ABSTRACT
This study was conducted to observe a potential formation and/or release of aerosol particles related to manufacturing processes inside a cleanroom. We introduce a novel technique to monitor airborne sub 2 nm particles in the cleanroom and present results from a measurement campaign during which the total particle number concentration (>1 nm and >7 nm) and the size resolved concentration in the 1 to 2 nm size range were measured. Measurements were carried out in locations where atomic layer deposition (ALD), sputtering, and lithography processes were conducted, with a wide variety of starting materials. During our campaign in the clean room, we observed several time periods when the particle number concentration was 105 cm−3 in the sub 2 nm size range and 104 cm−3 in the size class larger than 7 nm in one of the sampling locations. The highest concentrations were related to the maintenance processes of the manufacturing machines, which were conducted regularly in that specific location. Our measurements show that around 500 cm−3 sub 2 nm particles or clusters were in practice always present in this specific cleanroom, while the concentration of particles larger than 2 nm was less than 2 cm−3. During active processes, the concentrations of sub 2 nm particles could rise to over 105 cm−3 due to an active new particle formation. The new particle formation was most likely induced by a combination of the supersaturated vapors, released from the machines, and the very low existing condensation sink, leading to pretty high formation rates J1.4 nm = (9 ± 4) cm−3 s−1 and growth rates of particles (GR1.1–1.3 nm = (6 ± 3) nm/h and GR1.3–1.8 nm = (14 ± 3) nm/h).
Copyright © 2017 American Association for Aerosol Research
EDITOR:
Introduction
The main focus of this study was to investigate if new particle formation takes place in a cleanroom environment. The cleanroom is an environment where the number of particles transported into or generated inside the room is controlled to a required level relative to the intended use of the space. This is done by specific structural means and work practices instructed in the international ISO 14644 standard. The standard includes guidelines and limit values for contamination control in cleanrooms. For instance, it describes classification, monitoring, and construction and operations in cleanrooms. ISO 14644-1 provides the class designation in the form of “ISO Class N” from where N defines the maximum number concentration for particles equal or larger than the specific size in that class. The classification goes from number 1 to 9, ISO Class 1 being the highest level of cleanroom standards (ANSI/IEST/ISO Citation1999). Maximum concentrations are defined only for aerosol particles with diameter between 0.1 and 5 µm while nanoparticles (particles smaller than 100 nm) are not regulated by the cleanroom standards. The most common method for monitoring the particle number concentration in a cleanroom is optical particle counters, which are typically limited to particles larger than 300 nm (Ensor and Marie Dixon Citation2011). The amount of the aerosol particles (especially the number of coarse >1 µm particles) in the cleanroom is remarkably smaller than in ambient air or in normal room air.
Second aspect of the study is connected to the health effects of the aerosol particles as the measurements were conducted in a work environment. Workplace aerosol can have an impact to a worker's safety due to inhalation, ingestion, and dermal contact with the particles: for example, inhaled particles will deposit to different places in the respiratory system according to their aerodynamic diameter. The smallest, sub 2 nm particles, will deposit mostly in the very early parts of the respiratory system (nasopharyngeal compartment) due to their rapid diffusion. Up to the sizes of 20 nm, the probability of the particles to deposit in the deeper regions of respiratory system, tracheobronchial and alveolar region, increases. The particles with a diameter of around 20 nm have the highest probability to penetrate all the way down to the alveolar region (Oberdorster et al. Citation2005). For the larger particles, impaction and settling are the most effective processes depositing particles in the respiratory system. The chemical composition influences the health effects caused by airborne particles. Further, the chemical properties of the small particles may differ significantly from the bulk material with the same chemical composition, which can cause an unknown response in the human body. One example is the ratio of surface atoms in the particle, which increases when particle diameter decreases, which may affect the chemical and biological properties of the material, for example, the reactivity compared to bulk material of the same species (Nel et al. Citation2006).
Occupational health agencies and researchers have become more interested in the health effects of the ultrafine particles while the usage of nanomaterials has become more and more common in different industries. Different studies show that, e.g., TiO2 nanoparticles and carbon nanotubes and other ultrafine particles may potentially have harmful health effects (Pawar and Kaul Citation2014). Several toxicological (Elsaesser and Howard Citation2012) and epidemiological studies (Pekkanen et al. Citation1997; Peters et al. Citation1997; Borm et al. Citation2006; Strak et al. Citation2012; Meng et al. Citation2013) suggest that the nanoparticle fraction in ambient particulate air pollution is the most harmful part with respect to pulmonary uptake. Similarly, inhalation of engineered nanoparticles and nanomaterials is considered to be potentially toxic for humans (Savolainen et al. Citation2010; Yokel and MacPhail Citation2011). The inhalation pathway is considered as the predominant route of workplace exposure and uptake (Schmoll et al. Citation2009), however the health effects of inhaled particles are not completely understood.
