Abstract
The interest in theoretical modelling of radiation response has grown steadily from a fast method to estimate the gain of new treatment strategies to an individualisation tool that may be used as part of the treatment planning algorithms. While the advantages of biological optimisation of plans are obvious, accurate theoretical models and realistic information about the micro-environmental conditions in tissues are needed.
This paper aimed to investigate the clinical implications of taking into consideration the details of the tumour microenvironmental conditions. The focus was on the availability of oxygen and other nutrients to tumour cells and the relationship between cellular energy reserves and DNA repair ability as this is thought to influence the response of the various hypoxic cells. The choice of the theoretical models for predicting the response (the linear quadratic model or the inducible repair model) was also addressed.
The modelling performed in this project has shown that the postulated radiobiological differences between acute and chronic hypoxia have some important clinical implications which may help to understand the mechanism behind the current success rates of radiotherapy. The results also suggested that it is important to distinguish between the two types of hypoxia in predictive assays and other treatment simulations.
Theoretical modelling of radiation treatment is now widely used both for evaluating old treatments and for estimating the potential gain of new strategies. Identifying biologically relevant input parameters has allowed the use of theoretical modelling to predict the response to fractionated treatments. This has then led to the inclusion of biological endpoints into treatment planning algorithms. For this purpose it is important to have an accurate theoretical model to describe the radiation response as well as realistic information about the micro-environmental conditions knowing that they greatly influence the radiation response.
Many years ago it has been recognised that the tumour microenvironment differs considerably from that of normal tissues Citation[1] and that it influences greatly the result of cancer therapy Citation[2]. It is thus known that a brief interruption of the oxygen supply to the cells determines an increased radioresistance. However, less known is the effect of nutrient deprivation which results in a depletion of the cellular energy reserves Citation[3–5]. The DNA repair process requires the activation of many enzymes Citation[6] and therefore cells having low energy reserves would be less able to induce the repair processes and consequently they are expected to be more sensitive than those with plenty of energy Citation[7], Citation[8]. This was observed experimentally in cells lacking both oxygen and nutrients and in cells oxygenated briefly after chronic nutrient starvation Citation[9–15]. The incapacity of the starved cells to induce the repair mechanisms has some very interesting consequences for the hypoxic cells that have been deprived of other nutrients, some of which have been described elsewhere Citation[16], Citation[17]. Thus, the brief interruption of the oxygen supply to the acutely hypoxic cells determines an increased radioresistance, while the prolonged lack of oxygen and other nutrients in starved chronically hypoxic cells results in a radiosensitisation. Although this has been known or postulated for some time, its implications have generally been discussed only on a qualitative level. This study aims to bridge the gap and investigate in a quantitative fashion the clinical implications of taking into consideration these differences in the radiobiological responses of acutely and chronically hypoxic cells in order to get an estimation of the magnitude of the difference that will appear if this aspect is neglected for predictive purposes.
Materials and methods
Tumour microenvironment was simulated using the method described in Daşu et al. Citation[18]. Thus, a Monte Carlo method was used to generate tissues with blood vessels placed according to ranges of experimentally measured distributions of intervascular distances. The oxygenation of the tissue was calculated numerically from the differential equation describing the oxygen transport, the result being an oxygenation map which reflects the limited diffusion of oxygen into the tissue and hence the diffusion limited or chronic hypoxia demonstrated experimentally by Thomlinson and Gray Citation[19] and later by Tannock Citation[20]. The temporal component of hypoxia, the perfusion limited or acute hypoxia, suggested by Brown Citation[21] to occur due to the transient collapsing of the tumour blood vessels as the result of increased interstitial pressure or temporary vessel occlusion due to “rigidised” blood cells in the acid environment was simulated according to the method described in Daşu et al. Citation[18] by the random closure of some blood vessels in the simulated tissue. Several patterns of acute hypoxia were modelled for a treatment simulation as the time-scale for this type of hypoxia ranges from a few minutes to hours and therefore it is not likely to have the same oxygenation pattern through consequent radiation fractions. It was thus possible to simulate a realistic temporal variation of tumour oxygenation which may be encountered in clinical practice. Furthermore, being able to simulate both types of hypoxia which overlap in a real tumour has the advantage that it is possible to know at any moment the probable contribution of each hypoxia aspect to the oxygenation status of every cell in the tissue. This method was used to simulate the oxygenation of several tissues with various mean intervascular distances ranging from 60 µm to 120 µm and thus covering a whole range of tumours with various vascular densities. Low pO2 values were assumed for the boundary conditions at the tumour blood vessels as these originate on the venous side of the vasculature Citation[1].
The cellular survival was calculated theoretically using the models described below and taking into account the relationship between radiosensitivity and oxygenation on one hand and energy reserves on the other hand. As the same blood vessel network is used to transport both oxygen and other nutrients, it was assumed that diffusion-limited hypoxic cells are also nutrient deprived and hence energy deprived in contrast to the perfusion limited hypoxic cells that have quite high energy reserves. This assumption was made bearing in mind that the timescale for the depletion of the energy reserves is about one to two hours Citation[4], Citation[7], i.e., longer than the intrinsic timescale of a few minutes which characterises acute hypoxia and hence that it is extremely unlikely that acutely hypoxic cells would experience a drop in cellular energy which might impede their repair mechanisms. The equation proposed by Alper and Howard-Flanders Citation[22] was used to describe the variation in radiosensitivity with oxygen tension.
