Abstract
Background. The prognostic potential of HER2, TP53 mutations, PAI-1 protein levels, angiogenesis and proliferation were investigated in tumours from 408 patients with early breast cancer followed >10 years. One hundred and sixty seven patients (41%) died from breast cancer. Materials and methods. Tumour sections were stained for HER2, CD34, and MIB-1. HER2 scores were based on staining intensity, 3+ being considered HER2+. Angiogenesis was scored by the Chalkley method. MIB-1 was evaluated using systematic random sampling. PAI-1 was measured by ELISA. TP53 mutations were evaluated by DGGE analysis and DNA sequencing. Results. Ninety one patients (22%) were HER2 positive. TP53 was mutated in 101 cases (25%). Median PAI-1, Chalkley and MIB-1 was 0.72 ng/mg protein (range, 0–90 ng/mg protein), 5.00 (range, 2.67–12.00) and 15% (range, 1–83%). MIB-1 was correlated with HER2+, Chalkley counts, TP53 mutations (all p <0.0001), and PAI-1 (p =0.002). In univariate analyses with DSS as endpoint, HER2+ (p <0.0001), mutated TP53 (p <0.0001), high Chalkley (p =0.008), MIB-1 (p =0.002), tumour size (p =0.008), grade (p <0.0001), negative estrogen receptor (p =0.0001), and lymph node status (p <0.0001) were prognostic markers. Among node-negative patients, HER2+ (p =0.0002), mutated TP53 (p =0.001), high PAI-1 levels (p =0.02), and grade (p =0.03) indicated poor DSS. In node-positive patients, HER2+ (p =0.0002), mutated TP53 (p <0.0001), MIB-1 (p =0.01), Chalkley scores (p =0.007), negative estrogen receptor (p <0.0001) and grade (p =0.001) indicated poor prognosis. In multivariate analysis, metastatic nodes (1–3 positive: RR 1.56 95% CI 1.02–2.38; >3 positive: RR 3.70 95% CI 2.54–5.38), HER2+ (RR 1.91, 95% CI 1.35–2.70), mutated TP53 (RR 1.70, 95% CI 1.21–2.38), PAI-1 (RR 1.04, 95% CI 1.01–1.07) and grade 3 (RR 1.96, 95% CI 1.83–3.22) were independent markers of poor outcome. Conclusion. Compared to PAI-1 protein levels, Chalkley counts and MIB-1, HER2+ and mutations of TP53 were the strongest independent markers of poor prognosis irrespective of nodal status.
HER2, p53, Plasminogen Activator Inhibitor-1 (PAI-1), estimates of angiogenesis and proliferative activity (MIB-1) have all been identified as prognostic markers in early breast cancer Citation[1–3]. More than a decade ago a meeting took place in the tumour biology committee under the Danish Breast Cancer Cooperative Group, DBCG, and a study was designed to compare the prognostic influence of the above mentioned five variables in a well characterized group of patients diagnosed with early breast cancer. Here we present the final analysis of the study. Previously, we have published data regarding angiogenesis Citation[4], proliferative activity Citation[5], PAI-1 Citation[6], and in some of the patients also data on allelic loss of 16q23.2–24.2 Citation[7] and mutations of the gene TP53Citation[8], Citation[9]. Now we present additional data on HER2 evaluated by immunohistochemistry in 408 patients, and DNA sequencing for mutations of TP53 in 401 patients.
The main conclusions regarding estimates of angiogenesis in these tumours were that intense vascularisation was significantly associated with poor disease-specific survival (DSS) and overall survival (OS) in the whole cohort Citation[4]. In node-positive patients, high estimates of angiogenesis were also significantly associated with poor DSS and OS, however, this was not the case in tumours from node-negative patients. In multivariate analysis, high Chalkley scores were only independent markers of poor survival in node-positive patients, but not in the whole cohort nor in node-negative patients.
In our study of MIB-1 estimates in this patient group we found that increasing MIB-1 scores were significantly associated with poor DSS and OS in the whole cohort and poor DSS in node-positive patients but not in node-negative patients Citation[5]. MIB-1 was closely associated with malignancy grade and mitotic index; however, in multivariate analyses, both with and without malignancy grade and mitotic index included in the analyses, MIB-1 was not an independent marker of poor prognosis. Thus MIB-1 estimates did not contribute with additional prognostic information at diagnosis when evaluated together with classical prognostic markers in early breast cancer.
