Abstract
The main objective of the present study aims at comparing the long-term efficacy of breast conserving surgery (BCS) vs. mastectomy (M) based on a randomized design. The Danish Breast Cancer Cooperative Group (DBCG) conducted the trial (DBCG-82TM) from January 1983 to March 1989 recruiting 1154 patients with invasive breast carcinoma. Follow-up time ended 1st May 2006 with a median follow-up time of 19.6 years (time span 17.1–23.3 years). Eligibility criteria included a one-sided, unifocal, primary operable breast carcinoma, patient age below 70 years, probability of satisfactory cosmetic outcome with BCS, and no evidence of disseminated disease. The patients accrued were grouped into three subsets: correctly randomized, suspicion of randomization error, and declining randomization.
The main analyses focus on the subgroup of 793 correctly randomized patients representing 70% of the complete series. 10-year recurrence free survival (RFS) and 20-year overall survival (OS) based on intent to treat did not reveal significant differences in outcome between breast conserving surgery vs. mastectomy, p=0.95 and p=0.10, respectively. Including the complete series comprising 1133 eligible patients based on treatment in fact given similarly no significant difference between surgical options could be traced in outcome of 10-year RFS and 20-year OS, p=0.94 and p=0.24, respectively.
The pattern of recurrences as a first event in breast conservation vs. mastectomy did not differ significantly irrespective of site, p=0.27. Looking into the type of local relapse, viz., new primaries vs. true recurrences, it appeared that new primaries were significantly associated to BCS, while true recurrences dominated among M treated patients (p<0.001).
In conclusion, long-term data indicate that BCS in eligible patients proves as effective as mastectomy both regarding local tumour control, RFS and OS. Local failures as a first event consistent with new primaries are strongly associated with BCS, whereas true recurrence predominates after mastectomy.
The basic treatment of early stage breast cancer is surgery as a first step followed by loco-regional radiation and adjuvant systemic therapy – whenever indicated – according to current guidelines. The therapeutic objectives aim at reducing the risk of loco-regional and distant recurrence and thus to improve survival from the disease. Moreover, treatment modalities should take into account the demands for lowest morbidity and best available cosmetic outcome.
For at least a century, radical or modified radical mastectomy as the main treatment has been the approved therapeutic modality throughout the world complying with the Halstedian assumption of centrifugal growth of breast cancer. During the 1970s, however, the Fisher votaries gained ground assuming that the disease in the majority of patients has already propagated and disseminated subclinically at an early stage before diagnosis. The Fisher attitude put an emphasis on systemic therapy rather than extensive surgery. Due to increasing awareness and implementation of novel diagnostic technologies as well as mammographic screening services breast cancer is nowadays diagnosed at an earlier stage. Consequently, requirement of the mutilating mastectomies as loco-regional treatment seems to abate allowing introduction of conserving operations with improved cosmesis and less morbidity.
Based on the early innovative works by Keynes Citation[1], Atkins and co-workers Citation[2], Mustakallio Citation[3], Hayward Citation[4], and others, breast conserving techniques gained ground. In order to further clarify the therapeutic procedures, their applicability, and eligibility criteria for breast conserving therapy, a number of randomized type III studies were launched during the late 1970s and up through the 1980s comparing the outcome of breast conservation vs. mastectomy Citation[5–10]. The results originating from this scientific achievement were presented at the NIH Consensus Development Conference in 1990, Bethesda, MD Citation[11]. The final conclusion stated that “breast conservation treatment is an appropriate method of primary therapy for the majority of women with stage I and II breast cancer and is preferable because it provides survival rates equivalent to those of total mastectomy and axillary dissection while preserving the breast”.
