Aim
The aim of this treatment planning comparison study was to explore different spinal irradiation techniques with respect to the risk of late side-effects, particularly radiation-induced cancer. The radiotherapy techniques compared were conventional photon therapy, intensity modulated x-ray therapy (IMXT), conventional electron therapy, intensity/energy modulated electron therapy (IMET) and proton therapy (IMPT).
Material and methods
CT images for radiotherapy use from five children, median age 8 and diagnosed with medulloblastoma, were selected for this study. Target volumes and organs at risk were defined in 3-D. Treatment plans using conventional photon therapy, IMXT, conventional electron therapy, IMET and IMPT were set up. The probability of normal tissue complication (NTCP) and the risk of cancer induction were calculated using models with parameters-sets taken from published data for the general population; dose data were taken from dose volume histograms (DVH).
Results
Similar dose distributions in the targets were achieved with all techniques but the absorbed doses in the organs-at-risk varied significantly between the different techniques. The NTCP models based on available data predicted very low probabilities for side-effects in all cases. However, the effective mean doses outside the target volumes, and thus the predicted risk of cancer induction, varied significantly between the techniques. The highest lifetime risk of secondary cancers was estimated for IMXT (30%). The lowest risk was found with IMPT (4%). The risks associated with conventional photon therapy, electron therapy and IMET were 20%, 21% and 15%, respectively.
Conclusion
This model study shows that spinal irradiation of young children with photon and electron techniques results in a substantial risk of radiation-induced secondary cancers. Multiple beam IMXT seems to be associated with a particularly high risk of secondary cancer induction. To minimise this risk, IMPT should be the treatment of choice. If proton therapy is not available, advanced electron therapy may provide a better alternative.
Primitive neuroectodermal tumour (PNET) of the central nervous system is the most common malignant brain tumour in children. The majority of these embryonal tumours are located in the posterior fossa and classified as medulloblastomas. The tumour is characterised by a high frequency of seeding or metastases; up to 40% have been reported Citation[1]. The PNETs are usually sensitive to both chemo- and radiotherapy. Post-operative craniospinal radiotherapy results in a 5-year survival between 55 and 70% for all stage groups Citation[2]. The corresponding figure is 85% for standard-risk patients Citation[3]. Accompanying the improvement in long-term survival, there is increasing concern about the late side-effects associated with radiotherapy, such as hormonal deficits, decreased bone growth, hearing loss, neuro-cognitive deficits, cardiac dysfunction, pneumonitis, stenosis of the oesophagus and secondary cancers. Radiation-induced secondary cancer is a late side-effect of great concern in paediatric cancer treatment Citation[4], Citation[5]. Several conformal techniques for treating the cranial part of the target volume have been described Citation[6], Citation[7]. The classical conventional treatment of the spinal volume is a single posterior photon beam Citation[8]. On the other hand, information about conformal treatment methods for the spine that minimise the dose to the cervical, thoracic and abdominal organs is quite scanty. This study focuses on radiation therapy of the spinal canal.
One approach to reducing the late side-effects associated with radiotherapy is to reduce the absorbed dose to the craniospinal axis. To date, when radio- and chemotherapy are combined, the total dose to the craniospinal axis has been successfully reduced from 35–36 Gy to 23.4 Gy, while maintaining a dose of 54 Gy to the posterior fossa, and this treatment protocol results in a lower risk of long-term side-effects Citation[9]. Further dose reduction to 18 Gy led to an increased risk of recurrences and a similar risk of side-effects Citation[10]. Thus, improving radiotherapy techniques is essential.
Another approach to reduce the risk of late side-effects is to decrease the dose to the organs outside the target volume while maintaining the dose to the tumour target. Some techniques could potentially be used for this aim, i.e. intensity modulated x-ray therapy (IMXT), conventional electron beam therapy or intensity and energy modulated electron (IMET) or intensity modulated proton therapy (IMPT). IMXT can improve the conformity of the dose distribution to the target, compared with three-dimensional conformal radiotherapy (3-D CRT), but it delivers a larger integral dose to the surrounding normal tissues Citation[11]. This might be a critical risk factor for radiation-induced secondary cancer in long-term survivors Citation[12].
