ABSTRACT
Prevention of airborne contagious diseases depends on successful characterization of aerosols in the environment. The use of cascade impactors to characterize ambient aerosols is one of the most commonly used methods, providing data on both particle size and concentration. In this study, the use of a cascade impactor recently described in the literature using 8 mL of liquid in Petri dishes (CI-L) was compared with a new method that uses wet membrane filters on top of wax filled Petri dishes (CI-WWMF). Sampling efficiencies of the cascade impactors were evaluated using 0.5, 1, 3, and 5 μm polystyrene latex (PSL) microspheres and aerosol consisting of single spores of Bacillus atrophaeus var. globigii (BG). The sampling efficiency of the CI-L was 6%, 11%, 17%, 21%, and 58% for 0.5, 1, 3, 5 μm PSL microspheres and BG spores, respectively. Higher overall sampling efficiencies of 71%, 91%, 60%, 64%, and 104% were observed for the same size and type of particles for the CI-WWMF. This study indicates that using wet filters on top of wax-filled Petri dishes (CI-WWMF) in a viable cascade impactor is more efficient than the CI-L method for size-selectively collecting biological aerosols from the environment. The CI-WWMF method is useful when a liquid medium is required for identifying and quantifying organisms using polymerase chain reaction (PCR) and immuno-assay techniques.
Copyright © 2017 American Association for Aerosol Research
EDITOR:
Introduction
Contagious diseases have the potential to spread into outbreaks through a variety of mechanisms such as transfer of infectious agents by air or surfaces. Several major human infectious diseases are transmitted by aerosolized microorganisms, including bacterial diseases such as tuberculosis, legionellosis, and inhalation anthrax; viral diseases such as colds, flu, chicken pox, measles, and hantavirus pulmonary syndrome; and fungal diseases such as aspergillosis and histoplasmosis (Burge and Henningson Citation1993; Hinds Citation1999).
Commonly, infectious organisms within the airways' mucus material are released into the air during breathing, talking, singing, coughing, and sneezing. These aerosol particles range in size depending on the mechanism of release: submicron particles are released during breathing while micron size particles are released during sneezing and coughing (Fabian et al. Citation2008; Johnson and Morawska Citation2009). The incidence of infection and the type of infection depend on the organism as well as the particle size, which determines how long the organism stays airborne, how far it travels, and the deposition location in the respiratory system (McClellan et al. Citation2016). Therefore, when studying airborne transfer of infectious organisms it is important to determine the particle sizes as well as particle concentrations.
Filters, impingers, cyclones, and cascade impactors are traditionally used to capture biological aerosols from the air (Kesavan and Sagripanti Citation2015). Filters and impingers collect particles of all sizes together, while multistage cyclones (Blachere et al. Citation2009; Hsiao et al. Citation2010) and cascade impactors collect particles separated by size on different stages (Marple and Willeke Citation1976). The size separation depends on the design of the sampler and the sampling air flow rate. Sampling time is also an important parameter in selecting bioaerosol samplers in order to preserve biological integrity of the organisms.
Andersen viable cascade impactors were designed to collect biological aerosols into agar-filled plates to determine the type and concentration of culturable biological aerosols. The captured aerosol particles are separated by aerodynamic size, which takes into account the geometric size, density, and shape of the particle (Andersen Citation1958; Marple Citation2004). The Andersen viable cascade impactor consists of six stages, each containing 400 orifices. The orifice size is reduced progressively in each subsequent stage to further accelerate the air and collect smaller particles in lower stages of the impactor. Each stage of the Andersen cascade impactor has a size cut point range designed to represent deposition in various regions of the respiratory system (Thermo-Scientific Citation2009). Thus, particles larger than 7 μm in diameter are collected in the upper (1st) stage, 4.7–7.0 μm in the 2nd, 3.3–4.7 μm in the 3rd, 2.1–3.3 μm in the 4th, 1.1–2.1 μm in the 5th, and 0.6–1.1 μm in the bottom stage. This instrument is widely used to size-selectively enumerate culturable particles in the air (Bragoszewska et al. Citation2016; Pahari et al. Citation2016).