Recently, we have published data on PAI-1 measurements in this group of patients Citation[6]. The conclusions were that protein levels of PAI-1 were significantly associated with poor DSS in tumours from the whole cohort and in node-negative patients, but not correlated with prognosis in node-positive patients. Combining low/low versus high/high tertiles of PAI-1 and Chalkley scores showed actuarial 10-year survival rates of 82% versus 52%. Together with N-stage and malignancy grade, increasing PAI-1 was identified as an independent marker of poor prognosis in multivariate analysis using death from breast cancer as endpoint.
HER2 is a tyrosine kinase receptor in the epidermal growth factor family encoded by the HER2 gene on chromosome17q21. Activation of the receptor initializes a cascade reaction promoting cell growth. This is part of the uncontrolled expansion of cancers with pathological excess of HER2, based on either overexpression of the protein and/or amplification of the gene. In 20–30% of breast cancers HER2 is overexpressed, which is associated with an aggressive tumour behavior and poor prognosis Citation[10]. This constitutes the biological background for the targeted therapy directed against the receptor with the humanized antibody trastuzumab Citation[11].
TP53 is a tumour suppressor gene located on chromosome 17p. Mutation of the TP53 is the most common known genetic abnormality in malignant human tumours. Several studies have evaluated the prognostic and predictive value of p53, some using DNA sequencing for mutations of TP53, others using immunohistochemistry since the abnormal p53 protein produced by the mutant gene is more stable than the wild type. The prognostic role of p53 in early breast cancer has been studied several times earlier but the results based on immunohistochemistry are conflicting as reviewed by Petitjean et al. Citation[12]. By contrast, most studies using gene sequencing for TP53 mutations uniformly show poor outcome associated with different mutations, and these studies also indicate that some mutations are worse than others with regard to prognosis. The largest study included 1 794 patients with early breast cancer and identified TP53 mutation as an independent marker of poor outcome Citation[13].
A subgroup of the present patient cohort (n = 243) was included in a previous study which was analyzed for mutations in TP53Citation[8], Citation[9]. A significantly poorer prognosis was observed among patients whose tumours contained mutated TP53. Using death from breast cancer as endpoint, mutated TP53 was also an independent prognostic marker of poor prognosis in multivariate analysis Citation[8].
This study is a presentation of data on HER2 together with additional data on mutations of TP53, and in addition we show the final analysis of all five variables evaluated together. To our knowledge no studies have evaluated these five variables in the same cohort of patients diagnosed with early breast cancer.
Materials and methods
Patients and tumour samples
Tumour material was collected from 408 consecutive patients diagnosed with early breast cancer where adequate tumour material was available for analyses of the five variables, except for TP53 evaluation, where 401 tumours were available. Patients were included in the study from January 1990 to 1994 and fulfilled the following criteria: having primary unilateral breast carcinoma with no clinical evidence of metastasis; availability of complete clinical, histopathological and biological information; having no other malignancies; having received radical surgical therapy. Median age was 57 years (range, 29–89 years) and 167 patients (41%) died from breast cancer. The distribution of T1, T2 and T3 classification was 152 (37%), 223 (55%) and 33 cases (8%), respectively, and 276 patients (68%) were postmenopausal, Ductal carcinoma was found in 351 tumours (86%) with malignancy grades I, II and III seen in 76 (22%), 143 (41%), and 132 (38%) of these tumours, respectively. 290 patients (71%) had estrogen receptor (ER) positive tumours. Progesterone receptor analyses were not performed routinely. Forty seven percent of the patients were node-negative.