A major concern regarding breast conserving therapy refers primarily to whether or not local tumour control can be obtained equivalent to that of mastectomy. Several studies reveal that young age and the presence of extensive intraductal components are associated with an increased risk of local recurrence compared to mastectomy Citation[12–14]. Further, to improve the outcome of the method with special reference to local tumour control a number of precautions should be considered such as appropriate surgical techniques, width of free margins, quality of radiotherapy to residual breast tissue, pathological features of the tumour, and patient characteristics Citation[12], Citation[15–18]. Local relapse signifies a bad outcome due to the assumption that the finding might be an instigator to distant spread Citation[19–21]. Consequently, long-term results of local tumour control as well as final outcome in breast conservation vs. mastectomy are important to clarify the safety of the method.
Long-term results covering up to 20 years of follow-up have been presented recently from three of the four largest randomized trials on breast conservation vs. mastectomy (), Citation[5], Citation[7], Citation[8]. The present paper conveys 20-year follow-up results of the fourth of the four largest randomized studies on breast conserving therapy initially presented at the NIH Consensus Development Conference in 1990 Citation[10].
Table I. Survey of number of patients, patient- and tumour characteristics and treatment options in the four largest randomized studies comparing breast conserving surgery vs. mastectomy.
Material and methods
Enrolment of patients into the study (DBCG-82TM) took place from January 1983 to March 1989. The protocol was approved by the National Ethical Committee. Totally, 1 153 women were accrued. As enumerated earlier Citation[10], the eligibility criteria included: 1) primary operable breast carcinoma; 2) age below 70 years; 3) probability of satisfactory cosmetic outcome by excision of the tumour bearing part of the breast; 4) tumour confined to one breast and no signs of multifocality by palpation or mammography; 5) no evidence of disseminated disease as determined clinically, by chest radiography, and bone scintigraphy. Ineligible for the study were patients with Paget′s disease of the nipple, pure in situ lesions, clinical breast cancer stage IIIb and IV, or a history of previous or concomitant malignancy, apart from basal skin carcinoma and in situ carcinoma of the cervix uteri.
Among the 1 153 women initially accrued, 905 patients were randomly assigned to either breast conserving therapy or mastectomy, while 248 patients had surgery according to their own choice choosing breast conservation or mastectomy Citation[10]. Randomization took place decentralized in the participating surgical units applying a balanced closed envelope method as the standard procedure at that time. The randomization procedure followed the principle of Zelen using a preoperative one-arm information about the surgical option to which they were randomly assigned Citation[22]. However, the protocol held a proviso that information about randomization and both surgical options should be given at the patient′s request. There existed also a built-in proviso that patients randomly allocated to one surgical procedure could ask for the alternative operation in case of preference. In 1987 the Ethical Committee recommended a Zelen two-arm procedure allowing the patient full information about surgical options and randomization. However, the randomization was still executed prior to patient information. The change of information did not influence the accrual rate.
The patients accrued were recorded in the DBCG (Danish Breast Cancer Cooperative Group) Secretariat harbouring a national breast cancer data base. Registration of data related to surgery, histopathology, adjuvant therapy and follow-up were accomplished by the use of predetermined case report forms Citation[23].
Data review
During the mid-1990s all case report forms, clinical records-when appropriate -, all the original histological slides, and the sequence of randomization cards were carefully reviewed. No new histological slides were produced for the review.
The review work entailed minor changes as concerns number of patients excluded due to histological misclassification and protocol violation. Further, the number of patients allocated to the randomized series was reduced due to suspicion that irregularity of the randomization sequence occurred in two hospital units as elaborated in the following paragraph.
By reviewing the sequence of randomization cards a certain irregularity appeared concerning two surgical units, contributing 131 patients to the study. In the two units some of the randomization cards were not drawn in an uninterrupted succession. The Executive Committee of DBCG requested The Danish Committee on Scientific Dishonesty under the auspices of the Danish Research Councils to perform a further investigation on the matter. The Committee presented a comprehensive report in 1995 Citation[24]. In brief, the report concluded that there is every probability that irregularities of the sequence of randomization had taken place in the two surgical units. However, the motive could not be clarified. The report emphasized that scientific misconduct in the true sense of the word had not been performed. As a consequence, the DBCG Executive Committee decided to withdraw the patients coming from the two surgical units from future studies involving “the intent to treat” principle. On the other hand, the report issued by the Danish Committee on Scientific Dishonesty found no deviation from protocolized treatment given by the two units.