The response of the whole tumour tissue was described in terms of the tumour control probability (TCP) by assuming a Poisson function (Equation 1):1 where N is the number of clonogenic cells in the tumour and SFtot is the total cellular survival at the end of a fractionated treatment schedule. For calculations we have assumed that there are about 107–108 viable cells per gram of tumour, which corresponds to a reasonably low cell packing factor and clonogenic density. According to this assumption, a fairly small tumour of about 2.5–3 cm in diameter would contain 109 cells.
Mathematical models to describe tissue response to radiation
Among the models that are being considered for the biological optimisation of treatment planning, the linear quadratic (LQ) model Citation[23–27] would appear to be the first choice, as it has been used successfully for years for iso-effect calculations of fractionated treatments with a rather large range of fractional doses. According to the LQ model, the dependence between cell survival and radiation dose is given as:2 where D is the radiation dose and α and β are cell-specific parameters.
However, a series of experiments initiated in the mid-1980s Citation[28], Citation[29] have shown that the LQ model fails to predict the cell response to very small doses of radiation (below 0.5–1 Gy). This hypersensitivity to low doses has been shown to exist in vivo both in normal tissues Citation[28–31] and in tumours Citation[32], Citation[33] as well as in a wide range of cell lines studied in vitro Citation[34–42]. Experiments are still performed in order to establish the mechanism of the hypersensitivity at low doses, but it seems that it has a molecular origin, being an adaptive response to radiation damage, similar to other stress responses Citation[43–45]. It thus appears that unirradiated cells are in a radiosensitive ground state and the recognition of radiation-inflicted damage to the DNA by a checkpoint in the G2 phase of the cell cycle induces effective repair mechanisms to deal with the damage Citation[45]. The fact that the full induction the repair mechanisms seems to appear for radiation doses in the clinical range might have important implications for the clinical applications involving low levels of irradiation for the normal tissues such as intensity modulated radiation therapy.
Consequently, several alternative models Citation[29], Citation[36], Citation[46] have been proposed to replace the LQ model. All these models assume different mechanisms for the transition from the sensitive to the resistant state of the cell. Thus, the model proposed by Joiner and Johns Citation[29] is an adaptation of the LQ model which assumes a gradual transition that is consistent with the repair rate being related to the level of damage: the more damage is inflicted, the more efficient the induction of the repair mechanisms. By contrast, the model proposed by Wouters and Skarsgard Citation[36] assumes that the cells need a certain threshold level of damage to activate the repair mechanisms, while the activation has an “on-off” characteristic, activation meaning fully resistant response and inactivation fully sensitive response. Finally, Lind et al. Citation[46] used an approximation of a Poisson model to account for the accumulation of damage into the cells and the activation of the cellular repair.
Among these three models, the Joiner and Johns model Citation[29] is the most convenient to use, as it is closely related to the LQ model and can therefore use the large database of LQ parameters which have been derived along the many years of use of the latter model. It also has to be mentioned that the Joiner and Johns model Citation[29], which we will refer to as “the linear quadratic model with inducible repair” (LQ/IR) or simply as “the inducible repair model”, is virtually identical to the linear quadratic model above 1 Gy and up to several tens of Gy (the dose range where the LQ model has been used with reasonable accuracy) for oxic cells.
According to the Joiner and Johns model Citation[29] the hyperradiosensitivity at low doses is described mathematically by an exponential variation of the shoulder slope with dose. By this variation, one can assume that the competition between the repair and fixation of lesions is taken into account. The equation that gives the surviving fraction after dose D according to the LQ/IR model is:3 where IRR is the inducible repair ratio, a parameter describing the total inducibility of the cellular repair mechanisms, and DC is the dose at which 1 − 1/e (i.e. 63%) of the transition to the maximum repair capacity has occurred. The αS parameter is the initial slope of the dose survival curve at very low doses and gives the maximum sensitivity of the cells. The shoulder slope denoted by α in the classical LQ model is given by the ratio αS/IRR. It has to be mentioned that the IRR parameter is very important for the shape of the cell survival curve at low doses. It has been shown to vary considerably between 1 and 20 or even more Citation[42] for most of the cell lines investigated, even though a fraction of them (about one fifth of them) do not seem to exhibit inducible repair.
Energy starvation and repair impairment
We have previously studied the influence of energy starvation on radiation response by assuming that the full sensitisation has a magnitude given by the IRR parameter of the LQ/IR model Citation[16], Citation[17], Citation[47]. In order to account for the gradient of sensitivities resulting from the different degrees of starvation caused by the nutrient diffusion from the blood vessels, we have assumed a sigmoid function similar to that describing the oxygen effect with the maximum value equal to IRR for cells situated close to the blood vessels which have abundant supply of nutrient compared to those further away. This assumption was made taking into consideration that both oxygen and other nutrients such as glucose diffuse from the blood vessels and are gradually consumed in the tumour cells (preferentially through a glycolytic metabolism).