The formation of new aerosol particles includes several processes, for example, the formation of nanometer-sized clusters from gaseous precursors, growth of the newly formed particles, and the competition with removal processes for the gaseous precursors and growing clusters (McMurry and Friedlander Citation1979; Kulmala et al. Citation2004). Parameters describing the particle formation process are the formation rate, which is the difference between the production and loss rate for a specific size range; the growth rate including condensational growth and growth by coagulation; the coagulation sink for the formed particles; and the condensation sink for the gaseous compounds (Kulmala et al. Citation2012). Condensation sink is a quantity that describes how rapidly condensable vapors condense on existing aerosol, which acts as a sink for the gas molecules. In a cleanroom environment, the surface area of aerosol particles is small compared to ambient or normal room conditions. Therefore, under clean room conditions, the vapors are released, e.g., during manufacturing processes are not effectively scavenged by the background aerosol population. The condensation sink can be also used to estimate the coagulation sink for the smallest aerosol particles via an empirical correction (Lehtinen et al. Citation2007). In the absence of coarse particles, the loss rate of the smallest particles is only caused by self-coagulation and diffusion to surfaces, and these processes are not as efficient as the coagulation between the newly formed particles and the population of coarse particles (Lee and Chen Citation1984). In a cleanroom environment, the processes inhibiting the formation and growth of newly formed particles are thus not very effective, therefore a quite rapid and intense particle formation can occur if suitable vapors become available.
The aim of the present study is to explore the concentration of nanoparticles, especially sub 2 nm particles, in a cleanroom environment. Additionally, we want to find out if new particle formation can take place in cleanrooms, where the total existing particle mass and number is extremely low. The novel instrumentation to monitor particles enabled us to extend the measurements to sub 2 nm particles. We present the first results of the instrument's performance in detecting clusters consisting of materials relevant to the manufacturing processes (AlCl3, MnCl2, and ZnCl2).
Methods and measurements
Measurements in the cleanroom
Measurements of the particle number concentration and size distribution were carried out during autumn 2014 (29 September to 7 December 2014) in a production facility with cleanrooms with different requirements for particle number concentration (ISO Class 4–6). The aerosol number concentration measurements were conducted using an Airmodus A11 nCNC-system including an A10 particle size magnifier (PSM) and an A20 condensation particle counter (CPC) and a separate A20 CPC (Vanhanen et al. Citation2011; Kangasluoma et al. Citation2015). In the nCNC system, particles are grown in two stages via condensation of supersaturated vapors to optically detectable sizes. The A10 PSM is a continuous flow mixing type Particle Size Magnifier using diethylene glycol as a working fluid. Heated and saturated flow (0.1–1 liters per minute [lpm]) is mixed turbulently with the sample air (2.5 lpm), and subsequently the mixed flow is cooled down in the growth section where supersaturation of diethylene glycol takes place. By changing the saturator flow rate, the supersaturation, and thus the diameter of the smallest activated particles can be changed, which is used for getting size information of 1–2 nm particles (Lehtipalo et al. Citation2014). The particles are grown from the smallest initial diameter, around 1 nm, to around 90 nm in the PSM and then further in the A20 CPC by butanol, after which the particles are optically counted (Vanhanen et al. Citation2011). The A20 is a conventional laminar flow type butanol based CPC. The A20 has a cut-off diameter (diameter with 50% detection efficiency) of approximately 7 nm (electrical equivalent mobility diameter) according to the manufacturer's specification. The mobility diameter that was measured with the instrumentation is not strictly comparable with aerodynamic diameter because it does not take into account the inertia and the shape of the particles. However, in sub 2 nm size range mobility diameter is the diameter, which can be determined with electrical methods, unlike the aerodynamic diameter.
The aerosol was sampled directly from the room air to minimize wall losses in the sampling lines upstream of the instruments. The instruments were placed on a movable trolley in the way that the inlets of the two instruments were 25 cm from each enabling them to sample the same air mass. The instrument trolley was moved three times during the measurement period to sample next to different types of manufacturing processes and between the spaces with different requirements for cleanliness. The first location was in a large hall in the middle of several production machines. The second and third location represented the cleaner side of the facility and according to the prior expectations the third location was the cleanest site at the manufacturing facility. Machine operators work at the facility only during daytime on weekdays. The working hours at the facility are not strictly scheduled. The first operators begin their work around 5 AM and then the activities at the workplace continue until around 4 PM with a decreasing level of activity.
A more detailed description of the different locations is given below.
Atomic Layer Deposition (ALD) processing space, where there are altogether around 30 ALD machines (type P400, P800, and TFS200). Around two-thirds of them are in production use for making thin film electroluminescent (TFEL) displays and the rest of them are assigned in research and development (R&D) use. Several different kinds of precursors are used, for example, solid phase manganese, zinc, titanium chlorides, gaseous ammonia, hydrogen sulfide, nitrogen, and liquid/gaseous trimethylaluminium (TMA). This area corresponds to an ISO Class 6 type cleanroom. Sampling time in this area was 29 September to 18 October 2014 and it had an area of 312 m2 and volume of ca. 940 m3.
Indium Tin Oxide (ITO)-sputtering space, where non-reactive DC magnetron sputtering (physical vapor deposition, PVD) is conducted. More specifically, the substance is moved from a sintered and oxidized surface to a glass surface using argon or argon-oxygen carrier gas. The machine used for the sputtering is commercial, manufactured by Balzers. This area corresponds to an ISO Class 5 type cleanroom. The measurements at this location were performed between 18 October and 31 October 2014 and it had an area of 179 m2 and a volume of ca. 540 m3.