The use of high energy electrons in spinal cord irradiation was recommended already in the early 1980s Citation[13]. The expected benefit was related to the limited range of the electron beams and the accompanying dose reduction to the organs anterior to the vertebral column. However, due to dose calculation uncertainties inside the patient and poor delivery techniques, the use of electrons in medulloblastoma treatment has been limited. So far, only a few retrospective clinical reports using high energy electrons in craniospinal irradiation have been published Citation[14–17]. It is likely that electron therapy results in a tumour control rate comparable to that of conventional photon therapy, with less acute side-effects and a similar rate of late side-effects. IMET techniques are under development Citation[18–20]. High quality electron beams are available in racetrack microtrons (MM50, IBA, Belgium) Citation[21] or can be obtained by modification of the treatment head of general linear accelerators Citation[22]. Therefore, we aim to re-investigate the use of electrons in medulloblastoma radiotherapy.
Proton beams have physical properties that might be especially beneficial in paediatric patients Citation[23]. The usefulness of protons in medulloblastoma radiotherapy has been reported previously Citation[7], Citation[16], Citation[24].
The aim of this treatment planning comparison study was to explore different techniques for spinal irradiation with respect to the risk of some late side-effects, particularly radiation-induced secondary cancer. The radiotherapy techniques compared were conventional photon therapy, IMXT, conventional electron therapy, IMET and IMPT.
Material and methods
CT images for radiotherapy use from five children diagnosed with ‘standard-risk’ medulloblastoma were selected for this study. Two subjects were girls and three were boys; the median age was 8 (6–11) years. The planning CT was obtained with the children in the prone position in a treatment immobilization device. The increment of the CT slices was 10 mm in the thorax and 5 mm in the rest of the volume. Clinical target volumes (CTV) comprising the entire subarachnoid theca and the intervertebral foramina were outlined in each transverse CT slice from C5 to S2. The S2 was defined as the inferior border of the CTV for all patients Citation[25]. The planning target volume (PTV) was obtained by expanding a margin of 3 mm in the ventral direction from the CTV, and of 5 mm in other directions in the transversal plane. To prevent the risk of uneven bone growth, the vertebral bodies were considered to be a secondary target. The thyroid gland, lungs, heart, oesophagus, kidneys, liver, pancreas, stomach, abdominal cavity (i.e. the remaining normal tissue in the abdominal cavity) and mammary glands in girls were determined to be organs-at-risk (OARs). The patient outline, excluding the already defined targets and OARs, was also considered to be an OAR and was included in the treatment plan evaluation.
The total prescribed dose was 23.4 Gy, administered in 13 daily fractions of 1.8 Gy to the PTV as stipulated in the recent Société International d'Oncologie Pediatrique (SIOP) PNET4 study Citation[26]. The prescribed dose for IMPT treatment was converted to the biological equivalent dose (23.4 EGy) by applying a relative biological effectiveness (RBE) factor of 1.1 for proton therapy. All dose plans were normalised to the mean dose of the PTV for comparison, since the reference point defined in ICRU 50 Citation[27] could not be applied to all plans.
The 6 MV photon beam generated from a linear accelerator was used for the conventional photon (Primus, Siemens AG, Erlangen Germany) and IMXT (Clinac 2100,Varian Medical Systems inc, Palo Alto USA) planning. High energy electrons generated from a MM50 racetrack microtron (MM50, IBA, Belgium) were used for conventional electron and IMET planning. The electron energy range was 7.5–50 MeV with 2.5 MeV energy steps. Theoretical data for a proton beam with spot-scanning was used for the IMPT technique. The beam spots were modeled as a product of a realistic depth dose curve and a lateral Gaussian distribution with a depth-dependent variance σ. The initial σ on the patient surface was assumed to be 3 mm which broadens with increasing depth, according to the principle of multiple Coulomb scattering. More details about the dose model can be found in Citation[28].
Treatment techniques
Conventional photon technique
The treatment planning system (TPS) was Helax-TMS version 6.1 (Nucletron B. V., The Netherlands). A single posterior 6 MV photon beam was used to cover the PTV. Dose variation within the primary target was aimed at ±10%. In order to avoid irradiation of the thyroid, the cranial part of the PTV was treated with lateral opposed beams. A pencil beam algorithm was used for dose calculation and verified with a collapsed cone algorithm in Helax-TMS. The dose distributions were similar irrespective of the method used.