Thus, the general equation used to describe the cell survival in a point in tissue is:4 where DMFα, DMFD, DMFβ are dose modifying factors given by the Alper and Howard-Flander's Citation[22] equation according to the local oxygen tension or pO2 caused by the superposition of perfusion limited and diffusion limited hypoxia and IRRdl is the inducible repair ratio given by the local diffusion limited oxygenation.
Through Equation 4 it is therefore possible to account both for the radioresistance conferred by the oxygen depletion through the DMFα, DMFD and DMFβ factors and for the biochemical radiosensitisation linked to long term nutrient and oxygen starvation in the diffusion limited hypoxic cells. Thus chronically hypoxic cells are more sensitive than the acutely hypoxic cells due to reduced inducibility of repair. It may even happen that chronically hypoxic cells could be more sensitive than the glucose-fed oxic cells if IRR is greater than the radiochemical hypoxic protection (DMF of 2 to 3). By contrast, acutely hypoxic cells, for which the nutrient deprivation is relatively short, have high energy reserves and are thus capable of inducing the DNA repair mechanisms and are hence radioresistant. On the other hand, if oxygen supply is restored to the starved hypoxic cells, they lose the chemical radioresistance conferred by the absence of oxygen and provided that the oxygenation is very brief they cannot also gain the DNA repair capacity. Using the assumptions above, the response of tissues with various degrees of oxygenation was investigated for various fractionated radiation treatments. The results of the modelling were then compared to clinical situations.
Results
shows cell survival curves for the various assumptions regarding the chemical and biochemical modification of radiosensitivity for various extents of inducibility of the repair mechanisms. The curves were calculated with generic parameters which give a surviving fraction at 2 Gy (SF2) of 0.5 for the fully oxic cells, under the assumption that α/β = 10 Gy Citation[48]. These assumptions are universal enough to illustrate the concepts illustrated in this paper and have often been used for modelling the tumour response.
Figure 1. Cell survival curves for the various assumptions regarding the chemical and biochemical modification of radiosensitivity for various extents of inducibility of the repair mechanisms. Dashed lines – oxic cells; dotted lines – chronic (starved) hypoxic cells; solid lines – acutely (fed) hypoxic cells.
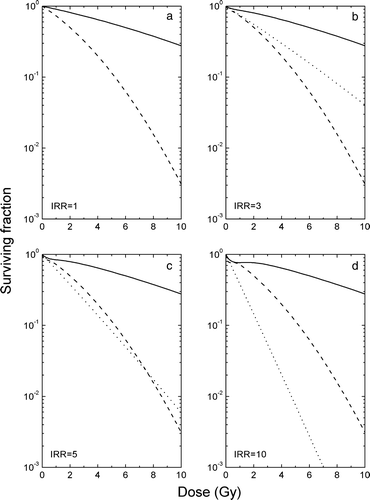
In panel 1a are presented survival curves for oxic and hypoxic cells having an IRR of 1, corresponding to either no or full induction of repair. The response of these cells is described by the classical LQ model. According to the predictions of this model, there is no difference between the responses of acutely and chronically hypoxic cells. By contrast, if the inducible repair ratio is larger than one, i.e. the response of the cells is described by the LQ/IR model (as in panels 1b–d), there is a clear difference between the predicted response of acutely and chronically hypoxic cells. The latter (starved cells) having low energy reserves, are unable to induce the repair mechanisms and therefore have an extremely sensitive response, while the former (fed cells) induce the repair mechanisms determining the appearance of a pronounced shoulder in the survival curve. As the value of the IRR increases progressively in panels b–d, the difference between the predicted responses of the two types of hypoxic subpopulations increases. Thus, the difference between the curve for acutely hypoxic cells and the curve for chronically hypoxic cells gradually increases. For the most extreme case presented in panel 1d, it may even happen that the fully chronically hypoxic cells are more sensitive than the oxic cells. It has to be mentioned that all the curves in represent the extreme radiosensitivities that may be encountered, as the radiosentivity of the cells varies from that described by the oxic curve for the well oxygenated and fed cells to that given by the acutely hypoxic curve for the poorly oxygenated cells but with strong energy reserves and eventually to that described by the chronically hypoxic curve for the poorly oxygenated and simultaneously starved cells.
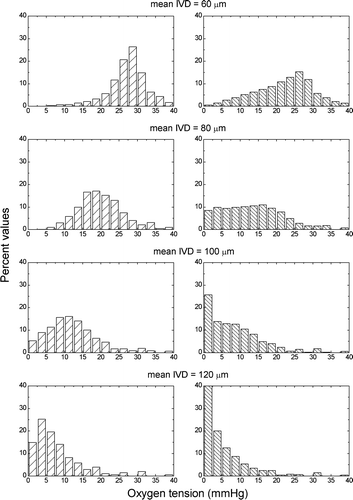
shows typical oxygenations in the modelled tissues caused by the diffusion limitations (left panels) or by the combined effects of diffusion limitations and perfusion limitations (right panels). As expected, a decrease in the vascular density (an increase in mean intervascular distance) leads to a deterioration of the tissue oxygenation. Furthermore, the temporary closure of some of the blood vessels in the tumour worsens the oxygenation of the tumours. However, it is not easy to predict the overall response of the tissues illustrated in as the two processes that lead to the appearance of tumour hypoxic influence differently the radiosensitivity of the cells.