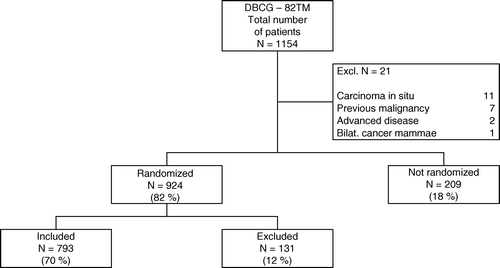
Consequently, after reallocation of patients according to review of data this presentation includes 793 randomized patients (70%), 131 excluded, albeit randomized patients (12%), and 209 non-randomized patients (18%), () vs. respectively 905 randomized and 248 non-randomized patients presented in the 1992 publication Citation[10].
Surgery
Ultimately, 20 surgical units took part in the study and about one third of patients diagnosed with invasive breast cancer fulfilled the eligibility criteria. No mammographic screening took place in Denmark at the time of study. Details have been described previously Citation[10]. The definitive surgical procedures were standardized according to national DBCG guidelines Citation[25].
Breast conserving surgery consisted of removal of the tumour-bearing part of the breast with enough surrounding normal tissue to ensure tumour free margins at gross examination. Segmental resection was used preferentially in case of peripheral location, while simple lumpectomy appeared the standard in more centrally located tumours. Axillary dissection was mandatory and generally performed through a separate transverse incision with removal of lymph nodes from the low- and mid-axillary levels.
Mastectomy comprised removal of the entire breast parenchyma and deep fascia through a transverse-oval incision including dissection of upper and lower skin flaps as well as removal of lymph nodes from low- and mid-axilla.
Histopathology
The pathological work-up was standardized according to the national DBCG guidelines Citation[25]. Details are given elsewhere Citation[10]. The pathologist determined whether specimen margins appeared tumour free at gross examination, and margins were reassessed microscopically on paraffin sections. Requirement of free margins at gross examination was obligatory, albeit not microscopically. The pathologist measured the size of largest tumour diameter and examined for invasion to skin and deep fascia, the number of lymph nodes retrieved from the axilla, and the number of involved nodes.
Patients were classified and divided into two risk groups on the basis of pathological work-up. Low-risk patients had tumour diameter of 50 mm or less, with no invasion to skin or deep fascia, and no metastatic axillary lymph nodes. High-risk patients contracted tumour diameter exceeding 50 mm, and/or invasion to skin or deep fascia, and/or involvement of axillary nodes Citation[25]. The review work-up did not involve additional histological slides.
Regarding local failures a distinction was made between true recurrences vs. new primary tumours. The criterion of a verifiable new primary tumour of invasive type included the finding of in situ components within and/or in immediate relation to the recurrent tumour or changed histological type compared with the original tumour. All other cases of local failures were classified as true recurrences Citation[26]. Loco-regional failures were confined to the residual breast/chest wall, axilla, and parasternal area. Supraclavicular recurrences belonged to the distant relapse group.
Radiation treatment
Patients assigned to breast conservation received radiotherapy within 2–4 weeks of their surgery. The target volume involved the entire residual breast. Regional lymph nodes were included only in high-risk patients (). The prescribed dose was a median absorbed dose in the target volume of 50 Gy in 25 fractions in 5 weeks according to ICRU-29. In addition, the tumour bed received a boost dose of 10–25 Gy in 5–12 fractions adjusted to whether or not the surgical margins were microscopically free.