Particle bounce is a problem in cascade impactors that have a hard collection surface (Chang et al. Citation1999; Cheng and Yeh Citation1979; Kuuluvainen et al. Citation2016). Many modifications have been made to the collection surface to reduce bounce. Coated surfaces and agar plates are used to reduce bounce of particles and to maintain biological organisms in a humid environment. Teflon tape, Nucleopore, and glass fiber filters have been also used as impaction surfaces to reduce bounce (Chang et al. Citation1999). In addition, Demokritou et al. (Citation2002) used polyurethane foam (Merryweather Foam, Baberton, OH, USA) to reduce bounce and to collect large quantities of material by impaction for toxicological, biological, and chemical characterization studies.
Andersen impactors are designed to work at an air flow rate of 28.3 L/min. The manufacturer recommended sampling times range from a few minutes up to 30 min. A longer sampling time may dry the agar, increase bounce, and damage the organisms. Since the 50% cut point of the last stage of this impactor is 0.6 μm, this sampler will not efficiently collect particles smaller than 0.6 μm. The manufacturer recommends pipetting 27 mL of agar into each of the six glass Petri dishes in an Andersen cascade impactor. Other non-glass Petri dishes can also be used if agar is filled to maintain the required distance between the accelerating orifice and the agar impaction surface.
Organisms collected on agar are typically analyzed by culturing. Organisms which are viable but not culturable, dead organisms, and some organisms that are complicated to culture cannot be quantified using agar-filled plates and therefore results may be underreported. Variables such as agar type and incubation conditions will also selectively encourage the growth of certain organisms and may negatively impact the identification of other organisms present in the air. Some of these drawbacks can be avoided by collecting organisms in a liquid and identifying and quantifying them through other methods, such as polymerase chain reaction (PCR) and immuno-assay techniques.
Several methods for collecting biological aerosols into liquid have been designed to keep the organisms viable. Collecting into liquid also provides flexibility for sample analysis, allowing the sample to be filtered (and concentrated) when air concentrations are low and serially diluted when air concentrations are high (e.g., agricultural environments). Traditional glass impingers limit sampling time due to the evaporation of liquid from the sampler and have been shown to stress environmentally sensitive organisms (Rule et al. Citation2007). Newer samplers such as the Biosampler® (SKC Inc., Eighty Four, PA, USA), and SASS 2300 (Research International Inc., Monroe, WA, USA) collect particles into liquid using centrifugal motion, thus reducing evaporation, extending sampling times, and reducing stress on the organisms. However, these samplers do not provide size-separation of aerosols like the Andersen cascade impactor.
Several studies have modified cascade impactor collection methods to acquire more information than what is provided by traditional use of agar-based impactors. Recent research found that each airborne biological particle can be comprised of many organisms; for example, a 3 μm bio-cluster particle may contain up to 32 organisms (Kesavan et al. Citation2014; King and McFarland Citation2012). King and McFarland (Citation2012) used a modified six-stage bio-cascade impactor method to determine both the aerosol size distribution and the number of organisms. In their study, a filter was placed on top of the agar to cover half the area. Particles were collected on both surfaces (agar and filter) to enumerate separately the biological particles (each containing many organisms) and biological organisms. The number of particles was determined using the results from incubating the agar plates, and the number of organisms was determined by removing the particles from the filters and plating the samples. In another recent study, Bischoff et al. (Citation2013) modified the traditional 6-stage cascade impactor by adding eight mL of liquid Hanks balanced Salt Solution (HBSS) into each collection Petri dish instead of the recommended volume of agar. This modified method was used in a hospital setting to sample airborne viruses.
The goal of this study was to evaluate the sampling efficiency of Bischoff's modified method utilizing impaction into liquid (further referred as CI-L), and to compare it with a new method that uses wax filled Petri dishes with wet membrane filters on top of the wax (CI-WWMF). Wax is used to maintain the distance required between the accelerating orifice and the impaction surface, while the wet membrane filters reduce particle bounce, provide a wet surface to collect aerosol into liquid, and facilitate particle extraction.
Methodology
Two modified cascade impactors, one containing liquid-filled Petri dishes, and one containing plates with wax and a wet filter (CI-L and CI-WWMF), were evaluated side-by-side to compare their sampling efficiencies. Impactors were tested using 0.5, 1, 3, and 5 μm polystyrene latex (PSL) microspheres (Duke Scientific Corp., Palo Alto, CA, USA) and Bacillus atrophaeus var. globigii (BG) spores, obtained from the U.S. Army, Dugway Proving Ground (UT, USA). Additional information about determining the sampling efficiency of samplers using PSL microspheres and BG is provided in a previous publication (Kesavan et al. Citation2008). The manufacturer reported PSL sizes and the median aerodynamic size of BG aerosol measured by an APS (0.8 µm) (King et al. Citation2011) were adopted. The fluorescent PSL test samples were quantified using a fluorometer (Sequoia-Turner Model 450, Abbott Diagnostic Division, Mountain View, CA, USA), and the biological test samples were quantified by standard microbiological plating.