Lithography site with a self-made production line. The process includes a coating of glass substrates in an automated line with a photoresist (AZ RFP-210K), drying the resist in an oven, and exposing it to ultraviolet light. This space corresponds to an ISO Class 4 type cleanroom. The measurements were conducted between 31 October and 8 November 2014. The area had an area of 124 m2 and volume of ca. 370 m3.
The estimated air exchange rate in the cleanroom is five times per hour, which was calculated taking into account the volumes and the constant flow velocity (0.5 m/s) through the high efficiency particulate air (HEPA) filters. However, the exact air flow patterns inside these clean rooms are unfortunately not known. Ultrafine aerosols in the clean room are distributed via convection, which strongly depend on the ventilation rate, and their concentration can have spatial variation inside the clean room due to natural convection caused by the temperature gradients in the room and localized emissions. For practical reasons, the instruments were placed to the locations so that they were close to the emission sources but not preventing the work around the machines.
Laboratory calibrations
The instruments were calibrated in the laboratory at the University of Helsinki after the in situ measurements at the production site. As the detection efficiency is a function of chemical composition (Kangasluoma et al. Citation2014), it is crucial to calibrate the instruments with the particles with a similar composition to the materials used in the manufacturing processes. Additionally, humidity has been shown to have an effect on the cut-off diameter and calibration of the PSM (Kangasluoma et al. Citation2013). To calibrate the PSM, we measured the maximum detection efficiency and the activation saturator flow rate (flow rate with 50% activation efficiency) for different sized monodisperse aerosol particles. The maximum detection efficiency and activation saturator flow can be used to invert the measured concentrations to a number size distribution in the 1–3 nm diameter size range (Lehtipalo et al. Citation2014).
A schema of the calibration setup is shown in . Three different metal chlorides (AlCl3, MnCl2, and ZnCl2) were heated up in a tube furnace to a temperature varying from 100 to 500°C depending on the melting point of the sample. A cooler and a 241Am neutralizer were placed downstream of the tube furnace to cool down the flow and charge the sample particles, as mobility classification and chemical composition measurement with a mass spectrometer can be done only for the charged particles. The composition of the sampled particles was measured with an atmospheric pressure interface time of flight mass spectrometer (APi-TOF) (Junninen et al. Citation2010). The APi-TOF, which allows sampling directly from atmospheric pressure, differentially pumps the pressure down to ∼2 × 10−6 mbar and measures the time of flight of the entering charged particles. The measured time of flight can be converted to mass, which is obtained at a high mass resolution R (describes the ability to resolve closely spaced peaks in the mass spectrum) of approximately 5000 (R = M/ΔM, where M is atomic mass and ΔM is width (FWHM) of the peak). The MATLAB toolbox, tofTools was used to analyze the APi-TOF data (Junninen et al. Citation2010).
Figure 1. Schematic figure of the calibration setup that was used in the laboratory measurements. Atmospheric pressure interface time-of-flight mass spectrometer (APi-ToF-MS) was not connected to setup simultaneously with DMA.
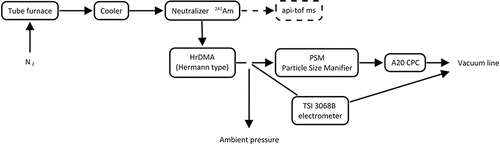
The mass spectrum was monitored online while tuning the calibration setup to find suitable operation conditions for the aerosol generation. When the suitable furnace temperatures were found and the APi-TOF showed a clean spectrum of the sample, the sample line was moved from APi-TOF setup to the differential mobility analyzer (DMA) setup. A Herrmann type high-resolution differential mobility analyzer DMA was used to classify particles according to their electrical mobility before guiding them to the nCNC and the reference electrometer (TSI 3068B). Ions generated by a radioactive source were mixed with the sampled clusters. These ions, mostly containing compounds with nitrate and chlorine, had smaller mass and consequently higher mobility than the other peaks, and thus they were easily distinguishable from the sample.
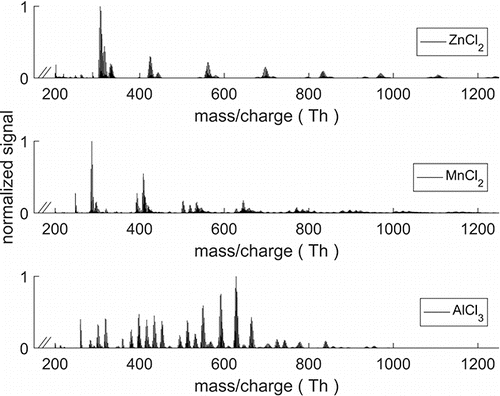
presents a mass spectrum of each of the three calibration materials. The isotopic patterns and exact measured masses were used to identify the composition of the detected clusters. The spectrum obtained with zinc and manganese chloride mostly consisted of (MnCl2)n or (ZnCl2)n clusters charged by Cl- or OH−. The spectrum obtained from heated AlCl3 produced cluster composition of (AlCl3)n with ions Cl−, OH−, and AlO2− giving the charge.