Treatment
Treatment of the patients has been described in detail previously Citation[4]. Locoregional treatment consisted of surgery, total mastectomy or lumpectomy combined with adjuvant radiotherapy (48 Gy/24 fx., 5 fx. weekly) to residual breast. The systemic adjuvant treatment was given according to the national Danish treatment policy described by The Danish Breast Cancer Cooperative Group (DBCG 89 protocols). In this way systemic therapy was offered to all women at increased risk of recurrence (node-positive, tumour size >5 cm, or ductal carcinoma grade II/III). Thus these women were allocated to one of three different protocols: DBCG 89b (premenopausal with ER+ tumour) was a randomization between CMF (inj. cyclophosphamide 600 mg/m2+methotrexate 40 mg/m2+5-fluorouracil 600 mg/m2) and castration; DBCG 89c (postmenopausal with ER+ tumour or unknown ER status) was a randomization among tamoxifen (30 mg daily) for 1 year, 2 years or 6 months followed by Megace for 6 months; DBCG 89d (administered to premenopausal ER- or unknown receptor status, or malignancy grade II/III, and to postmenopausal patients <70 years with ER- tumours) was a randomization among CMF, CEF (inj. cyclophosphamide 600 mg/m2+epirubicin 60 mg/m2+5-fluorouracil 600 mg/m2), CMF + pamidronate (tabl. 150 mg×2 daily for 4 years), and CEF + pamidronate administered 9 times at intervals of 3 weeks.
Follow-up
According to the DBCG recommendations all patients were followed by clinical examination every third month the first year, then twice yearly until the fifth year, then once yearly from the sixth to the tenth year. After this period, information about recurrence was achieved from the clinical records at the treating hospital. Some older patients were followed by their general practitioner and referred to hospital if recurrence was suspected. All patients were followed from the date of operation and at least for 10 years or until death. Furthermore, all records were revised and survival status assured by contact to the National Population Register, and in case of death we contacted the Danish Cancer Register and the National Causes of Death Register. In this way complete information was available regarding disease-specific survival (DSS). Information regarding first relapse was also available, however, with shorter follow-up, and thus is not presented in this paper.
HER2 evaluation
The paraffin-embedded tumours were cut in 4 µm sections for immunohistchemical staining. Antigen retrieval was performed by microwave heating in 10 mM citrate buffer at pH 6.0 for 11 min at 900 W followed by 15 min at 400 W. The slides were incubated with HER2 antibody 1:4000 (DAKO, Glostrup, Denmark). The immunostaining was automated using the ChemMate HRP/DAB detection kit K5001 on the TechMate 500 (DAKO, Glostrup, Denmark). Counter staining was done with Mayers hematoxylin.
Scoring in four groups was performed regarding the intensity and membrane completeness of the staining reaction as follows: score 0 – no staining or staining in less than 10% of the cells; for staining of more than 10% of the cells score 1+ was given for weak and incomplete membrane reaction; score 2+ for complete membrane staining of weak to moderate intensity; and score 3+ for strong and complete membrane reaction in more than 10% of the tumour cells. Tumours with score 3+ were considered HER2 positive in the final analysis, whereas tumours with score 2+ were analysed further by fluorescence in situ hybridization (FISH) Citation[14] or by quantitative PCR Citation[15]. These tumors were considered HER2 positive in case the HER2 gene was amplified ≥2.
TP53 evaluation for gene mutations by DGGE analysis and DNA sequencing
DNA was extracted from the pellet left over from estrogen receptor analysis Citation[16]. The entire coding region and all exon/intron boundaries of TP53 were analyzed by DGGE (denaturing gradient gel electrophoresis). The method has been described in detail previously Citation[9]. Amplification reactions were carried out by 38 rounds of thermal cycling (94°C for 20 s, 62°C for 20 s, 72°C for 20 s) in final volumes of 15 µl containing 10 mM Tris-HCL (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.02% gelatine, 0.2 mM cresol red, 12% sucrose, 5% DMSO, 100 µM of each deoxynucleotide triphosphate, 0.6 µM of each primer, 100 ng of DNA, and 0.5 unit of AmpliTaq DNA polymerase (Perkin-Elmer Cetus, Emeryville, CA). For DGGE analysis, PCR products were run on 6% polyacrylamide gels containing various gradients of urea and formamide (100% denaturant consisted of 7 M urea and 40% formamide). As a modification to a previous approach, the gradient ranges were narrowed and adapted to each exon to increase the separation between mutant and wild-type alleles. The gradient ranges were 35–75% for exons 2, 3, 4, 7, 8, and 10; 20–60% for exon 9; 25–65% for exon 6; and 40–80% for exon 5, respectively. The gels were run at 160 V for 5 h in 1×TAE buffer at a constant temperature of 58°C. After electrophoresis, gels were stained in 1×TAE buffer containing 2 µg/ml ethidium bromide and analyzed under UV transillumination. Each sample was analyzed once by DGGE.