IMXT
The treatment plans were created with inverse planning technique using Helios software in the Eclipse treatment planning system (Varian Medical Systems, Finland OY, Finland). The intensity-modulated beams were delivered with a dynamic MLC technique. Five isocentric beams, at gantry angles of 300, 325, 0, 35 and 60 degrees, were used for best avoidance of the OARs. The same beam setting was used for both isocentres. The maximal obtained length of the optimised cranial-caudal dose distribution in the TPS was about 35 cm. Due to this limitation, two isocentres had to be selected in order to cover the PTV. The gap on the patients’ skin was between 4 and 9 mm, and it was moved 20 mm downward or upward after half of the treatment. The gap was positioned below the heart level. The isocentres were placed close to the matching line in order to minimise dose divergence in the beam junction. The dose constraints for PTVs and OARs are shown in .
Table I. The dose constraints for OARs in IMXT, IMPT and IMET.
Conventional electron technique
The TPS was Helax-TMS 6.1 (Nucletron B. V., The Netherlands) with the electron dose calculated by a pencil beam algorithm. A single posterior high energy electron beam was divided into 2–3 sub-fields. Either 20 MeV or 25 MeV were selected for each sub-field according to the depth of the PTV. The beam weight of each sub-field was adjusted to reach the dose constraints for the PTV and the vertebral bodies. The dose variation within the primary target was aimed at ±10%. The dose distribution in the vertebral bodies was required to be similar to that of the conventional photon plans. No dose constraints were used for OARs. Maximum electron energy of 25 MeV was chosen because most clinical accelerators cannot produce energies above that level. In cases of insufficient dose to the distal part of the PTV, a 6 MV photon beam was added through the same beam portal as the electron beam Citation[19]. In two of the five cases, mixed photon and electron beams were used to cover the lumbo-sacral spine.
IMET
The IMET plans were produced in a modified research version of the KonRad program (DKFZ-Heidelberg, Department of Medical Physics, Germany) with Monte Carlo pre-calculated pencil kernals and density corrections performed by density-range scaling. Primary energy selection for the single posterior beam was performed according to a previously described method Citation[20]. The energy was modulated by adding two additional energy layers consisting of −2.5 MeV and +2.5 MeV, respectively. The applied dose constraints are shown in . In order to compare the calculations of electron doses by the KonRad and TMS systems used in this study, the conventional electron plans produced with the TMS system were recalculated in the KonRad system. The differences between the calculated mean doses in the various structures were typically less than 5%.
IMPT
The optimisation system used for IMET was also applied for proton optimisation. The system uses an intensity modulated proton pencil beam algorithm Citation[28]. The dose constraints applied are shown in . A single posterior beam was used. The maximum energy was 160 MeV and the beam energy was modulated with a range shifter. The spatial grid size for the lateral scanning of beam spots was chosen to be 3 mm projected on the isocentre plane. The same size, 3 mm, was also adopted for the scanning distance in depth.
Dose plan evaluation
Since the conventional photon technique has a long history and has been used extensively, it was used as a baseline for comparisons. Dose volume histograms (DVH) for targets and OARs were obtained for dose planning evaluation. The average mean doses to the OARs for the five patients were calculated and are presented in .
Table II. Mean absorbed dose in the volumes of interest (average for the five cases).
The normal tissue complication probability (NTCP) was estimated for a number of OARs and endpoints of interest. The NTCP models used in this study were the relative seriality model (RS model) Citation[29] and the Lyman-Kutcher-Burman model (LKB model) Citation[30–32]. The calculation was performed with the BioPlan program Citation[33]. The probabilities of acute side-effects, such as radiation pneumonitis Citation[34] and radiation hepatitis Citation[35], and late side-effects, such as radiation-induced ischemic heart disease Citation[36], oesophagus stricture Citation[37] and small and large bowel obstruction (the abdominal cavity volume was used for calculation) Citation[32], respectively, were estimated. The DVHs were converted to 2 Gy/fraction regimes by application of the linear quadratic model Citation[38], since the parameters of NTCP models were derived based on the assumption that the fraction dose is close to 2 Gy. The α/β values used for DVH conversion were taken from Citation[39].