Figure 2. Mass spectrum of ZnCl2, MnCl2, and AlCl3 used for calibrating the A11 system. Spectra obtained with zinc and manganese chloride were very similar and the peaks after 200 Th consisted of n amount of (MnCl2)n or (ZnCl2)n clusters with ion (Cl−,OH−) as charge carrier. (AlCl3)n clusters were charged with the same two ions, and also with AlO2−.
The results of the calibration for the nCNC, i.e., the activation saturator flow as function of the particle diameter for different calibration materials, is shown in . The particles produced from ZnCl2 and MnCl2 samples were quite similar in their chemical composition and were detected equally well by the instrument. The particles produced from AlCl3 needed a slightly higher saturator flow rates (supersaturations) to activate than same sized particles from the two other compounds. The calibration obtained with MnCl2 particles was utilized for analyzing the data from the cleanroom, because this calibration curve was in between the other two calibration curves, thus giving a good average for the cleanroom particles, which probably consisted of a mixture of the different compositions.
Figure 3. Activation saturator flow rate for different particle types as a function of mobility diameter. This is the saturator flow rate at which half of the maximum detection efficiency is achieved in the nCNC. Larger activation saturator flow rate means that higher supersaturations are needed to start the condensational growth. Particles produced from ZnCl2 and MnCl2 sample behaved quite similarly compared to each other. With AlCl3 slightly higher saturator flow rates were two-day example of the particle size distribution at each of the three sampling sites: (I) ALD, (II) ITO-sputtering, and (III) Lithography site. A band of sub 1.4 nm clusters is visible in all of the sites.
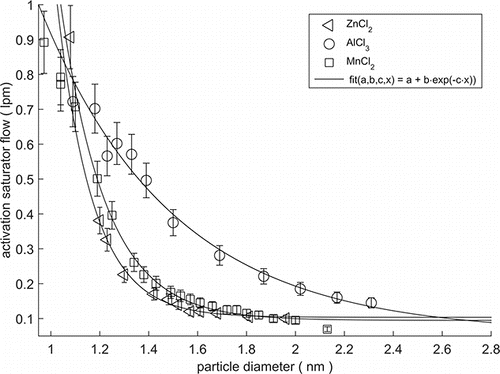
The calibration was done with negatively charged particles since those are less prone to contaminants than positively charged particles during the particle generation. Depending on the system, the activation of the positive or negative particles may be easier due to their different chemical composition (Kangasluoma et al. Citation2016). Therefore, the calibration curves in may shift horizontally (±0.15 nm) for the positively charged particles. There is also a difference, when comparing charged particles to neutral particles. A difference of 0.2–0.5 nm in the cut-off diameter was reported between the charged and neutralized sample with diethylene glycol as a working fluid (Kangasluoma et al. Citation2016). It should be noted that the actual chemical composition of the particles in the cleanroom may differ from the particles produced with the calibration setup even though the starting materials were similar to the ones used in the processes in the cleanroom. Kangasluoma et al. (Citation2014) have shown approximately 1 nm variation in the PSM cut-off diameter for particles of different composition. The particles composed of oxidation products of organic precursor vapors exhibit the largest cut-off diameter while inorganic salts and metal oxides are detected with the lowest cut-off size. In our study, the particles in the clean room can be expected to be composed of a combination of inorganic metal and metal chlorine species, which translates to an approximate uncertainty of ±0.2 nm in the PSM cut-offs. Nevertheless, the tailored calibration measurements enabled us to use a calibration that is more accurate for the specific conditions of the facility than a standard calibration.
Results
In all the three sampling locations, a constant band of small 1.1–1.4 nm particles/clusters/large molecules was present at all times (), with a concentration varying between 200 and 700 cm−3. A similar population of the small charged and neutral clusters is present also in ambient (outdoor) conditions (Hirsikko et al. Citation2011; Kulmala et al. Citation2013). The number concentration of the particles larger than 1.4 nm was less than 10 cm−3 in all of the locations (<0.1 cm−3 in ALD and ITO site) except during particle release/formation episodes. This means that, if there were no active processes ongoing to generate the particles or condensing vapors, the observed clusters were not growing out of the 1.1–1.4 nm size range. The concentration of these small clusters does not have an obvious diurnal cycle or dependence on the working hours. Thus, it seems unlike that the measured concentrations are caused by an outside infiltration. A likely source is ion production inside the room caused by natural gamma radiation including cosmic radiation, external radiation from the ground, and radon. Earlier Indoor study shows the ion concentrations and ion production rates can exceed the corresponding outdoor levels (Hirsikko et al. Citation2007). Similar processes are likely to produce the (charged) particles also in the clean rooms.
Figure 4. Two-day example of the particle size distribution at each of the three sampling sites: (I) ALD, (II) ITO-sputtering, and (III) lithography site. A band of sub 1.4 nm clusters is visible in all of the sites.