Mutant heteroduplex and homoduplex bands were excised and reamplified. Sequencing of PCR products was performed either with 33P-end-labeled primers using ThermoPrime Cycle Sequencing Kit (Amersham, Cleveland, OH) or with the BigDye DyeTerminator Cycle Sequencing Kit and analyzed on an ABI 310 (Perkin-Elmer Cetus). Only excised bands were sequenced.
PAI-1 ELISA
The method used has been described in detail previously Citation[17]. In short, cytosol extracts originally prepared for oestrogen receptor analyses using a standard procedure, including precooling in liquid nitrogen, pulverization with a Micro-Dismembrator, and extraction at 4°C with a buffer consisting of 10 mM K2HPO4/KH2PO4, 1.5 mM K2EDTA, 10mM monothioglycerol, 10% glycerol (v/v), and 10mM sodium molybdate (pH 7.5), followed by centrifugation at 105 000×g for 1 h at 4°C. The supernatants were stored at −70°C. PAI-1 was determined using a sandwich ELISA kit (Monozyme, Horsholm, Denmark) with monoclonal catching and detecting antibodies. This assay recognizes active PAI-1, latent PAI-1, and PAI-1 complexed with urokinase Plasminogen Activator (uPA).
Immunohistochemical staining and microvessel quantification
This method has been presented in detail previously Citation[4]. Briefly, 4 µm sections from formalin-fixed, paraffin-embedded tumour blocks were microwaved in 10 mM citrate buffer and immunostained with anti-CD34 monoclonal antibody (clone QB-END 10, Immunotech, France) diluted 1:50. Primary antibody was detected with LSAB (K681, Dako, Denmark) and visualized with DAB.
At×40–100 magnification the area of the tumour with highest microvascular density was found, and at×200 magnification estimates of angiogenesis were counted using a Chalkley eyepiece graticule placed in the ocular. The mean of three individual hot spots was reported to characterize the vascular density of the individual tumour.
MIB-1 staining and evaluation
As previously described 4 µm sections from formalin-fixed, paraffin-embedded tumour blocks were microwaved in a buffer of 10 nM Tris and 0.5 mM EGTA (Titriplex® VI, Merck, Eurolab, Copenhagen, Denmark) (pH 9.0) three times for 5 min followed by overnight incubation at 4°C with MIB-1 (monoclonal antibody against recombinant parts of the Ki67 antigen, cat. no. 505; Immunotech, Marseilles, France) diluted 1:800 Citation[5]. Bound primary antibody was detected using EnVision + /HRP, mouse (K4001; Dako) following the manufacturer′s instructions, and visualized with DAB.
Stereological counting was done using a light microscope equipped with a CAST-grid software package (Olympus Denmark A/S, Ballerup, Denmark) for manual interactive counting on a computer screen. Using systematic random sampling of counting fields, the stereological estimates were obtained by covering the whole invasive tumour area in the section. A motorized object stage, controlled by the computer, sampled the first field of interest at random and then moved systematically throughout the histological section. Counting of all stained and unstained carcinoma cell nuclei was done on the computer screen using a two-dimensional unbiased counting frame, and the fraction of positively stained nuclei was calculated. Intra- and interobserver variability was tested and found acceptable Citation[5].
Statistical analysis
The correlations among tertiles of the variables and other known ordinal clinicopathological parameters were investigated by Spearman's rho, whereas a χ2-test was used to investigate the correlations among tertiles of the factors and nominal parameters, i.e. age and histopathology. Survival functions were made according to the Kaplan-Meier method and the differences among the survival curves were calculated with a log-rank test with a test for trend. Follow-up time was calculated using the date of primary operation as initial value.