The risk of secondary lethal cancer (SLC) was estimated according to the model recommended in the ICRP recommendations Citation[40]. The probability of SLC is the product of the effective dose (E) and the nominal probability coefficient of SLC, and E can be expressed byin which DTR denotes the mean absorbed dose in a tissue or organ (T), due to incident radiation (R), and WR and WT are the radiation modality and tissue weighting factors, respectively.
The dose to red marrow and bone surfaces were not included in the calculation of the effective dose; thus, only the risk of lethal solid cancers was considered. The probability of solid SLC has been estimated to 0.045 based on ICRP (Table IV) for the entire population and has been further adjusted for children by multiplying with a factor of 2.5 [40].
Based on the assumption of a linear dose-response relationship up to 4 Gy and of a dose-independent relationship for doses exceeding 4 Gy Citation[12], a corrected mean dose to each outlined structure was calculated from DVH data. This means that all doses of > 4Gy in the DVH were given the value of 4 Gy. For use in models relating to radiation protection, a dose-rate effectiveness factor of 2 was included by ICRP Citation[40]. This factor has been retained and considered as relevant for the present purpose as well Citation[12]. A value of 1 for WR was used for photon and electron beams, and 2 for proton beams Citation[41]. The WT factors recommended in the ICRP 60 Citation[40] were used (). The bone (red marrow and bone surface) and gonad factors were not included; thus, the sum of the weighting factors was equal to 0.67 (it would have been 1, had the entire body been considered). Therefore, the sum of the weighted effective doses in considered organs was renormalized (divided by 0.67). The interleaf leakage in IMXT was not included in the estimate.
Table III. Estimates of the risk of secondary lethal cancer (solid cancers).
Statistics
The two-sided Wilcoxon signed ranks test was used for statistical inference of paired samples.
Results
shows some dose distribution parameters for the volumes of interest in this study. All five techniques produced a clinically acceptable dose distribution in the primary and secondary targets. The mean, maximum and minimum doses were similar for the PTV, as was the case for the vertebral bodies, but the minimum doses were slightly lower for all techniques, compared with conventional photons. However, the volume of the vertebral target receiving the low doses was small in all plans. CT sections and dose distributions are shown in .
Figure 1. Dose distributions for Patient 2, showing the conventional photon (a1–a3), IMXT (b1–b3), conventional electron (c1–c3), IMET (d1–d3), IMPT (e1–e3) plan in a transverse (a1–e1), sagittal (a2–e2), and coronal (a3–e3) sections. The PTV and CTV are delineated with the dark dotted lines. Isodoses are, in percentage, 5%, 10%, 30%, 50%, 70%, 90%, and 100%. Isodose lines of 50% and 90% are in solid black lines, others are in white.
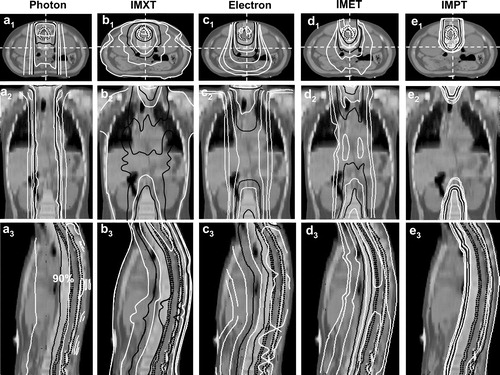
The absorbed doses in the OARs varied considerably with the treatment technique used (). IMPT resulted in a significantly lower mean dose to the different OARs, except for the lungs, compared with all other techniques, and many of the OARs escaped irradiation. IMXT delivered significantly higher mean doses to the breast, lung, spleen, stomach and kidney than the conventional photon and electron techniques. As might be expected, conventional photon and electron techniques yielded the highest doses to the thyroid and oesophagus. Lower mean doses were observed to most OARs with IMET than with the IMXT, photon and electron beam techniques.