Figure 2. Typical oxygenations caused by the diffusion limitations (left panels) or by the combined effects of diffusion limitations and perfusion limitations (right panels) in tumours with the specified mean intervascular distances (IVD).
shows the estimated tumour control probabilities for a relatively small tumour of 109 cells treated with a fractionated treatment of 33 fractions of 2 Gy each under the assumption that the tumour contains only diffusion limited hypoxia. The results show that for no or small inducible repair (IRR = 1–3) the decrease in vascular density correlates with a decrease in probability to control the tumour. Furthermore, the presence of the relatively radioresistant chronic hypoxic cells decrease the overall probability of control which is about 89% for a population of fully oxic cells. In contrast, in the more extreme case when the inducibility of repair is moderate to large (IRR = 5–10), the diffusion limited hypoxic cells are quite radiosensitive and their presence in the tumour might increase the probability of control even beyond the predictions for a purely oxic population. This may appear at first quite improbable, but one has to bear in mind that there are quite a number of tumours characterised by much larger intervascular distances Citation[1], Citation[49] which are successfully irradiated in spite of the theoretical predictions of zero probability of control (see for example the third and fourth row in ). These results show quite clearly the relative effect of a subpopulation of chronically hypoxic cells. Even though the absolute levels of local control will change for differently-sized tumours (e.g., larger tumours would have lower TCP values for the same dose level or would require higher doses to achieve the same TCP level), the presence of a radiosensitive sub-population of chronically hypoxic cells would improve the probability of local control.
Table I. Tumour control probabilities for tissues with various degrees of diffusion limited hypoxia. The calculations were performed for various assumptions regarding the inducibility of the repair of the cells.
The effect of perfusion limited hypoxia is presented in which gives the corresponding TCP values if acute hypoxia appears in the considered tumours due to the temporary collapse of some vessels. Thus, the presence of perfusion limited hypoxia worsens the response as it is associated with an increase in cellular radioresistance. This is seen by a decrease in the predicted TCP when compared to the corresponding values in , as well as a progressive decrease of the TCP for increasing intervascular distances. It has to be mentioned however that if one takes inducible repair into consideration, for the case of the more repair competent cells the presence of the radiosensitive chronically hypoxic cells actually increases the overall probability of control, and as such the existence of the repair incompetent subpopulation of chronically hypoxic cells in tumours might provide one of the explanations for the clinical success of radiotherapy, as illustrated in an earlier study Citation[16] which made use of a simple model with a three-compartment population.
Table II. Tumour control probabilities for tissues with various perfusion limited hypoxia superimposed onto the diffusion limited hypoxia. The calculations were performed for various assumptions regarding the inducibility of the repair of the cells.
Discussion
Taking inducible repair into consideration for modelling purposes has many clinical implications in spite of it not being present directly in the clinical dose range. Based on the experimentally demonstrated difference in the radiobiological response of the two types of hypoxia, we have analysed its implications for clinical applications in an attempt to obtain a better understanding of tumour response to radiation. In this respect we have tried to integrate the cellular effects of oxygen deprivation with those resulting from a shortage of nutrients as it occurs in the tumours. In particular, the mechanism of induction of repair that is behind the LQ/IR model was assumed to quantify the extent of radiosensitisation for the repair incompetent, starved hypoxic cells in comparison to the well-fed hypoxic cells or even the oxic cells.
It is well recognised that tumour vasculature is rather poor compared to that of normal tissues, the former having an almost chaotic structure characterised by low vascular density, poor blood flow and low nutrient content Citation[1], Citation[49], Citation[50]. Low vascular density leads to a chronic insufficiency of oxygen and nutrients for some cells, named either chronically hypoxic or diffusion limited hypoxic. While there are some molecular mechanisms which trigger the formation of new blood vessels into the starved regions, the intense proliferation of tumour cells outgrows the angiogenesis process and hence perpetuates the existence of starved cells in the tumours. Superimposed onto the regions with chronically low supplies of oxygen and nutrients, the transient collapsing of the tumour blood vessels due to increased interstitial pressure or temporary vessel occlusion due to “rigidised” blood cells in the acid environment determines variations in the blood flow that translate into further poor supply of oxygen and other nutrients. It has to be mentioned however that these latter effects have a much shorter life-time than the former ones, an aspect usually ignored when considering their influence on the radiosensitivity of the cells. Thus, it has usually been assumed that the response of the cells is given by the oxygen starvation and therefore that starved diffusion-limited hypoxic cells are equally radiosensitive with the fed perfusion-limited hypoxic cells. Many experimental studies have however shown that starved cells have low energy reserves Citation[3–5] which result in poor repair of DNA damage Citation[7], Citation[8] and hence in an overall radiosensitisation Citation[9–15] compared to the well fed cells. The contribution of the nutrient starvation is essential for the sensitisation process as it has been shown that oxygen removal alone for a long period of time does not lead to radiosensitisation Citation[51]. These results are usually overlooked when modelling or analysing the clinical implications of tumour hypoxia.