A multivariate Cox proportional hazards regression analysis was used to investigate the prognostic value of the different parameters regarding death from cancer. The Cox analysis was stratified according to ductal versus non-ductal histology since non-ductal carcinomas had no malignancy grade. The statistical method was backward Likelihood Ratio. Univariate and multivariate analyses were performed using the SPSS 13.00 program package. All p-values were based on two-sided testing and the level of statistical significance was 5%.
Results
HER2 and clinicopathological parameters
In the whole cohort 91 patients (22%) had HER2 positive tumours. Tables show the association between HER2+ and other parameters in the whole cohort and after separating the cohort into node-negative and node-positive. HER2+ status was significantly associated with lymph node status (stratified as 0, 1–3, or >3 positive nodes). In the whole cohort and in the node-positive cohort, HER2+ status was significantly associated with high malignancy grade (ductal only), high MIB-1 score, and negative estrogen receptor status, respectively, and furthermore in the whole cohort, increasing number of lymph node metastases. Among node-negative, HER2+ status was significantly associated with high MIB-1 score and high Chalkley score. There was no association between HER2 status and age, menopausal status, tumour size, PAI-1 protein levels and TP53 mutations in the whole study group () neither after separating the patients into node-negative () nor node-positive ().
Table I. PAI-1, Chalkley, HER2, MIB-1, and TP53 in relation to clinicopathological parameters in 408 patients diagnosed with early breast cancer.
Table II. PAI-1, Chalkley, HER2, MIB-1, and TP53 in relation to clinicopathological parameters in 191 patients diagnosed with node-negative early breast cancer.
Table III. PAI-1, Chalkley, HER2, MIB-1, and TP53 in relation to clinicopathological parameters in 217 patients diagnosed with node-positive early breast cancer.
TP53 mutations and clinicopathological parameters
The analysis was performed in 401 tumours, and TP53 was mutated in 101 cases (25%). Tables shows the correlation of mutated TP53 and classical prognostic markers in early breast cancer in the whole cohort and after separation in node-negative and -positive. Mutated TP53 was significantly associated with lymph node status (stratified as 0, 1–3, or >3 positive nodes). In the whole cohort and also among N0 and N+, mutated TP53 was significantly correlated with high malignancy grade, lack of estrogen receptor, and high Chalkley and MIB-1 estimates. In the node-negative cohort, mutated TP53 was also significantly correlated to high PAI-1 levels.
HER2, TP53 mutations and survival
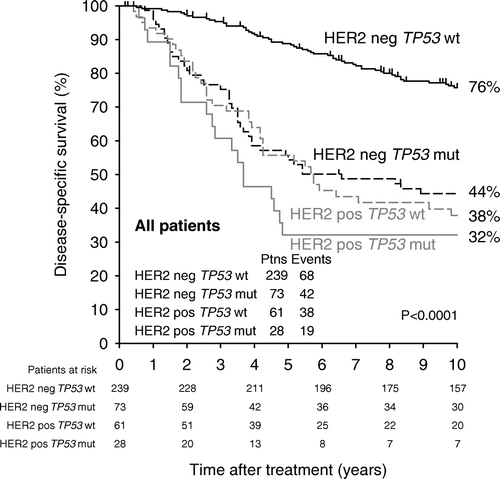
Patients with HER2+ tumours had a poorer disease-specific survival as compared to HER2 negative status in the whole cohort (p < 0.0001), and this was also the case after separating the patients into node-negative and node-positive (p = 0.0002), (, ). The same pattern was seen in data from mutational analyses of TP53, where mutated TP53 was significantly associated with poor DSS in the whole cohort (p < 0.0001), and among node-negative (p = 0.001) and node-positive (p < 0.0001) patients, (, ).
Figure 1. Kaplan-Meier survival plot illustrating the disease-specific survival probability according to years of follow-up. The plots are based on the whole cohort, the node-negative cohort, and the node-positive cohort.
