Development. J. Nuclear Mater., 378(1):98–104.
- Ku, B. K., and Maynard, A. D. (2005). Comparing Aerosol Surface-Area Measurements of Monodisperse Ultrafine Silver Agglomerates by Mobility Analysis, Transmission Electron Microscopy and Diffusion Charging. J. Aerosol Sci., 36(9):1108–1124.
- Kulkarni, P., Baron, P. A., and Willeke, K. (Eds.). (2011). Aerosol Measurement: Principles, Techniques, and Applications. John Wiley & Sons, Hoboken, New Jersey, USA.
- Lide, D. R. (2005). CRC Handbook of Chemistry and Physics, Internet Version 2005, CRC Press, Boca Raton, FL. Available at: http://www.hbcpnetbase.com.
- Liu, W. J., Hu, B. Y., and Li, Q. Z. (2011). Review of Plutonium Aerosol Source-Term in Nuclear Accident. J. Safety Environ., 11(5):259–263. (in Chinese)
- Liu, W. X., Tan, S. S., Jing, J. Y., Zhang, X. L., Zhu, Y. R., Liu, G. L., and Zhang, Q. M. (2013). Internal Loads and Structure Responses of Spherical Explosive Vessel. Explos. Shock Waves, 33(6):594–600.
- Luna, R. E., and Church, H. W. (1969). Difout: A Model for Computation of Aerosol Transport and Diffusion in the Atmosphere (No. SC-RR–68-555). Sandia Labs., Albuquerque, NM.
- Luna, R. E., Church, H. W., and Shreve, J. D. (1971). Variability of Air Sampler Data. Atmos. Environ. (1967), 5(8):579–597.
- Martz, J. C., and Haschke, J. M. (1998). A Mechanism for Combustive Heating and Explosive Dispersal of Plutonium. J. Alloy Compound, 266(1):90–103.
- Mishima, J., and Pinkston, D. (1994). DOE Handbook: Airborne Release Fractions/Rates and Respirable Fractions for Nonreactor Nuclear Facilities (No. DOE-HDBK-3010-94), Vol. 1, US Department of Energy, Washington, DC.
- Olson, C., Landsberger, S., and Braisted, J. (2007). Neutron Activation Analysis of Aerosolized Sodium Chloride to Simulate Size-Fractionation of Plutonium in a Glovebox. J. Radioanalyt. Nucl. Chem., 276(1):157–160.
- Sagartz, M. J. (1995). Violent Reaction Source Term Study (No. SAND-943252). Sandia National Laboratory, Albuquerque, NM, United States.
- Shinn, J. H. (2002). Complementary Pu Resuspension Study at Palomares, Spain (No. UCRL-ID-150980). Lawrence Livermore National Laboratory, San Francisco, CA, United States.
- Shinn, J. H., Homan, D. N., and Hofmann, C. B. (1986). A Summary of Plutonium Aerosol Studies: Resuspension at the Nevada Test Site (No. UCRL-90746). Lawrence Livermore National Laboratory, CA, United States.
- Shinonaga, T., Steier, P., Lagos, M., and Ohkura, T. (2014). Airborne Plutonium and Non-Natural Uranium from the Fukushima DNPP Found at 120 km Distance a Few Days After Reactor Hydrogen Explosions. Environ. Sci. Technol., 48(7):3808–3814.
- Simones, M. P., Gutti, V. R., Meyer, R. M., and Loyalka, S. K. (2011). Measurements of Aerosol Charge and Size Distribution for Graphite, Gold, Palladium, and Silver Nanoparticles. Nucl. Technol., 176(2):211–226.
- Simones, M. P., and Loyalka, S. K. (2015). Measurements of Charged Aerosol Coagulation. Nucl. Technol., 189(1):45–62.
- Steele, C. M., Wald, T. L., and Chanin, D. I. (1998). Plutonium Explosive Dispersal Modeling using the MACCS2 Computer Code (No. LA-UR–98-1901). Los Alamos National Lab., NM United States.
- Stephens, D. R. (1995). Source Terms for Plutonium Aerosolization From Nuclear Weapon Accidents (No. UCRL-ID–119303). Lawrence Livermore National Lab., CA, United States.
- Stewart, K., Thomas, D. M. C., Terry, J. L., and Wilson, R. H. (1965). A Preliminary Evaluation of the Biological Measurements on Operation Roller Coaster (Joint US/UK Experiments) (No. AWRE-0-29/65). Atomic Weapons Research Establishment, Aldermaston, England.
- Titus, R. W. (1965). Operation Roller Coaster. Project Officers Report-Project 2.4. Micro-Meteorological Measurements (U) (No. POR-2504). United States Weather Bureau Research Station, Las Vegas, NV.
- Voelz, G. L., and Buican, I. G. (2000). Plutonium and Health: How Great is the Risk? Los Alamos Sci., 26:74–89.
- Wendel, C. C., Fifield, L. K., Oughton, D. H., Lind, O. C., Skipperud, L., Bartnicki, J., Tims, S. G., Høibråten, S., and Salbu, B. (2013). Long-Range Tropospheric Transport of Uranium and Plutonium Weapons Fallout from Semipalatinsk Nuclear Test Site to Norway. Environ. Int., 59:92–102.
- Williams, M. M. R., and Loyalka, S. K. (1991). Aerosol Science: Theory and Practice. Pergamon Press, Oxford, U.K.
- World Health Organization. (1999). Hazard Prevention and Control in the Work Environment: Airborne Dust (WHO/SDE/OEH/99.14). WHO, Geneva.
- Zamoryanskaya, M. V., and Burakov, B. E. (2000). Feasibility Limits in Using Cerium as a Surrogate for Plutonium Incorporation in Zircon, Zirconia and Pyrochlore, in MRS Proceedings, K. P. Hart and G. R. Lumpkin, eds., Vol. 663, Cambridge University Press, pp. 301. Available at http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=8232433&fulltextType=RA&fileId=S1946427400646535
- Zhou, H. S., and Dong, F. F. (1989). Performance of High Explosives and Related Materials (in Chinese). Science Press, Beijing.