Milled BG in powder form was processed to remove soluble materials as described by Kesavan et al. (Citation2014). Briefly, approximately 1 g of BG powder was added to a 50 mL centrifuge tube and filled with 45 mL deionized water. The tube was vortexed for 5 min and then centrifuged at 4000 RPM for 20 min. The supernatant with dissolved materials was removed and the process was repeated two more times to remove the soluble components of the BG suspension. The cleaned BG suspension was stored in the refrigerator and was used within a month of preparation.
Glass Petri dishes for the CI-WWMF were prepared by pouring hot wax (Paraffin wax, Aldrich Chemical Company, Inc., Milwaukee, WI, USA) into them to provide the required distance between the accelerating orifice of the previous stage and the impaction surface (Thermo-Scientific Citation2009). Before each test, one 90 mm diameter membrane filter (0.2 μm pore, GVS Life Sciences, Sanford, ME, USA) was placed on each Petri dish to cover the wax surface, and then 2 mL of liquid were added on top of each filter to provide a wet collection surface. Each stage of the CI-WWMF was loaded with one of these plates. Each stage of the CI-L was loaded with a glass Petri dish containing 8 mL of liquid (Bischoff, personal communication). The liquid used for the PSL tests was deionized water, and the liquid used for the BG tests was phosphate buffered saline with 0.01% Triton X-100 (PBST). One CI-L and one CI-WWMF were used side-by-side in each of the tests.
Experiments with PSL microspheres and BG spores were conducted in a 27 ft3 Plexiglass chamber (). A 24-jet Collison nebulizer (BGI, Inc., Waltham, MA, USA) was used to aerosolize the PSL microspheres for 10 min and BG for 3–5 min. All aerosols were neutralized by passing them through a Kr-85 neutralizer (TSI Inc., Shoreview, MN, USA) before they were delivered to the chamber. The chamber air was mixed for 1 minute after aerosolization to obtain uniform aerosol concentration in the chamber. The aerosol concentration, mass mean diameter, and standard deviation of the aerosolized PSL microspheres were measured with an aerodynamic particle sizer (APS 3321, TSI Inc.) inside the chamber. The PSL aerosol concentrations inside the chamber ranged from 7.5 to 16.3 particles/cm3. Based on the culture results of the reference filters, the average concentration of culturable BG was 265 cfu/ cm3 of air (range: 141–444 cfu/cm3). The Collison nebulizer was cleaned thoroughly before switching to a different size particle, and the chamber air was purged for approximately 10 minutes between experiments until concentrations were back to background levels. APS measurements were used to confirm no carry over of particles from one test to the other.
Two 47 mm membrane filters (0.2 μm pore size, BGI Inc., Butler, NJ, USA) were used simultaneously at 20 L/min as reference samplers to measure particle concentration inside the chamber (Kesavan et al. Citation2010). The air flow rate through the cascade impactors was adjusted to sample at 28.3 L/min. All the flow rates were measured using an air flow meter (4000 series, TSI Inc.). Both cascade impactors and the reference filters sampled the air for 10 min to collect samples for analysis. Each particle size test was repeated 3–5 times to assess variability.
After each sample, the cascade impactors were disassembled. The remaining liquid from each liquid-filled Petri dish was transferred to a 50 mL test tube and measured. Additional liquid (8 mL) was added to each test tube to increase the liquid volume for fluorometric analysis. CI-L Petri dishes were not washed to remove any remaining particles as this was not mentioned in the original paper by Bischoff et al. (Citation2013) Similarly, the filters and remaining liquid were collected from wax-filled Petri dishes and transferred to a 50 mL centrifuge tube. Eight mL of liquid was added to each of the wax-filled plates, swirled, and pipetted out to remove remaining particles from the plates. This wash volume was added to the centrifuge tube containing the filter from the same stage. An additional 8 mL of liquid was added to all filter tubes to improve the removal of particles from filters. Each reference filter was placed in 20 mL of deionized water (for PSL) or PBST (for BG). All filter samples were vortexed for 10 min to remove particles from filters. An aliquot of approximately 5 mL of each fluorescent PSL test sample was transferred to a test tube (12 mm diameter by 75 mm long, Borosilicate glass, Fisher Scientific) and placed in the measurement well of the fluorometer. The fluorometer was zeroed every five measurements with deionized water. All test and reference samples from each particle size were measured one after the other to eliminate error from change in temperature of the room. For tests with BG, three 100 µL aliquots from each sample were plated on tryptic soy agar (TSA) plates and incubated at 37°C for 24 h for colony enumeration.