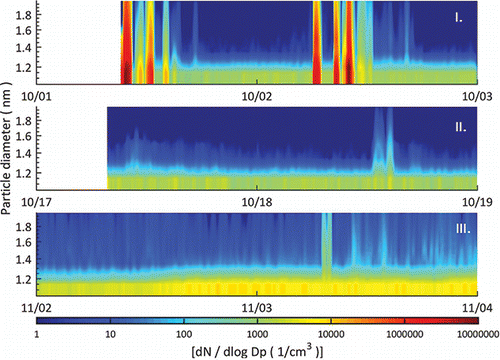
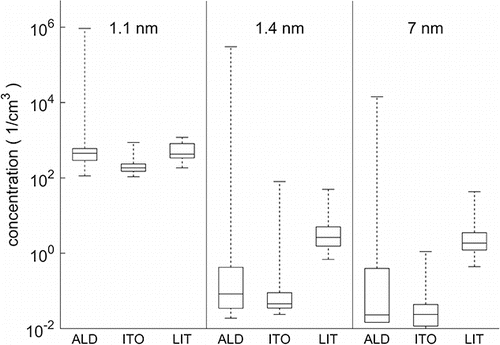
On one hand, the ALD processing site had by far the highest peak concentrations (>105 cm−3). The ITO-sputtering site, on the other hand, had the smallest peak and average concentration. presents a summary of the measured particle concentrations in all of the locations with three different cut-off diameters (1.1 nm, 1.4 nm, and 7 nm) showing the median concentrations, and the full variance of the particle concentration. The highest peak concentrations in the ITO and lithography site were at a similar level (102–103 cm−3), which is several orders of magnitude lower than at the ALD site. The lithography site had a slightly higher concentration for the particles larger than 1.4 nm and 7 nm than the other locations. At the ALD and ITO site, the A20 CPC was showing most of the time less than 0.1 cm−3 but at the lithography site the concentration was usually between 1 and 10 cm−3. The duration of elevated concentrations in the ITO and lithography site was shorter than in the ALD site.
Figure 5. Summary of the measured number concentrations from each site including median values (line within the boxes), 25th and 75th percentile (boxes) and full range of variation (whiskers). The different cut-off diameters are marked in the figure (the diameter from which upward instruments detects more than half of the particles) and the different sampling locations are indicated with abbreviations on the x-axis (ALD, ITO, LIT = lithography). Concentrations are 15 min average values that are corrected for internal losses according to the calibration for particles smaller than 2 nm (for the larger particles the losses are not significant). First (ALD) location had by far the greatest number of particles during release of particles.
In the ALD processing space, we observed several short periods when the number concentration was more than 105 cm−3 (). During these bursts, the concentration increased rapidly to the maximum concentration and then decreased fast due to dilution and mixing. The highest concentrations were so high that the Airmodus A11 nCNC-system was not able to definitely resolve them. The A20 is calibrated up to 100,000 cm−3 by the manufacturer. Therefore, the highest concentrations should be regarded as order of magnitude estimations for the number concentration. These kinds of concentrations were observed usually in the morning hours during weekdays. The duration of these events was from few minutes to few hours, the longer ones consisting of several individual bursts. This is an indication that these bursts of particles were related to a production or maintenance process or chemicals that are exposed to air in the workspace. Some of the instruments in this sampling location were opened regularly for maintenance, during which the materials for the manufacturing processes were exposed to room air. Some parts of the machines were at elevated temperatures while exposed to room air. At this site, our instruments were located in the middle of several production and R&D machines. Due to this, there have been a wide variety of different maintenance operations with different cycles. The operations include, for example, cleaning and replacement of valves and chancing the precursors (metal chloride starting materials). Unfortunately, there is no exact record of these operations. However, on a typical day the coating runs that were started on a previous day were completed in the morning (6 AM to 8 AM). This is when machines are vented and opened for the next runs. As the particle bursts were observed mainly in the morning, it is likely that they are related to the maintenance cycles.
Figure 6. Time series of the number concentration measured at the ALD site. (a) Number concentration in different size classes in 1–2 nm size range. (b) Number concentration for particles above 7 nm measured with the A20 CPC and with the nCNC at 1.4 nm cut-off diameter. Periods when the nCNC was offline are marked in the figure with light gray.
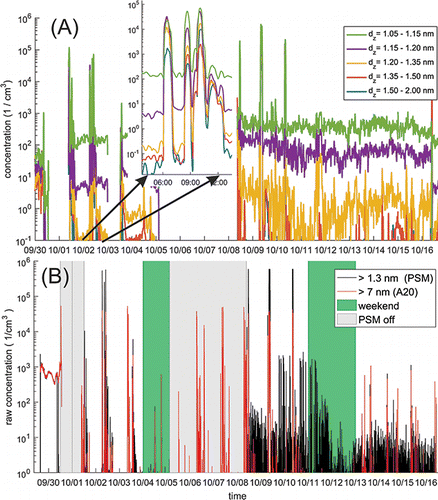
The increase in the particle concentration during the particle bursts was observed in most of the cases almost simultaneously with both particle counters having different cut-off sizes (). One option is that the particles were formed locally near the source and transported to the sampling location while growing rapidly. Another plausible explanation is that the particles with a wide size range were released at the same time. The distinction between these two possibilities is difficult because the time resolution (2 min) of the size classification does not allow to resolve growth rates faster than ∼15 nm/h.