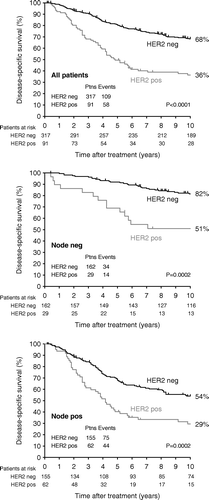
Figure 2. Kaplan-Meier survival plot illustrating the disease-specific survival probability according to years of follow-up. The plots are based on the whole cohort, the node-negative cohort, and the node-positive cohort.
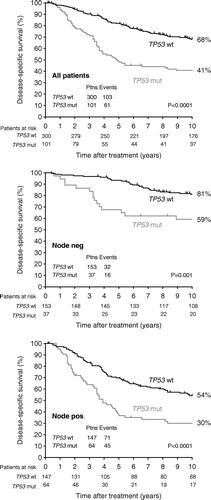
Table IV. 10 year disease-specific survival in all patients, and in node-negative and −positive patients.
Survival plots for Chalkley counts, MIB-1 estimates and PAI-1 protein levels, respectively, have been published previously Citation[4–6], and their prognostic potentials are listed in .
Additionally, shows the 10 year disease-specific survival in relation to known clinicopathological parameters. A significant correlation to poor DSS in the whole cohort is seen in cases of large tumour size (p = 0.008), poor malignancy grade in ductal carcinoma (p < 0.0001), negative oestrogen receptor status (p = 0.0001), increasing number of positive lymph nodes (p < 0.0001), high Chalkley counts (p = 0.008) and high MIB-1 estimates (p = 0.002). Age, menopausal status and high PAI-1 protein levels did not reach prognostic significance. In node-negative patients, prognostic impact was documented in high malignancy grade (p = 0.03) and high PAI-1 protein levels (p = 0.02), whilst age, large tumour size, oestrogen receptor status, Chalkley counts, and MIB-1 estimates offered no prognostic information. In node-positive patients, malignancy grade (p = 0.001), negative oestrogen receptor status (p < 0.0001), increasing tertiles of Chalkley counts (p = 0.007) and MIB-1 (p = 0.01), respectively, indicated poor DSS at 10 years follow-up, whereas no prognostic information was detected by age, menopausal status, large tumour size, and PAI-1 protein levels ().
To further investigate the relation between HER2 status and mutated TP53, we looked at the prognosis for those patients who had tumours positive for HER2 and contained mutated TP53, (). Twenty-eight patients had HER2+ tumours with mutated TP53, whilst 239 patients had HER2 negative tumours with no mutated TP53. The 10 year disease-specific survival was 32±9% versus 76±3%, (p < 0.0001), respectively, and the survival curves are illustrated in . It is seen that patients with tumours both positive for HER2 and mutated TP53 have a very grave prognosis, and the last breast cancer related death in that group is at 58 months. Furthermore, shows that TP53 status adds no further prognostic information to HER2 positive tumours, whilst among the 239 HER2 negative tumours mutated TP53 can identify a subgroup of 73 tumours with a prognosis just as poor as had the tumour been HER2 positive.
HER2, TP53, PAI-1 protein levels, Chalkley estimates, MIB-1 and multivariate analysis
Finally, a Cox multivariate analysis using death from breast cancer as endpoint was made, and the results of the analysis are shown in . In all 408 patients (401 when analysing for TP53), independent prognostic information was found by increasing number of metastatic lymph nodes, HER2+ status, TP53 mutation, increasing malignancy score, and increasing PAI-1 evaluated as a continuous parameter. In node-negative patients, HER2+ status, TP53 mutation, and increasing PAI-1 evaluated as a continuous parameter were independent significant markers of cancer death, whereas in node-positive patients malignancy grade, lymph node status, HER2+ status, and TP53 mutation were significant markers of poor prognosis. PAI-1 levels and Chalkley scores showed only borderline prognostic significance in these patients. In addition, a multivariate analysis was done without PAI-1 and TP53, since these two variables were made in supernatant from fresh frozen tumour tissue, which is not routinely available. In that analysis, no significant changes appeared regarding the remaining variables.
Table V. Cox multivariate analysis in all patients and in patients separated into node-negative and -positive. Endpoint is death from breast cancer.