The sampling efficiency for each stage was determined using Equation (Equation1[1] ). Student's t-tests were conducted to determine whether the sampling efficiency of the CI-L and CI-WWMF were different for the tested particles sizes.
[1]
Results
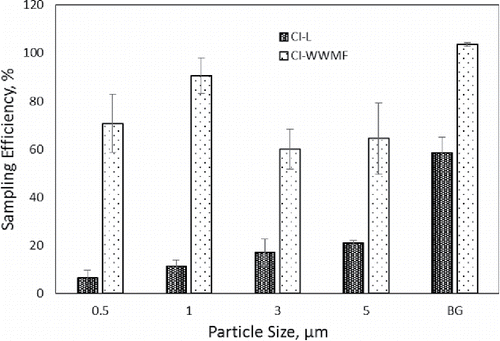
Total sampling efficiencies of the CI-L and CI-WWMF cascade impactors as a function of particle size are provided in . The total sampling efficiency of the CI-WWMF was significantly higher (p < 0.004) compared to the sampling efficiency of the CI-L. The CI-WWMF sampling efficiency for 0.5 PSL particles was more than ten times higher than that of the CI-L, more than eight times for 1 μm PSL particles, more than 3 times for 3 and 5 µm PSL particles, and more than 1.7 times for BG spores. With both samplers, the sampling efficiency for bacterial particles (median aerodynamic particle size of 0.8 µm) was higher than the sampling efficiency for PSL microspheres of comparable size.
Figure 2. Total sampling efficiency of the CI-L and CI-WWMF. The X-axis lists the particle sizes of polystyrene latex (PSL) microspheres and Bacillus atrophaeus var. globigii (BG) spores (median aerodynamic particle size of 0.8 µm) used to determine the sampling efficiency.
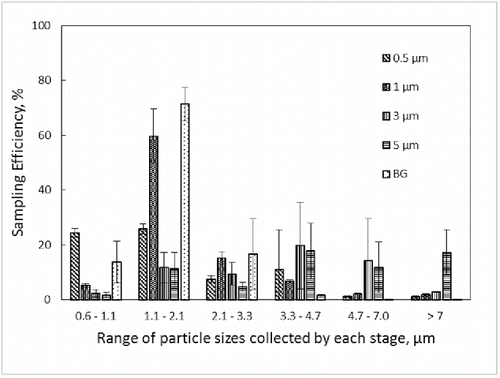
Sampling efficiencies by stage for the CI-L and CI-WWMF are provided in and , respectively. As expected, most of the 0.5 μm PSL particles were collected in stage 6 in the CI-L sampler, but only with 2% (±1) efficiency, whereas the same 0.5 μm PSL particles were collected with higher efficiency (24 and 25%) in stages 5 and 6 of the CI-WWMF. The majority of 1 μm particles were collected in stage 5 in both impactors, which is designed to collect particles in the range of 1.1 to 2.1 μm. The majority of BG spores were also collected in stage 5 (1.1–2.1 μm) on both impactors, with 24.5% collected in the CI-L, and 71% in the CI-WWMF. When compared by stage, the CI-WWMF consistently sampled with higher efficiency than the CI-L.
Figure 3. Sampling efficiency by stage of the CI-L. The X-axis provides the range of particle sizes collected by each stage. The different bar markings correspond to the size of monodisperse PSL microspheres and Bacillus atrophaeus var. globigii (BG) spores used in the sampling efficiency testing.