In practice, many human tumours are eradicated within a therapeutic window of 60–70 Gy with the preservation of the function of the adjacent tissue. Due to the largely non-organised structure of the tumour tissue and considering that in tumours the tissue function is growth and/or regrowth, all the clonogenic tumour cells are potential rescuers. This means that all the clonogenic cells in a tumour should be destroyed in order to eradicate it. It is likely that clinical tumours have rather large number of cells of the order of 108–109 or maybe even more. Not only the number of clonogenic cells represents a serious obstacle for the success of radiotherapy, but the increased proliferation in tumours and the radioresistance conferred by hypoxia to the nutrient fed cells are further motives for tumours to escape eradication with radiation doses that allow the preservation of the normal tissue function. Indeed it has previously been shown that assuming full radioresitance for the tumour hypoxic cells could increase the dose needed to eradicate the tumours up to 200 Gy or more in the intrinsically radioresistant cells Citation[16]. However, in spite of the general correlation between intrinsic radiosensitivity and success of radiotherapy described by Fertil and Malaise Citation[52], the dose levels implied in practice are nowhere near the values derived from simulations. Thus, it seems that the difference in radiobiological response of the various hypoxic cells in tumours may provide one of the explanations of the clinical success of radiotherapy.
In this respect we have shown that integrating the different radiobiological response of starved and fed cells into the modelling of tumour response could bring the predictions of the probability to control the tumour closer to the clinical observations. Thus, the presence of tumour hypoxia (especially the well fed repair competent type) worsens the overall response to radiation, but the radiosensitisation of the starved hypoxic cells narrows the dose range needed for a favourable response to radiation treatment. Furthermore, for the cells with high capacity of inducing the repair mechanisms (high IRR), it may happen that the presence of radiosensitive cells might be the key to successful understanding of the differential between tumour and normal tissues Citation[16]. The relatively fast disappearance from the tumours of the chronically hypoxic cells, although related to the nutrient starvation, has to be treated separately, as it is part of the huge cell loss that characterises the tumours. In the steady state of an unirradiated tumour, the death of the chronically hypoxic cells counteracts only part of the increase in cell number through proliferation. On the other hand, it has been suggested that cellular loss in irradiated tumours might improve the nutrient supply and thus rescue the otherwise doomed hypoxic cells Citation[53]. Thus, cell death due to starvation alone does not seem to account for the narrowing of the therapeutic dose range.
The assumption that tumours contain repair deficient hypoxic cells does not contradict other experimental results regarding tumour oxygenation. The postulated radiosensitisation of starved hypoxic cells is superimposed over the chemical radioresistance conferred by the absence of oxygen. Thus, an improvement in the oxygenation of the repair deficient hypoxic cells would result in increased radiosensitivity due to the oxygen effect. However, the timescale of the oxygenation is very important as illustrated by Foster et al. Citation[54]. If the oxygen was available to the starved hypoxic cells only for a brief period before irradiation, the energy charge of the cell is unlikely to have been modified and consequently the repair deficiency is retained leading to a sensitisation as observed in the experimental studies of Pettersen and Wang Citation[14] or Zölzer and Streffer Citation[15]. On the other hand, if the oxygen was available for the cells for a time long enough so that the energy reserves of the cell are increased through the metabolisation of other nutrients, the radiosensitisation due to impaired repair mechanisms might decrease and it will compete with the chemical radiosensitisation process due to the presence of oxygen. In this case, the response of the tumour on the whole might not modify much as has indeed been seen in clinical practice with hyperbaric oxygen or normobaric carbogen when prolonged pre-irradiation breathing times showed relatively little benefit in comparison to short ones Citation[55], Citation[56]. On the other hand this might also explain the clinical efficiency of oxygen mimetic sensitizers which are not used in cellular metabolism Citation[57].
The different radiobiological response of starved and fed hypoxic cells could also explain why there have been found only few correlations between energy charge parameters and tumour oxygenation or radiobiological hypoxic fraction Citation[58]. Low energy charge parameters are correlated only with chronic hypoxia, while tumour oxygenation refers to the oxygen availability, not taking into consideration other nutrients. On the other hand radiobiological hypoxia is a complex parameter that is given by the combined response to radiation of all the subpopulations in the tumour (oxic, fed hypoxic and starved hypoxic). It is therefore unlikely that an a priori correlation will be found between these parameters.