Applying the different NTCP models resulted in calculated probabilities for acute and late side-effects, for all techniques, that approached zero for all OARs.
shows the mean doses (DTR) to OARs and the corresponding risks of SLC. There is a large variation in the estimated risk depending on the technique used. The highest estimated risk of SLC was equal to 30% after IMXT, while the lowest was 4%, after IMPT. In the case of the proton technique we also estimated the potential contributions from secondary neutrons by using measurements and Monte-Carlo simulations performed by Schneider et al. 2002 for the PSI treatment facility Citation[42]. They observed an additional dose burden to OARs, due to secondary neutrons, ranging from 0.002 Sv to 0.004 Sv per treatment Gy. Based on these numbers, a conservative estimate of the effective neutron dose accumulated in all OARs leads to an additional dose burden of 0.002 Sv (i.e. the total effective dose was 0.047 Sv); the contribution of neutrons to the risk of SLC should thus be in the order of 1%. The effective dose from IMXT was significantly higher than that from the other techniques (p = 0.002 in all patients). IMET resulted in a lower effective dose, compared with conventional photon beam, IMXT and conventional electron beam (p = 0.002 in all patients). IMPT resulted in the lowest effective dose in all comparisons (p = 0.002 in all patients). Conventional photon and electron techniques resulted in similar effective doses.
Discussion
The NTCPs predicted by different biological models for the considered endpoints were very low for all five techniques, despite the considerable variation in absorbed dose to the surrounding OARs. This is probably due to the low total dose. However, the predictive ability of the NTCP models has actually not been fully tested as yet, especially in the case of late side-effects (e.g. cardiac mortality). Another noteworthy aspect when using NTCP models is that the dose distribution and clinical data on which the models are based reflect irradiation conditions in the specific patient groups. To our knowledge, no available NTCP parameters are based on data sets derived from a paediatric population. Furthermore, the relevant end-points in a paediatric population might not always be the same as in an adult population. In paediatric populations more data for end-points such as, hormonal deficits, retardation of bone growth etc. are needed. Under these circumstances, minimization of the dose outside the target volumes is the appropriate general therapy principle. The largest reduction of absorbed dose outside the target volume was achieved by IMPT. IMXT also reduced the mean dose to heart and thyroid to 60% and 50% respectively, compared with conventional photon technique. The conventional electron technique also reduced the mean dose to heart and thyroid to 50% and 25%, respectively, and IMET reduced it further. This might be a clinical advantage of IMXT and electron techniques, i.e. improving the rates of radiation-induced hypothyroidism and cardiovascular disease Citation[5], in the absence of available more advanced techniques.
Several reports have been published concerning secondary cancer in long-term survivors of childhood cancer Citation[4], Citation[5], Citation[43]. Radiation therapy is the most important risk factor for secondary cancer; the risk is further increased when chemotherapy is added Citation[44]. Low age at the time of treatment, high radiation dose and longer follow-up are factors that increase the risk of secondary cancers. The interval between primary cancer treatment and secondary cancer diagnosis is usually 5–15 years, but the risk keeps rising at 20–30 years follow-up Citation[43]. The estimated risk of SLC was about 20% for conventional photon therapy and similar for conventional electrons. The corresponding risk was about 30% for IMXT, without including the extra absorbed dose due to interleaf leakage etc. The main reason for this is the large number of beams used for achieving a highly conformed, high radiation dose to the target, whilst a low radiation dose is delivered to a large volume of normal tissue. IMET treatment was predicted to yield a lifetime risk of 15%. The difference between conventional electrons and this technique is probably due to the use of a larger number of segments, allowing better dose distribution conformity. Another important factor might be that higher electron energies (maximum 40 MeV), which make the use of additional photon beams for target coverage, are obsolete. However, the vast majority of clinical accelerators do not currently have the capacity to deliver these high electron energies. In this context, the IMET technique was used to explore the potential of advanced electron therapy. IMPT resulted in the lowest risk estimates, 4%. The physical properties of protons seem almost optimal for this patient group. However, the production of neutrons may be a problem in the treatment rooms; even low physical doses of neutrons might increase the risk substantially. In the case of spot-scanned proton beams, the neutron contribution can be very low Citation[42].