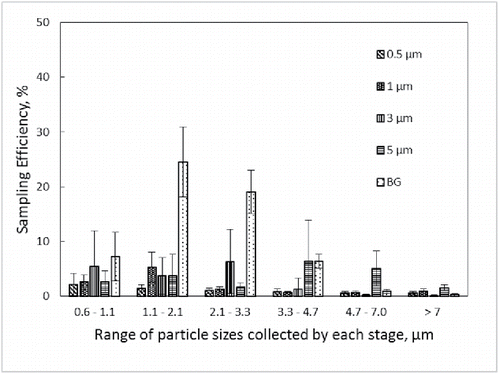
Figure 4. Sampling efficiency by stage of the CI-WWMF. The X-axis provides the range of particle sizes collected by each stage. The different bar markings correspond to the size of monodisperse PSL microspheres and Bacillus atrophaeus var. globigii (BG) spores used in the sampling efficiency testing.
The amount of liquid remaining in the Petri dishes of the CI-L after 10 min of sampling depends on the relative humidity (RH) of the air. Approximately 7 mL of liquid (range 6.97 to 7.45) remained after 10 min of sampling at 60% RH and 21°C; however, an average of 3.8 ± 0.28 mL of liquid remained in the Petri dishes at 19% RH and 23°C.
Discussion
This project evaluated the sampling efficiencies of two modified 6-stage cascade impactors, one containing only liquid (CI-L) and one containing wax and wet membrane filters (CI-WWMF). Sampling efficiency of the CI-L was 6%, 11%, 17%, 21%, and 58% for 0.5, 1, 3, 5 μm PSL microspheres and aerosol consisting of single spore BG, respectively. Higher overall sampling efficiency was observed for the CI-WWMF, with efficiencies of 71%, 91%, 60%, 64%, and 104% for 0.5, 1, 3, and 5 µm PSL microspheres and BG spores, respectively. We believe the improved sampling efficiency of the CI-WWMF was a result of a softer impaction surface that can be maintained wet over the 10-min sampling period. An additional washing of the surface after removing the wet filter may have also increased particle recovery.
In some cases, particles were collected in stages designed to capture larger sizes. Similar results were also seen by King and McFarland (Citation2012), where 1.8 µm particles deposited on the 2.1 to 3.3 μm stage. This may be due to the particles not completely drying and therefore having a larger aerodynamic size compared to dry PSL particles. This phenomenon has also been observed with aqueous aerosols in high and low humidity environments (Hiller et al. Citation1980; Rovelli et al. Citation2016). For example, the sampling efficiency of the CI-WWMF for 0.5 µm particles was 71%, which was probably due to this phenomenon. However, the sampling efficiency for 0.5 μm particles was only 6% for the CI-L. This was probably because of liquid displacement; lower stages of the cascade impactor have smaller accelerating nozzles and higher air velocities compared to the stages above. The high velocity of the air displaces the liquid and exposes glass as the impaction surface, so particles impacting the lower stages have a higher potential for bounce. In addition, some 0.5 µm particles were observed to be impacted onto the bottom of the glass Petri dishes of the CI-L, and were difficult to remove. Researchers interested in collecting particles smaller than 0.6 μm should consider using low pressure impactors that collect particles as small as 0.08 µm.
Sampling efficiencies for PSL microspheres were lower in general than the sampling efficiencies for BG spores of comparable size. This is likely due to the higher bounce of solid PSL microspheres compared to the “stickier” BG spores. In addition, the surfactant used in the PBST solution used in the BG tests helps wet the surface of the Petri dish better compared to the water used for the PSL tests and may help retain particles more efficiently. The sampling efficiencies measured in these tests are lower than the sampling efficiency reported by the manufacturer, except for BG spores in the CI-WWMF. This is likely because agar surfaces are able to prevent bounce and thus retain particles more efficiently than a wet filter and glass surfaces. In addition, not all particles collected may have been recovered for analysis; some may be retained within the filter or remained stuck to the glass plate, whereas every particle that impacts on agar is available for evaluation, provided it is culturable. The 50% cut-off size of the systems are not provided as the sampling efficiency of the CI-L did not reach above 50%, and the sampling efficiency of the CI-WWMF did not reach below 50% for the tested PSL microspheres.
Bischoff et al. (Citation2013) used HBSS in their cascade impactor study; however, since HBSS affects fluorescence measurements, the cascade impactors in this study were filled with deionized water for the PSL microsphere tests and with PBST for the BG tests, as these solutions are commonly used in laboratory testing. HBSS and water covered a similar area of the Petri dish when filled with similar volumes of liquid; however, PBST contains a surfactant that helped completely cover the Petri dish's collection surface.