Other clinical implications of the process of induction of repair relate to the reduced effective hypoxic protection (OER') of the acutely hypoxic cells that has been observed in hyperfractionated experiments. Early experiments performed by Littbrand and his co-workers Citation[59], Citation[60]--who compared the survival at the same dose (per fraction) for oxic and hypoxic cells over the dose range 0.5–1.5 Gy--showed a reduced hypoxic protection at these low doses and sometimes irradiations in extreme hypoxia produced the same cell kill as in oxic conditions. Several years later, Taylor and Brown Citation[61] have seen that fractionated experiments performed with 1.7 Gy per fraction in oxic and hypoxic conditions yielded an OER' of only 1.34, much lower than the commonly reported values of 1.8–2.5 that were obtained in single dose experiments Citation[62–65]. Taylor and Brown Citation[61] attributed the reduced OER' to a decreased repair capacity of the hypoxic cells, but they failed to identify the reasons for this impaired repair. These observations have been explained elsewhere Citation[66], Citation[67] by using the LQ/IR model through a different activation of the repair mechanisms in oxic and hypoxic cells. Thus, in the clinical dose range, the repair mechanisms are more or less fully activated in the oxic cells, while for the hypoxic cells they are closer to the ground state and thus the cells are far from their full radioresistance. This means that for the hypoxic cells, radiation in this small-dose range is more damaging per unit dose than for the oxic cells. In terms of survival curves this means that hypoxic cells would have a less shouldered curve that could be interpreted as a decreased ability to repair potential and/or sublethal damage as was proposed by Taylor and Brown Citation[61] using a target theory model. This means that hyperfractionating a treatment would result in less hypoxic protection even in the acutely hypoxic cells and therefore in improved tumour control. Conversely, increasing the dose per fraction would lead to an increase of the hypoxic protection as observed experimentally by Yaromina and co-workers Citation[68].
It thus appears that the dependence of the DNA repair rates on the cellular energy charge and oxygenation plays an important role in determining the radiation response and thus might have potentially serious clinical implications.
Conclusions
The use of the inducible repair for simulations of the tumour response to radiation has many clinical implications. Thus, it could explain some unusual reports in the literature with respect to hypoxic protection and it could also predict more accurately the tissue response in the low dose region.
More important, the distinction between the different types of tumour hypoxia based on their physiological and radiobiological characteristics could provide a better understanding of the mechanism behind clinical radiation therapy and a re-evaluation of some conflicting results or puzzling anomalies. In particular, the use of the LQ/IR model allowed an easy quantification of the radiobiological differences between acutely and chronically hypoxic cells. The clinical implications of the postulated physiological and radiobiological differences between acute and chronic hypoxia also suggested that it is important to distinguish between the two types of hypoxia in predictive assays and other treatment simulations.
Acknowledgements
We would want to thank to Prof. Jack F. Fowler, to Prof. Michael Joiner and to Prof. Bo Littbrand for discussions around this paper.
References
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res 1989; 49: 6449–65
- Denekamp J, Daşu A, Waites A. Vasculature and microenvironmental gradients: The missing links in novel approaches to cancer therapy?. Adv Enzyme Regul 1998; 38: 281–99
- Gerweck LE, Dahlberg WK, Epstein LF, Shimm DS. Influence of nutrient and energy deprivation on cellular response to single and fractionated heat treatments. Radiat Res 1984; 99: 573–81
- Gerweck LE, Seneviratne T, Gerweck KK. Energy status and radiobiological hypoxia at specified oxygen concentrations. Radiat Res 1993; 135: 69–74
- Skøyum R, Eide K, Berg K, Rofstad EK. Energy metabolism in human melanoma cells under hypoxic and acidic conditions in vitro. Br J Cancer 1997; 76: 421–8
- Ward JF. Mechanisms of DNA repair and their potential modification for radiotherapy. Int J Radiat Oncol Biol Phys 1986; 12: 1027–32
- Nagle WA, Moss AJ, Jr, Roberts HG, Jr, Baker ML. Effects of 5-thio-D-glucose on cellular adenosine triphosphate levels and deoxyribonucleic acid rejoining in hypoxic and aerobic Chinese hamster cells. Radiology 1980; 137: 203–11
- Spiro IJ, Kennedy KA, Stickler R, Ling CC. Cellular and molecular repair of X-ray-induced damage: Dependence on oxygen tension and nutritional status. Radiat Res 1985; 101: 144–55
- Hall EJ, Bedford JS, Oliver R. Extreme hypoxia; its effect on the survival of mammalian cells irradiated at high and low dose-rates. Br J Radiol 1966; 39: 302–7
- Berry RJ, Hall EJ, Cavanagh J. Radiosensitivity and the oxygen effect for mammalian cells cultured in vitro in stationary phase. Br J Radiol 1970; 43: 81–90
- Franko AJ, Sutherland RM. Radiation survival of cells from spheroids grown in different oxygen concentrations. Radiat Res 1979; 79: 454–67