Discussion
This study was designed more than a decade ago by the Translational Research committee under DBCG in order to investigate the prognostic potential of HER2, TP53, Chalkley estimates of angiogenesis, MIB-1 and PAI-1 protein levels in a well-characterized group of patients with early breast cancer. All analyses are hereby presented, and HER2 status and TP53 mutation were found to be the strongest indicators of death from breast cancer. HER2+ status and TP53 mutation were significantly correlated to poor outcome in all patients and also in the subgroups of both node-negative and -positive. In multivariate analysis, these markers were very strong independent markers of poor prognosis also in node-negative and node-positive patients, thus HER2 and TP53 mutation can potentially identify patients in an otherwise good prognostic group who should be offered additional therapy.
This conclusion supports the rationale of changing the risk categorizing of Danish patients diagnosed with early breast cancer; from January 1, 2007, all patients diagnosed with HER2+ tumours have been classified as high risk irrespective of other markers. Patients with HER2+ tumours deemed to receive chemotherapy are also routinely offered adjuvant Trastuzumab every 3 weeks for one year, in that Trastuzumab has been shown to significantly lower the risk of relapse Citation[18]. There is an overlap among the different variables used when the patient is being risk stratified, and in the present study, only one patient was identified who was classified high risk based solely on HER2+, i.e. the patient had low risk on the other parameters (pN0, T ≤ 20 mm, grade I, ER+ and >35 years of age).
Besides being a prognostic variable, HER2 is also a predictive marker, since only patients with HER2+ tumours benefit from Trastuzumab Citation[19]. It has furthermore been demonstrated that amplification of the HER2 gene is associated with improved responsiveness to adjuvant anthracycline-based chemotherapy Citation[20].
The method used in the present study both regarding immunohistochemistry and HER2 evaluation is the same as used routinely in Denmark at the present time. Relatively few tumours were scored HER2 2+ and therefore needed further analysis with FISH or quantitative PCR, and this may be attributable to the fact that only one experienced pathologist performed all immunohistochemical evaluations.
The findings in the present study regarding the prognostic potential of mutations of TP53 are in accordance with previous studies in breast cancer Citation[12]. Data on 243 of the 401 patients with TP53 data has been presented previously as part of a study on the heterogeneity in the clinical phenotype of different mutations Citation[9]. In the extended cohort presented here, identical associations were found between outcome and different types and position of mutations Citation[21]. For simplicity, however, mutations were categorized as being either present or absent based on the criteria previously described Citation[9].
In conclusion we hereby present the final analysis of a study of HER2, TP53, PAI-1, estimates of angiogenesis, and MIB-1 in a well characterized group of 408 patients diagnosed with early breast cancer. All patients alive were followed at minimum 10 years. The findings in this final analysis confirm the prognostic influence of the classical markers such as lymph node status, tumour size, estrogen receptor status, and malignancy grade. Today, HER2 status is used to aid in the classification of tumours into low and high risk. HER2 status was a strong prognostic marker in the whole cohort and among node-negative and node-positive patients. In multivariate analysis, HER2 status was an independent marker in the whole cohort and in both the node-negative and node-positive group implying that the marker can indeed identify patients who potentially will benefit from aggressive adjuvant therapy. Mutation in TP53 had a similar prognostic impact as HER2 status, and was also an independent marker in the whole cohort and among node-negative and node-positive patients. Both HER2 status and mutation in TP53, the latter in particular, were strongly associated with malignancy grade, however, in the multivariate analyses in the whole cohort and among node-positive patients, malignancy grade was also an independent prognostic marker as well as HER2 and TP53. Thus malignancy grade may add valuable information to TP53 and HER2 status, and it is therefore still not possible to replace the “subjective” malignancy grade with any of these new markers as previously discussed Citation[5]. Although TP53 status adds important biological information, all tumours with mutated TP53 already were classified as high risk by other means. The present guidelines for risk stratification of patients with early breast cancer together with the lack of specific targeted therapy aimed at mutated TP53 do therefore not support mutational analysis for TP53 as routine.
Acknowledgements
Funding has been received from The Danish Cancer Society, The Fund of M.L.Jørgensen and Gunnar Hansen, The Danish Ministry of Health and The Danish Medical Research Council. This manuscript was presented as oral presentation at ECCO 14, Barcelona, September 2007.