After 10 min of sampling with the CI-L using 8 mL of liquid, only between 3 and 7 mL of liquid were left in each Petri dish, exposing part of the glass bottom for both HBSS and deionized water tests. Having a higher volume of liquid in the Petri dish to cover the bottom is not possible because the liquid is pulled out of the Petri dishes by the vacuum required to run the cascade impactor. The use of other liquids to fill the Petri dishes or addition of a surfactant should be investigated for their ability to increase sampling efficiency. Furthermore, liquid droplets were observed on all metal surfaces of the CI-L after sampling. This liquid could not be collected for analysis and may have further contributed to the lower sampling efficiency of the CI-L. Future research is needed to evaluate the different losses and other collection media to minimize this problem; oils and dissolvable or peelable gels may be better candidates to increase the sampling efficiency of airborne organisms.
In a recent study that evaluated 29 samplers (Kesavan and Sagripanti Citation2015), the authors found that the average sampling efficiency for 1 to 5 µm particles ranges from 14–93%. In the current study, the CI-WWMF had an average sampling efficiency of 72% for 1 to 5 µm particles which falls in the middle of the range of the 29 evaluated samplers, whereas the CI-L, with 22% efficiency, is at the lower end. The sampling efficiency of BG in the CI-WWMF (104%) was higher than what is reported for efficient samplers such as the BioSampler that has an efficiency of 96 to 98% for 1–3 µm particles. However, in contrast to the samplers evaluated by Kesavan and Sagripanti (Citation2015), CI-WWMF collects particles size-selectively, which is an advantage for hazard assessment.
The two evaluated cascade impactors collect bioaerosols into liquid instead of an agar surface. The method reported by Bischoff et al. (CI-L) is assumed to collect particles directly into liquid; however, a solid dry surface is effectively being used for collection, since liquid is displaced by the air jet streams during sampling, exposing the glass for impaction. The use of the new CI-WMMF method described in this article is recommended over the use of only liquid for those researchers who need to collect particles into a liquid. A drawback of the CI-WWMF is that it requires additional steps of rinsing the wax and vortexing the filters to recover particles, which adds time to the analysis. However, this is justified by the improved sampling efficiency.
Conclusion
We have shown that with slight modifications, the efficiency of the cascade impactor reported by Bischoff in 2013 can be improved to provide size separated particle collection into liquid for various analysis methods such as PCR, and immuno-assays. The results indicate that using CI-WWMF is an efficient method for size-selectively collecting biological aerosols from environments like health care settings. Other modifications such as the use of surfactants, oils, or peelable gels may provide additional improvements in the sampling efficiency and should be evaluated in future studies.
Acknowledgments
The authors thank Amelia Stephens, Pamela Humphreys, Daniel Mcgrady, Paul Deluca, and Garrett Nelson for help with some of the experiments.
References
- Andersen, A. A. (1958). New Sampler for the Collection, Sizing, and Enumeration of Viable Airborne Particles. J. Bacterio., 76:471–484.
- Bischoff, W. E., Swett, K., Leng, I., and Peters, T. R. (2013). Exposure to Influenza Virus Aerosols during Routine Patient Care. J. Infect. Dis., 207:1037–1046.
- Blachere, F. M., Lindsley, W. G., Pearce, T. A., Anderson, S. E., Fisher, M., Khakoo, R., Meade, B. J., Lander, O., Davis, S., Thewlis, R., Celik, I., Chaen, B. T., and Beezhold, D. H. (2009). Measurement of Airborne Influenza Virus in a Hospital emergency department. Clin. Infect. Dis., 48:438–440.
- Bragoszewska, E., Mainka, A., and Pastuszka, J. S. (2016). Bacterial Aerosols in an Urban Nursery School in Gliwice, Poland: a Case Study. Aerobiologia, 32:469–480.
- Burge, H. A., and Henningson, E. (1993). Bioaerosol Sampling, in Aerosol Measurement: Principles, Techniques, and Applications, K. Willeke and P. A. Baron, eds., Van Nostrand Reinhold, New York, pp. 471–492.
- Chang, M., Kim, S., and Sioutas, C. (1999). Experimental Studies on Particle Impaction and Bounce: Effects of Substrate Design and Material. Atmosph. Envir., 33:2313–2322.
- Cheng, Y.-S., and Yeh, H.-C. (1979). Particle Bounce in Cascade Impactors. Envir. Sci. Techno., 13:1392–1396.