- Shrieve DC, Harris JW. The in vitro sensitivity of chronically hypoxic EMT6/SF cells to X-radiation and hypoxic cell radiosensitizers. Int J Radiat Biol 1985; 48: 127–38
- Ling CC, Robinson E, Shrieve DC. Repair of radiation induced damage–dependence on oxygen and energy status. Int J Radiat Oncol Biol Phys 1988; 15: 1179–86
- Pettersen EO, Wang H. Radiation-modifying effect of oxygen in synchronized cells pre-treated with acute or prolonged hypoxia. Int J Radiat Biol 1996; 70: 319–26
- Zölzer F, Streffer C. Increased radiosensitivity with chronic hypoxia in four human tumor cell lines. Int J Radiat Oncol Biol Phys 2002; 54: 910–20
- Denekamp J, Daşu A. Inducible repair and the two forms of tumour hypoxia--time for a paradigm shift. Acta Oncol 1999; 38: 903–18
- Daşu A, Denekamp J. The impact of tissue microenvironment on treatment simulation. Adv Exp Med Biol 2003; 510: 63–7
- Daşu A, Toma-Daşu I, Karlsson M. Theoretical simulation of tumour oxygenation and results from acute and chronic hypoxia. Phys Med Biol 2003; 48: 2829–42
- Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer 1955; 9: 539–49
- Tannock IF. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer 1968; 22: 258–73
- Brown JM. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol 1979; 52: 650–6
- Alper T, Howard-Flanders P. Role of oxygen in modifying the radiosensitivity of E. Coli B. Nature 1956; 178: 978–9
- Kellerer AM, Rossi HH. The theory of dual radiation action. Curr Top Radiat Res Q 1972; 8: 85–158
- Chadwick KH, Leenhouts HP. A molecular theory of cell survival. Phys Med Biol 1973; 18: 78–87
- Douglas BG, Fowler JF. Fractionation schedules and a quadratic dose-effect relationship. Br J Radiol 1975; 48: 502–4
- Barendsen GW. Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys 1982; 8: 1981–97
- Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 1989; 62: 679–94
- Joiner MC, Denekamp J, Maughan RL. The use of ‘top-up’ experiments to investigate the effect of very small doses per fraction in mouse skin. Int J Radiat Biol 1986; 49: 565–80
- Joiner MC, Johns H. Renal damage in the mouse: The response to very small doses per fraction. Radiat Res 1988; 114: 385–98
- Hamilton CS, Denham JW, O'Brien M, Ostwald P, Kron T, Wright S, et al. Underprediction of human skin erythema at low doses per fraction by the linear quadratic model. Radiother Oncol 1996; 40: 23–30
- Turesson I, Johansson K-A, Nyman J, Flogegard M, Wahlgren T. A clinical study on the effect of low dose per fraction. Radiother Oncol 1998; 48(Suppl 1)S4
- Beck-Bornholdt H-P, Maurer T, Becker S, Omniczynski M, Vogler H, Würschmidt F. Radiotherapy of the rhabdomyosarcoma R1H of the rat: Hyperfractionation-126 fractions applied within 6 weeks. Int J Radiat Oncol Biol Phys 1989; 16: 701–5
- Harney J, Short SC, Shah N, Joiner M, Saunders MI. Low dose hyper-radiosensitivity in metastatic tumors. Int J Radiat Oncol Biol Phys 2004; 59: 1190–5
- Marples B, Joiner MC. The response of Chinese hamster V79 cells to low radiation doses: Evidence of enhanced sensitivity of the whole cell population. Radiat Res 1993; 133: 41–51
- Lambin P, Marples B, Fertil B, Malaise EP, Joiner MC. Hypersensitivity of a human tumour cell line to very low radiation doses. Int J Radiat Biol 1993; 63: 639–50
- Wouters BG, Skarsgard LD. The response of a human tumor cell line to low radiation doses: Evidence of enhanced sensitivity. Radiat Res 1994; 138: S76–S80
- Singh B, Arrand JE, Joiner MC. Hypersensitive response of normal human lung epithelial cells at low radiation doses. Int J Radiat Biol 1994; 65: 457–64
- Wouters BG, Sy AM, Skarsgard LD. Low-dose hypersensitivity and increased radioresistance in a panel of human tumor cell lines with different radiosensitivity. Radiat Res 1996; 146: 399–413
- Marples B, Adomat H, Koch CJ, Skov KA. Response of V79 cells to low doses of X-rays and negative π-mesons: clonogenic survival and DNA strand breaks. Int J Radiat Biol 1996; 70: 429–36
- Wouters BG, Skarsgard LD. Low-dose radiation sensitivity and induced radioresistance to cell killing in HT-29 cells is distinct from the “adaptive response” and cannot be explained by a subpopulation of sensitive cells. Radiat Res 1997; 148: 435–42
- Short S, Mayes C, Woodcock M, Johns H, Joiner MC. Low dose hypersensitivity in the T98G human glioblastoma cell line. Int J Radiat Biol 1999; 75: 847–55
- Joiner MC, Marples B, Lambin P, Short SC, Turesson I. Low-dose hypersensitivity: Current status and possible mechanisms. Int J Radiat Oncol Biol Phys 2001; 49: 379–89
- Joiner MC, Lambin P, Malaise EP, Robson T, Arrand JE, Skov KA, et al. Hypersensitivity to very-low single radiation doses: Its relationship to the adaptive response and induced radioresistance. Mutat Res 1996; 358: 171–83
- Robson T, Joiner MC, Wilson GD, McCullough W, Price ME, Logan I, et al. A novel human stress response-related gene with a potential role in induced radioresistance. Radiat Res 1999; 152: 451–61
- Marples B, Wouters BG, Collis SJ, Chalmers AJ, Joiner MC. Low-dose hyper-radiosensitivity: A consequence of ineffective cell cycle arrest of radiation-damaged G2-phase cells. Radiat Res 2004; 161: 247–55
- Lind BK, Persson LM, Edgren MR, Hedlof I, Brahme A. Repairable-conditionally repairable damage model based on dual Poisson processes. Radiat Res 2003; 160: 366–75
- Daşu A. Modelling the impact of two forms of hypoxia on novel radiotherapy approaches. 2000. PhD thesis, Umeå University.