Although the ICRP model for cancer induction was not originally intended for radiotherapy Citation[40], it is one of the few models available today that can be applied in this situation. In radiation protection, lower doses than those relevant for radiotherapy are usually considered. However, in Citation[12], the dose range in which the risk of cancer increases linearly with dose is discussed. Their conclusion is that the risk of cancer induction levels out at doses above about 4 Gy. This hypothesis was taken as an assumption in this study, which might make the estimates of risk slightly conservative. On the other hand, any effects of cell kill and a consequent decrease in cancer risk at high doses were not taken into consideration due to the lack of available data on which to base such a correction. The absolute risks in this study should probably be regarded with caution due to uncertainties in the estimates. However, the relative differences between the treatments are probably only slightly influenced by these uncertainties.
In this model study, the estimated lifetime risk of SLC after conventional photon or electron radiotherapy is 20%. In a study of 1 262 medulloblastoma patients, 20 second cancers were diagnosed as compared with the expected 3.7 Citation[48]. The average follow up was less than 5 years. For children exposed to radiation, the peak incidence of SLC is expected to occur between the sixth and the eighth decades of their lives Citation[40]. Thus, an observation time longer than 50 years would be necessary for confirming the incidence of SLC in this population. In a later study on 446 patients that had undergone radiotherapy in childhood and survived for a minimum of 5 years, 26 developed second cancers whereas 5 would be expected [4]. The same authors estimate the cumulative risk for developing a second cancer to13% after 30 years, and still the cumulative risk curve does not reach the plateau. It is not possible to verify the estimates of the risk for SLC in this model study by comparisons with published clinical data since the observation times are still too short. However, it can be concluded that radiotherapy in childhood leads to a significant risk for second cancers.
Bone deformity after electron radiotherapy is rarely reported in the literature Citation[14]. The dose gradient to the vertebral bodies has usually been considered to be a cause of bone deformity. In this study, the vertebral bodies were considered as a secondary target, and a sufficient dose was delivered for avoiding uneven bone growth. It was reported that a dose of 20 Gy was considered as a critical dose to arrest bone growth Citation[45], Citation[46]. In addition, the mean absorbed dose in the vertebral bodies was about 20 Gy with conventional photon treatment. Therefore, a mean dose of 20 Gy was used as a goal for the other treatments in this study. The low minimum dose to the secondary target might cause concerns about uneven bone growth; however, the volume of vertebral bodies receiving the low dose might be too small for any effects to be noticeable. Furthermore, when kilovoltage X-ray was used for spinal canal radiotherapy, the minimum dose to the vertebral bodies was down to 9–11 Gy. No bone deformity was found in the long-term survivors Citation[47].
In conclusion, this model study shows that spinal irradiation of young children results in a substantial risk of radiation-induced secondary cancers after treatment with photon and electron techniques. Multiple beam IMXT seems to be associated with a particularly high risk of secondary lethal cancer induction. To minimise this risk, IMPT should be the treatment of choice. If proton therapy is not available, advanced electron therapy may provide a better alternative to conventional photon therapy, provided that the algorithms for dose calculations are valid.
This work was funded by the Cancer Research Foundation in Northern Sweden and the Swedish Cancer Society. The work was also partly conducted using the resources of the High Performance Computing Centre North (HPC2N). The BioPlan program was kindly provided by Dr. Alan Nahum, Clatterbridge Centre for Oncology, UK. Valuable suggestions have been forwarded from members of the Swedish Proton Therapy Centre (SPTC) network. The authors also wish to thank Ms Agneta Sundberg, dosimetrist at the Department of Radiophysics, Sahlgrenska University Hospital, Gothenburg, Sweden, for IMRT dose-planning and production of images.