- Demokritou, P., Kavouras, I. G., Ferguson, S. T., and Koutrakis, P. (2002). Development of a High Volume Cascade Impactor for Toxicological and Chemical Characterization Studies. Aero, Sci. Techno., 36:925–933.
- Fabian, P., DcDevitt, J. J., DeHaan, W. H., Fung, R. O., Cowling, B. J., Chan, K. H., Leung, G. M., and Milton, D. K. (2008). Influenza Virus in Human Exhaled Breath: An Observational Study. PLOS ONE, 3:e2691.
- Hiller, F. C., Mazumder, M. K., Wilson, J. D., and Bone, R. C. (1980). Effect of Low and High Relative Humidity on Metered-Dose Bronchodilator Solution and Powder Aerosols. J. Pharmace. Sci., 69:334–337.
- Hinds, W. C. (1999). Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. John Wiley & Sons, Inc., New York.
- Hsiao, T.-C., Chen, D.-R., Li, L., Greenberg, P., and Street, K. W. (2010). Development of a Multi-Stage Axial Flow Cyclone. Aero. Sci. Techno., 44:253–261.
- Johnson, G. R., and Morawska, I. (2009). The Mechanism of Breath Aerosol Formation. J. Aero. Med. Pulmo. Drug Deli., 22:229–237.
- Kesavan, J., Bottiger, J. R., and McFarland, A. R. (2008). Bioaerosol Concentrator Performance: Comparative Tests with Viable and with Solid and Liquid Nonviable Particles. J. Appl. Microbio., 104:285–295.
- Kesavan, J., and Sagripanti, J. L. (2015). Evaluation Criteria for Bioaerosol Samplers. Envir. Sci. Proc. Impact, 17(3):638–645.
- Kesavan, J., Schepers, D., Bottiger, J., and Edmonds, J. (2014). UV-C Decontamination of Aerosolized and Surface Bound Single Spores and Bioclusters. Aero. Sci. Techno., 48:450–457.
- Kesavan, J., Schepers, D., and McFarland, A. R. (2010). Sampling and Retention Efficiencies of Batch-Type Liquid-Based Bioaerosol Samplers. Aero. Sci. Techno., 44:817–829.
- King, B., Kesavan, J., and Sagripanti, J.-L. (2011). Germicidal UV Sensitivity of Bacteria in Aerosols and on Contaminated Surfaces. Aero. Sci. Techno., 45:1–9.
- King, M. D., and McFarland, A. R. (2012). Use of an Andersen Bioaerosol Sampler to Simultaneously Provide Culturable Particle and Culturable Organism Size Distribution. Aero. Sci. Techno., 46:852–861.
- Kuuluvainen, H., Saari, S., Mensah-Attipoe, J., Arffman, A., Pasanen, P., Reponen, T., and Keskinen, J. (2016). Triboelectric Charging of Fungal Spores During Resuspension and Rebound. Aero. Sci. Techno., 50:187–192.
- Marple, V. A. (2004). History of Impactors - The first 110 years. Aero. Sci. Techno., 38:247–292.
- Marple, V. A., and Willeke, K. (1976). Impactor Design. Atmosph. Envir., 10:891–896.
- McClellan, G. E., Millage, K. K., and Asgharian, B. (2016). An Improved Model of Human Response to Bioaerosol Exposure, in Aerobiology: The Toxicology of Airborne Pathogens and Toxins, H. Salem and S. Katz, eds., Royal Society of Chemistry, Cambridge, UK, 400–445.
- Pahari, A. K., Dasgupta, D., Patil, R. S., and Mukherji, S. (2016). Emission of Bacterial Bioaerosols from a Composting Facility in Maharashtra, India. Waste Manage., 53:22–31.
- Rovelli, G., Miles, R. E. H., Reid, J. P., and Clegg, S. L. (2016). Accurate Measurements of Aerosol Hygroscopic Growth over a Wide Range in Relative Humidity. J. Phys. Chem., 120:4376–4388.
- Rule, A. M., Kesavan, J., Schwab, k. J., and Buckley, T. J. (2007). Application of Flow Cytometry for the Assessment of Preservation and Recovery Efficiency of Bioaerosol Samplers Spiked with Pantoea Agglomerans. Env. Sci. Tech., 41(7):2467–2472.
- Thermo-Scientific. (2009). Instruction Manual: six and two stage viable samplers, Thermo Fisher Scientific, Franklin, MA.