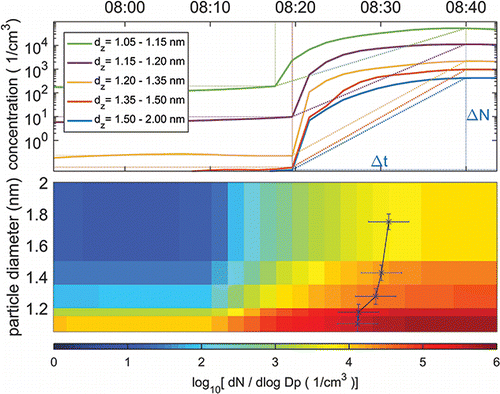
For nine of these bursts, it was possible to measure the growth rate (GRsize range) of aerosol particles comparing the appearance time (corresponding to a time, when a 50% concentration increase was achieved) at different size bins. The propagation of uncertainty is used to calculate the estimate of the uncertainty of the growth rate. The average growth rate for these events was GR 1.1–1.3 nm = (6 ± 3) and GR 1.3–1.8 nm = (14 ± 3) nm/h. These values are greater than the growth rates measured outdoors at remote boreal forest (Yli-Juuti et al. Citation2011; Kulmala et al. Citation2013) or even at metropolis like Shanghai (Yli-Juuti et al. Citation2011; Xiao et al. Citation2015). One of these bursts (a moderate one) is shown in , where the appearance time is marked as the leading edge of the curve on top of the particle size distribution. The lower limit of the average nucleation rate was calculated for these same nine events assuming the sink terms (coagulation loss and growth out of a size bin) to be negligible compared to the formation rate. In this case, the formation rate is J =(cm−3 s−1). The uncertainty of the average formation rate is estimated with the standard error of the mean (SEM). The average formation rate calculated in this way was J (avg,1.4 nm) = 9 ± 4 (cm−3 s−1). Actually, in the case of the highest peak concentrations (>105 cm−3), the concentrations were factor of 5–10 higher and the time window was shorter indicating that the formation rate was higher by at least the same factor. The growth rates and the formation rates were calculated only for the ALD site since at the ITO and lithography sites the duration of the peaks was too short.
Figure 7. One of the bursts at the ALD site shown with concentrations in different size ranges (upper) and as a size distribution with normalized dN/dlogDp concentration (lower). Appearance time (time when concentration has reached to 50% from the total increase) is marked to the leading edge of the curve (x) on the surface plot. Lower limit for the formation rates was estimated comparing the increase in the concentrations ΔN in the time Δt (marked in the upper panel).
Discussion and conclusions
We investigated the existence and formation of nanoparticles (sub 2 nm size range) in a cleanroom facility at three different locations (ALD, ITO, and lithography site). During the experiments, we observed a rather constant population of sub 1.4 nm particles with a fairly low particle number concentration (around 1000 cm−3) at all of the locations, while the concentration of the particles larger than 1.4 nm was most of the time really low (<0.1–3 cm−3) as was expected in a clean room facility. Concentrations above 105 cm−3 were observed inside the cleanroom environment in the midst of the manufacturing machines, which were opened regularly for maintenance. The formation and growth rates during the fast particle formation bursts were remarkable high (J1.4 nm = (9 ± 4) cm−3s−1, GR 1.1–1.3 nm = (6 ± 3) nm/h, and GR 1.3-1.8 nm = (14 ± 3) nm/h). The vast differences between the different sampling locations were most likely due to the technical features of the site, which influence the ability of the clean room to dilute or to remove the formed particles and possible precursors rather than due to the differences in the processes occurring in the space. The two cleaner spaces (ITO and lithography site) were physically smaller and had a faster circulation of air than the ALD site.
Based on our experiments, the nCNC (Airmodus A11) was proven to be suitable for measurements of particle concentration and sub 3 nm size distribution in a cleanroom. Our calibration of the instrument with nano-particles having similar composition to what we expect from the manufacturing processes in the cleanroom further improved the interpretation of the measurements. In subsequent deployments, the operating mode and settings of the instrument should be adjusted to be optimal, e.g., for the specific location and manufacturing processes.
Although the highest measured particle number concentrations in the cleanroom were remarkably high, the occupational exposure time for people working in there would be rather short (maximum a few tens of minutes), keeping the actual exposure reasonably small. It is still important to expand the particle monitoring to cover also the smallest particles as the health effects of these particles are not accurately known. There are indications that the nanoparticles may have significant health impacts and therefore it is useful to build up a database of concentrations and exposure of various particle types in different occupational environments.
Funding
This work was supported by the Academy of Finland Center of Excellence (project no. 272041) and Beneq Oy (P.O. Box 4, FI-02201 Espoo, Finland).
References
- ANSI/IEST/ISO (1999). ANSI/IEST/ISO 14644-1: 1999. Cleanrooms and Associated Controlled Environments—Part 1: Classification of Air Cleanliness. International Organization for Standardization, Geneva, Switzerland.