References
- Colozza M, Cardoso F, Sotiriou C, Larsimont D, Piccart MJ. Bringing molecular prognosis and prediction to the clinic. Clin Breast Cancer 2005; 6: 61–76
- Erdem O, Dursun A, Coskun U, Gunel N. The prognostic value of p53 and c-erbB-2 expression, proliferative activity and angiogenesis in node-negative breast carcinoma. Tumori 2005; 91: 46–52
- Look M, van PW, Duffy M, Harbeck N, Christensen IJ, Thomssen C, et al. Pooled analysis of prognostic impact of uPA and PAI-1 in breast cancer patients. Thromb Haemost 2003; 90: 538–48
- Offersen BV, Sørensen FB, Yilmaz M, Knoop A, Overgaard J. Chalkley estimates of angiogenesis in early breast cancer–relevance to prognosis. Acta Oncol 2002; 41: 695–703
- Offersen BV, Sørensen FB, Knoop A, Overgaard J. The prognostic relevance of estimates of proliferative activity in early breast cancer. Histopathology 2003; 43: 573–82
- Offersen BV, Riisbro R, Knoop A, Brunner N, Overgaard J. Lack of association between level of Plasminogen Activator Inhibitor-1 and estimates of tumor angiogenesis in early breast cancer. Acta Oncol 2007; 46: 782–91
- Hansen LL, Yilmaz M, Overgaard J, Andersen J, Kruse TA. Allelic loss of 16q23.2-24.2 is an independent marker of good prognosis in primary breast cancer. Cancer Res 1998; 58: 2166–9
- Overgaard J, Yilmaz M, Guldberg P, Hansen LL, Alsner J. TP53 mutation is an independent prognostic marker for poor outcome in both node-negative and node-positive breast cancer. Acta Oncol 2000; 39: 327–33
- Alsner J, Yilmaz M, Guldberg P, Hansen LL, Overgaard J. Heterogeneity in the clinical phenotype of TP53 mutations in breast cancer patients. Clin Cancer Res 2000; 6: 3923–31
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244: 707–12
- Moasser, MM. Targeting the function of the HER2 oncogene in human cancer therapeutics. Oncogene 2007.
- Petitjean A, Achatz MI, Børresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene 2007; 26: 2157–65
- Olivier M, Langerod A, Carrieri P, Bergh J, Klaar S, Eyfjord J, et al. The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clin Cancer Res 2006; 12: 1157–67
- Olsen KE, Knudsen H, Rasmussen BB, Balslev E, Knoop A, Ejlertsen B, et al. Amplification of HER2 and TOP2A and deletion of TOP2A genes in breast cancer investigated by new FISH probes. Acta Oncol 2004; 43: 35–42
- Schlemmer BO, Sørensen BS, Overgaard J, Olsen KE, Gjerdrum LM, Nexø E. Quantitative PCR–new diagnostic tool for quantifying specific mRNA and DNA molecules: HER2/neu DNA quantification with LightCycler real-time PCR in comparison with immunohistochemistry and fluorescence in situ hybridization. Scand J Clin Lab Invest 2004; 64: 511–22
- Hansen LL, Andersen J, Overgaard J, Kruse TA. Molecular genetic analysis of easily accessible breast tumour DNA, purified from tissue left over from hormone receptor measurement. APMIS 1998; 106: 371–7
- Grøndahl-Hansen J, Christensen IJ, Rosenquist C, Brünner N, Mouridsen HT, Danø K, Blichert-Toft M. High levels of urokinase-type plasminogen activator and its inhibitor PAI-1 in cytosolic extracts of breast carcinomas are associated with poor prognosis. Cancer Res 1993; 53: 2513–21
- Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet 2007; 369: 29–36
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002; 20: 719–26
- Pritchard KI, Shepherd LE, O'Malley FP, Andrulis IL, Tu D, Bramwell VH, Levine MN. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med 2006; 354: 2103–11
- Alsner, J , Jensen, V , Overgaard, J. A comparison among p53 expression and TP53 mutations as prognostic variables in tumours from 401 patients diagnosed with early breast cancer. Acta Oncol 2008;47:600–7.