References
- Harisiadis L, Chang CH. Medulloblastoma in children: a correlation between staging and results of treatment. Int J Radiat Oncol Biol Phys 1977; 2: 833–41
- Packer RJ, Sutton LN, Elterman R, Lange B, Goldwein J, Nicholson HS, et al. Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J Neurosurg 1994; 81: 690–8
- Taylor RE, Bailey CC, Robinson K, Weston CL, Ellison D, Ironside J, et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 Study. J Clin Oncol 2003; 21: 1581–91
- Gold DG, Neglia JP, Dusenbery KE. Second neoplasms after megavoltage radiation for pediatric tumours. Cancer 2003; 97: 2588–96
- Oeffinger KC, Hudson MM. Long-term complications following childhood and adolescent cancer: foundations for providing risk-based health care for survivors. CA Cancer J Clin 2004; 54: 208–36
- Huang E, Teh BS, Strother DR, Davis QG, Chiu JK, Lu HH, et al. Intensity-modulated radiation therapy for pediatric medulloblastoma: early report on the reduction of ototoxicity. Int J Radiat Oncol Biol Phys 2002; 52: 599–605
- St Clair WH, Adams JA, Bues M, Fullerton BC, La Shell S, Kooy HM, et al. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys 2004; 58: 727–34
- Carlos, AP, Edward, HC. Principles and practice of radiation oncology. 4th ed.,. Philadelphia: Lippincott Williams & Wilkins, Corp.; 2004. p, 2224–9.
- Paulino AC. Hypothyroidism in children with medulloblastoma: a comparison of 3600 and 2340 cGy craniospinal radiotherapy. Int J Radiat Oncol Biol Phys 2002; 53: 543–7
- Jakacki RI, Feldman H, Jamison C, Boaz JC, Luerssen TG, Timmerman R. A pilot study of preirradiation chemotherapy and 1800 cGy craniospinal irradiation in young children with medulloblastoma. Int J Radiat Oncol Biol Phys 2004; 60: 531–6
- Glatstein E. Intensity-modulated radiation therapy: the inverse, the converse, and the perverse. Semin Radiat Oncol 2002; 12: 272–81
- Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys 2003; 56: 83–8
- Maor MH, Fields RS, Hogstrom KR, van Eys J. Improving the therapeutic ratio of craniospinal irradiation in medulloblastoma. Int J Radiat Oncol Biol Phys 1985; 11: 687–97
- Gaspar LE, Dawson DJ, Tilley-Gulliford SA, Banerjee P. Medulloblastoma: long-term follow-up of patients treated with electron irradiation of the spinal field. Radiology 1991; 180: 867–70
- Goldwein JW, Radcliffe J, Johnson J, Moshang T, Packer RJ, Sutton LN, et al. Updated results of a pilot study of low dose craniospinal irradiation plus chemotherapy for children under five with cerebellar primitive neuroectodermal tumours (medulloblastoma). Int J Radiat Oncol Biol Phys 1996; 34: 899–904
- Miralbell R, Lomax A, Russo M. Potential role of proton therapy in the treatment of pediatric medulloblastoma/primitive neuro-ectodermal tumours: spinal theca irradiation. Int J Radiat Oncol Biol Phys 1997; 38: 805–11
- Endicott TJ, Fisher BJ, Wong E, Paterson NA, Gaspar LE, Bauman M. Pulmonary sequelae after electron spinal irradiation. Radiother Oncol 2001; 60: 267–72
- Ma CM, Pawlicki T, Lee MC, et al. Energy- and intensity-modulated electron beams for radiotherapy. Phys Med Biol 2000; 45: 2293–311
- Mu X, Olofsson L, Karlsson M, Sjögren R, Zackrisson B. Can photon IMRT be improved by combination with mixed electron and photon techniques?. Acta Oncol 2004; 43: 727–35
- Olofsson L, Mu X, Nill S, Oelfke U, Zackrisson B, Karlsson M. Intensity modulated radiation therapy with electrons using algorithm based energy/range selection methods. Radiother Oncol 2004; 73: 223–31
- Karlsson M, Nyström H, Svensson H. Electron beam characteristics of the 50-MeV racetrack microtron. Med Phys 1992; 19: 307–15
- Karlsson MG, Karlsson M, Ma CM. Treatment head design for multileaf collimated high-energy electrons. Med Phys 1999; 26: 2161–7
- Suit H, Goldberg S, Niemierko A, Trofimov A, Adams J, Paganetti H, et al. Proton beams to replace photon beams in radical dose treatments. Acta Oncol 2003; 42: 800–8
- Miralbell R, Lomax A, Cella L, Schneider U. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumours. Int J Radiat Oncol Biol Phys 2002; 54: 824–9
- Carrie C, Hoffstetter S, Gomez F, Moncho V, Doz F, Alapetite C, et al. Impact of targeting deviations on outcome in medulloblastoma: study of the French Society of Pediatric Oncology (SFOP). Int J Radiat Oncol Biol Phys 1999; 45: 435–9
- Coles CE, Hoole AC, Harden SV, Burnet NG, Twyman N, Taylor RE, et al. Quantitative assessment of inter-clinician variability of target volume delineation for medulloblastoma: quality assurance for the SIOP PNET 4 trial protocol. Radiother Oncol 2003; 69: 189–94
- ICRU. International Commission on Radiation Units and Measurements (ICRU). Prescribing, recording, and reporting photon beam therapy. ICRU Report 50. 1993.