- Borm, P. J., Robbins, D., Haubold, S., Kuhlbusch, T., Fissan, H., Donaldson, K., Schins, R., Stone, V., Kreyling, W., Lademann, J., Krutmann, J., Warheit, D., and Oberdorster, E. (2006). The Potential Risks of Nanomaterials: A Review Carried Out for ECETOC. Part Fibre Toxicol., 3:11.
- Elsaesser, A., and Howard, C. V. (2012). Toxicology of Nanoparticles. Adv. Drug. Del. Rev., 64:129–137.
- Ensor, D. S., and Marie Dixon, A. (2011). Aerosol Measurements in Cleanrooms, in Aerosol Measurement, P. Kulkarni, P. A. Baron, and K. Willeke, eds., John Wiley & Sons, Inc., New York, pp. 771–784.
- Hirsikko, A., Nieminen, T., Gagne, S., Lehtipalo, K., Manninen, H. E., Ehn, M., Horrak, U., Kerminen, V. M., Laakso, L., McMurry, P. H., Mirme, A., Mirme, S., Petäjä, T., Tammet, H., Vakkari, V., Vana, M., and Kulmala, M. (2011). Atmospheric Ions and Nucleation: A Review of Observations. Atmos. Chem. Phys., 11:767–798.
- Hirsikko, A., Yli-Juuti, T., Nieminen, T., Vartiainen, E., Laakso, L., Hussein, T., and Kulmala, M. (2007). Indoor and Outdoor Air Ions and Aerosol Particles in the Urban Atmosphere of Helsinki: Characteristics, Sources and Formation. Boreal Environ. Res., 12:295–310.
- Junninen, H., Ehn, M., Petäjä, T., Luosujärvi, L., Kotiaho, T., Kostiainen, R., Rohner, U., Gonin, M., Fuhrer, K., Kulmala, M., and Worsnop, D. R. (2010). A High-Resolution Mass Spectrometer to Measure Atmospheric Ion Composition. Atmos. Meas. Tech., 3:1039–1053.
- Kangasluoma, J., Ahonen, L., Attoui, M., Vuollekoski, H., Kulmala, M., and Petäjä, T. (2015). Sub-3 nm Particle Detection with Commercial TSI 3772 and Airmodus A20 Fine Condensation Particle Counters. Aerosol Sci. Technol., 49:674–681.
- Kangasluoma, J., Junninen, H., Lehtipalo, K., Mikkilä, J., Vanhanen, J., Attoui, M., Sipilä, M., Worsnop, D., Kulmala, M., and Petäjä, T. (2013). Remarks on Ion Generation for CPC Detection Efficiency Studies in Sub-3-nm Size Range. Aerosol Science and Technology 47:556–563.
- Kangasluoma, J., Kuang, C., Wimmer, D., Rissanen, M. P., Lehtipalo, K., Ehn, M., Worsnop, D. R., Wang, J., Kulmala, M., and Petäjä, T. (2014). Sub-3 nm Particle Size and Composition Dependent Response of a Nano-CPC Battery. Atmos. Meas. Tech., 7:689–700.
- Kangasluoma, J., Samodurov, A., Attoui, M., Franchin, A., Junninen, H., Korhonen, F., Kurtén, T., Vehkamäki, H., Sipilä, M., Lehtipalo, K., Worsnop, D. R., Petäjä, T., and Kulmala, M. (2016). Heterogeneous Nucleation onto Ions and Neutralized Ions: Insights into Sign-Preference. The Journal of Physical Chemistry C 120:7444–7450.
- Kulmala, M., Kontkanen, J., Junninen, H., Lehtipalo, K., Manninen, H. E., Nieminen, T., Petäjä, T., Sipilä, M., Schobesberger, S., Rantala, P., Franchin, A., Jokinen, T., Järvinen, E., Äijala, M., Kangasluoma, J., Hakala, J., Aalto, P. P., Paasonen, P., Mikkilä, J., Vanhanen, J., Aalto, J., Hakola, H., Makkonen, U., Ruuskanen, T., Mauldin, R. L., Duplissy, J., Vehkamäki, H., Back, J., Kortelainen, A., Riipinen, I., Kurten, T., Johnston, M. V., Smith, J. N., Ehn, M., Mentel, T. F., Lehtinen, K. E. J., Laaksonen, A., Kerminen, V. M., and Worsnop, D. R. (2013). Direct Observations of Atmospheric Aerosol Nucleation. Science, 339:943–946.
- Kulmala, M., Petäjä, T., Nieminen, T., Sipilä, M., Manninen, H. E., Lehtipalo, K., Dal Maso, M., Aalto, P.P., Junninen, H., Paasonen, P., Riipinen, I., Lehtinen, K. E. J., Laaksonen, A., and Kerminen, V. M. (2012). Measurement of the Nucleation of Atmospheric Aerosol Particles. Nat. Protoc., 7:1651–1667.
- Kulmala, M., Vehkamäki, H., Petäjä, T., Dal Maso, M., Lauri, A., Kerminen, V. M., Birmili, W., and McMurry, P. H. (2004). Formation and Growth Rates of Ultrafine Atmospheric Particles: A Review of Observations. J. Aerosol Sci., 35:143–176.