- Thames HD, Bentzen SM, Turesson I, Overgaard M, Van den Bogaert W. Time-dose factors in radiotherapy: A review of the human data. Radiother Oncol 1990; 19: 219–35
- Konerding MA, Malkusch W, Klapthor B, van Ackern C, Fait E, Hill SA, et al. Evidence for characteristic vascular patterns in solid tumours: Quantitative studies using corrosion casts. Br J Cancer 1999; 80: 724–32
- Jain RK. Determinants of tumor blood flow: A review. Cancer Res 1988; 48: 2641–58
- Vordermark D, Menke DR, Brown JM. Similar radiation sensitivities of acutely and chronically hypoxic cells in HT 1080 fibrosarcoma xenografts. Radiat Res 2003; 159: 94–101
- Fertil B, Malaise EP. Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: Analysis of 101 published survival curves. Int J Radiat Oncol Biol Phys 1985; 11: 1699–707
- Ljungkvist AS, Bussink J, Kaanders JH, Wiedenmann NE, Vlasman R, van der Kogel AJ. Dynamics of hypoxia, proliferation and apoptosis after irradiation in a murine tumor model. Radiat Res 2006; 165: 326–36
- Foster CJ, Malone J, Orr JS, Macfarlane DE. The recovery of the survival curve shoulder after protracted hypoxia. Br J Radiol 1971; 44: 540–5
- Siemann DW, Hill RP, Bush RS. The importance of the pre-irradiation breathing times of oxygen and carbogen (5% CO2: 95% O2) on the in vivo radiation response of a murine sarcoma. Int J Radiat Oncol Biol Phys 1977; 2: 903–11
- Kaanders JH, Pop LA, Marres HA, Liefers J, van den Hoogen FJ, van Daal WA, et al. Accelerated radiotherapy with carbogen and nicotinamide (ARCON) for laryngeal cancer. Radiother Oncol 1998; 48: 115–22
- Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5–85. Radiother Oncol 1998; 46: 135–46
- Stubbs M. Application of magnetic resonance techniques for imaging tumour physiology. Acta Oncol 1999; 38: 845–53
- Littbrand B. Survival characteristics of mammalian cell lines after single or multiple exposures to roentgen radiation under oxic or anoxic conditions. Acta Radiol Ther Phys Biol 1970; 9: 257–81
- Littbrand B, Edsmyr F, Révész L. A low dose-fractionation scheme for the radiotherapy of carcinoma of the bladder. Experimental background and preliminary results. Bull Cancer 1975; 62: 241–8
- Taylor YC, Brown JM. Radiosensitization in multifraction schedules. I. Evidence for an extremely low oxygen enhancement ratio. Radiat Res 1987; 112: 124–33
- Palcic B, Brosing JW, Skarsgard LD. Survival measurements at low doses: Oxygen enhancement ratio. Br J Cancer 1982; 46: 980–4
- Watts ME, Hodgkiss RJ, Jones NR, Fowler JF. Radiosensitization of Chinese hamster cells by oxygen and misonidazole at low X-ray doses. Int J Radiat Biol 1986; 50: 1009–21
- Skarsgard LD, Harrison I. Dose dependence of the oxygen enhancement ratio (OER) in radiation inactivation of Chinese hamster V79–171 cells. Radiat Res 1991; 127: 243–7
- Freyer JP, Jarrett K, Carpenter S, Raju MR. Oxygen enhancement ratio as a function of dose and cell cycle phase for radiation-resistant and sensitive CHO cells. Radiat Res 1991; 127: 297–307
- Daşu A, Denekamp J. New insights into factors influencing the clinically relevant oxygen enhancement ratio. Radiother Oncol 1998; 46: 269–77
- Daşu A, Denekamp J. Superfractionation as a potential hypoxic cell radiosensitizer: Prediction of an optimum dose per fraction. Int J Radiat Oncol Biol Phys 1999; 43: 1083–94
- Yaromina A, Zips D, Thames HD, Eicheler W, Krause M, Rosner A, et al. Pimonidazole labelling and response to fractionated irradiation of five human squamous cell carcinoma (hSCC) lines in nude mice: The need for a multivariate approach in biomarker studies. Radiother Oncol 2006; 81: 122–9