- Nill S, Bortfeld T, Oelfke U. Inverse planning of intensity modulated proton therapy. Z Med Phys 2004; 14: 35–40
- Källman P, Ågren A, Brahme A. Tumour and normal tissue responses to fractionated non-uniform dose delivery. Int J Radiat Biol 1992; 62: 249–62
- Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl 1985; 8: S13–9
- Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys 1989; 16: 1623–30
- Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys 1991; 21: 123–35
- Sanchez-Nieto B, Nahum AE. BIOPLAN: software for the biological evaluation of. Radiotherapy treatment plans. Med Dosim 2000; 25: 71–6
- Gagliardi, G. Modeling heart and lung complication data in radiation therapy of the breast. Ph D Thesis; Stockholm University: 1998. p. 63–71.
- Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 2002; 53: 810–21
- Gagliardi G, Lax I, Ottolenghi A, Rutqvist LE. Long-term cardiac mortality after radiotherapy of breast cancer–application of the relative seriality model. Br J Radiol 1996; 69: 839–46
- Mavroidis P, Laurell G, Kraepelien T, Fernberg JO, Lind BK, Brahme A. Determination and clinical verification of dose-response parameters for esophageal stricture from head and neck radiotherapy. Acta Oncol 2003; 42: 865–81
- Wheldon TE, Deehan C, Wheldon EG, Barrett A. The linear-quadratic transformation of dose-volume histograms in fractionated radiotherapy. Radiother Oncol 1998; 46: 285–95
- Steel, GG. Basic clinical radiobiology. 3rd ed. London: Arnold; 2002.
- ICRP (1990). Recommendations of the International Commission on Radiological Protection. ICRP. Publication 60. Ann ICRP 1990;21(1–3).
- ICRP (2003). Recommendations of the International Commission on Radiological Protection. ICRP. Publication 92. Ann ICRP 2003;33(4).
- Schneider U, Agosteo S, Pedroni E, Besserer J. Secondary neutron dose during proton therapy using spot scanning. Int J Radiat Oncol Biol Phys 2002; 53: 244–51
- Neglia JP, Friedman DL, Yasui Y, Mertens AC, Hammond S, Stovall M, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst 2001; 93: 618–29
- Garwicz S, Anderson H, Olsen JH, Dollner H, Hertz H, Jonmundsson G, et al. Second malignant neoplasms after cancer in childhood and adolescence: a population-based case-control study in the 5 Nordic countries. The Nordic Society for Pediatric Hematology and Oncology. The Association of the Nordic Cancer Registries. Int J Cancer 2000; 88: 672–8
- Eifel PJ, Donaldson SS, Thomas PR. Response of growing bone to irradiation: a proposed late effects scoring system. Int J Radiat Oncol Biol Phys 1995; 31: 1301–7
- Neuhauser EB, Wittenborg MH, Berman CZ, Cohen J. Irradiation effects of roentgen therapy on the growing spine. Radiology 1952; 59: 637–50
- Bloom HJ, Wallace EN, Henk JM. The treatment and prognosis of medulloblastoma in children. A study of 82 verified cases. Am J Roentgenol Radium Ther Nucl Med 1969; 105: 43–62
- Goldstein AM, Yuen J, Tucker MA. Second cancers after medulloblastoma: population-based results from the United States and Sweden. Cancer Causes Control 1997; 8: 865–71