- Lee, K. W., and Chen, H. (1984). Coagulation Rate of Polydisperse Particles. Aerosol Sci. Technol., 3:327–334.
- Lehtinen, K. E. J., Dal Maso, M., Kulmala, M., and Kerminen, V. M. (2007). Estimating Nucleation Rates from Apparent Particle Formation Rates and Vice Versa: Revised Formulation of the Kerminen-Kulmala Equation. J. Aerosol Sci., 38:988–994.
- Lehtipalo, K., Leppä, J., Kontkanen, J., Kangasluoma, J., Franchin, A., Wimnner, D., Schobesberger, S., Junninen, H., Petäjä, T., Sipilä, M., Mikkilä, J., Vanhanen, J., Worsnop, D. R., and Kulmala, M. (2014). Methods for Determining Particle Size Distribution and Growth Rates Between 1 and 3 nm using the Particle Size Magnifier. Boreal Environ. Res., 19:215–236.
- McMurry, P. H., and Friedlander, S. K. (1979). New Particle Formation in the Presence of an Aerosol. Atmos. Environ., 13:1635–1651.
- Meng, X., Ma, Y., Chen, R., Zhou, Z., Chen, B., and Kan, H. (2013). Size-Fractionated Particle Number Concentrations and Daily Mortality in a Chinese city. Environ. Health Perspect., 121:1174–1178.
- Nel, A., Xia, T., Madler, L., and Li, N. (2006). Toxic Potential of Materials at the Nanolevel. Science, 311:622–627.
- Oberdorster, G., Oberdorster, E., and Oberdorster, J. (2005). Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Persp., 113:823–839.
- Pawar, K., and Kaul, G. (2014). Toxicity of Titanium Oxide Nanoparticles Causes Functionality and DNA Damage in Buffalo (Bubalus bubalis) Sperm In Vitro. Toxicol. Ind. Health, 30:520–533.
- Pekkanen, J., Timonen, K. L., Ruuskanen, J., Reponen, A., and Mirme, A. (1997). Effects of Ultrafine and Fine Particles in Urban Air on Peak Expiratory Flow Among Children with Asthmatic Symptoms. Environ. Res., 74:24–33.
- Peters, A., Wichmann, H. E., Tuch, T., Heinrich, J., and Heyder, J. (1997). Respiratory Effects are Associated with the Number of Ultrafine Particles. Am. J. Resp. Crit. Care, 155:1376–1383.
- Savolainen, K., Pylkkanen, L., Norppa, H., Falck, G., Lindberg, H., Tuomi, T., Vippola, M., Alenius, H., Hameri, K., Koivisto, J., Brouwer, D., Mark, D., Bard, D., Berges, M., Jankowska, E., Posniak, M., Farmer, P., Singh, R., Krombach, F., Bihari, P., Kasper, G., and Seipenbusch, M. (2010). Nanotechnologies, Engineered Nanomaterials and Occupational Health and Safety - A Review. Safety Sci., 48:957–963.
- Schmoll, L. H., Elzey, S., Grassian, V. H., and O'Shaughnessy, P. T. (2009). Nanoparticle Aerosol Generation Methods from Bulk Powders for Inhalation Exposure Studies. Nanotoxicology, 3:265–275.
- Strak, M., Janssen, N. A. H., Godri, K. J., Gosens, I., Mudway, I. S., Cassee, F. R., Lebret, E., Kelly, F. J., Harrison, R. M., Brunekreef, B., Steenhof, M., and Hoek, G. (2012). Respiratory Health Effects of Airborne Particulate Matter: The Role of Particle Size, Composition, and Oxidative Potential-The RAPTES Project. Environ. Health Persp., 120:1183–1189.
- Vanhanen, J., Mikkilä, J., Lehtipalo, K., Sipilä, M., Manninen, H. E., Siivola, E., Petäjä, T., and Kulmala, M. (2011). Particle Size Magnifier for Nano-CN Detection. Aerosol Sci. Technol., 45:533–542.
- Xiao, S., Wang, M. Y., Yao, L., Kulmala, M., Zhou, B., Yang, X., Chen, J. M., Wang, D. F., Fu, Q. Y., Worsnop, D. R., and Wang, L. (2015). Strong Atmospheric New Particle Formation in Winter in Urban Shanghai, China. Atmos. Chem. Phys., 15:1769–1781.
- Yli-Juuti, T., Nieminen, T., Hirsikko, A., Aalto, P. P., Asmi, E., Horrak, U., Manninen, H. E., Patokoski, J., Dal Maso, M., Petäjä, T., Rinne, J., Kulmala, M., and Riipinen, I. (2011). Growth Rates of Nucleation Mode Particles in Hyytiälä During 2003–2009: Variation with Particle Size, Season, Data Analysis Method and Ambient Conditions. Atmos. Chem. Phys., 11:12865–12886.
- Yokel, R. A., and MacPhail, R. C. (2011). Engineered Nanomaterials: Exposures, Hazards, and Risk Prevention. J. Occup. Med. Toxicol., 6.