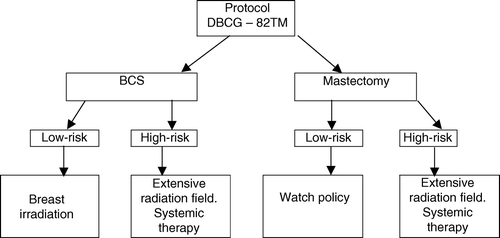
Figure 2. Study design and treatment modalities of patients entering the DBCG-82TM protocol. (BCS = Breast conserving surgery).
Two different techniques were applied to irradiate the residual breast. Three of the six radiotherapy centres used mainly anterior electron fields with energies 6–20 MeV against the breast. The electron energy was chosen to get the target volume within the 85% isodose curve. The remaining three centres allowed medial and lateral tangential photon fields, 6–8 MV. Details are presented in the report published earlier Citation[10].
The radiation treatment of regional lymph nodes in high-risk patients was similar in all six centres. The supraclavicular/infraclavicular and axillary nodes were treated by anterior photon fields, 6–16 MV. Posterior axillary fields were recommended in patients with large anterior-posterior diameters to reduce the maximum absorbed target dose to 55 Gy. The internal mammary chain was included in the fields to the breast in either radiation technique.
Mastectomy patients in the low-risk group did not receive radiotherapy (), while high-risk patients had standard postoperative radiotherapy including thoracic wall and regional lymph node areas. The target volume in high-risk mastectomy patients was analogous to the target volume in high-risk breast-conservation patients. The target dose was either a median absorbed dose of 50 Gy in 25 fractions in 5 weeks or 48 Gy in 22 fractions in 51/2 weeks Citation[10].
Adjuvant systemic treatment
All high-risk patients received adjuvant systemic therapy in addition to the extended radiation field (). The systemic regimens administered were those considered standard at the time. Premenopausal high-risk patients had 8 cycles of CMF (Cyclophosphamide 600 mg/m2, Methotrexate 40 mg/m2, and fluorouracil 600 mg/m2) given as intravenous bolus on day 1 every fourth week. Chemotherapy and radiation were administered sequentially. Postmenopausal patients received 30 mg of tamoxifen daily for one year. A further description has been given previously Citation[10].
Follow-up
All patients were seen at regular intervals according to the national guidelines, and the clinical results of each follow-up visit were reported on case report forms to the DBCG data base for data processing. Regular clinical follow-up continued for 10 years or until first event of recurrence, second malignancy, death, or the patient′s wish to discontinue controls Citation[25]. After discontinuation of clinical follow-up, status of survival was achieved by linkage to the Danish Death Certificate Registry. The follow-up time is defined as the time span between the date of primary operation and the date of most recent evaluation. In the present study, the follow-up time regarding overall survival (OS) ended May 1, 2006 and, thus, varied from maximum 23.3 years to minimum 17.1 years with a median of 19.6 years. As for recurrence free-survival (RFS), the observation time was limited to a maximum of 10 years according to schedule.
Statistical analysis
Comparison of patient- and histological characteristics between groups was obtained by the χ2 test in relevant contingency tables. Recurrence free-survival and overall survival estimates involved the Kaplan-Meier method and assessed by applying a log-rank test Citation[27]. The median follow-up time was quantified in terms of a Kaplan-Meier estimate of potential follow-up. The level of significance was set at less than 5% and implied two-sided analysis.
Results
The revised data set ended up with 1 154 patients for the DBCG-82TM protocol. Hereof 21 patients were rejected up-front due to inconsistency with inclusion criteria () leaving 1 133 patients entering the study. The patients were subsequently organized into subgroups, viz., the randomised patients of whom some were excluded due to inappropriate randomization and the non-randomized patients. In the following analyses, the main objective focuses on the correctly randomized group comprising 793 patients (70%), ().
Analyses of the randomized series, N = 793 patients
From the algorithm () it appears that 51% of patients were allocated at random to receive breast conserving surgery (BCS) vs. 49% of patients allocated to mastectomy (M). In accordance with the built-in proviso of the protocol giving the patient access to decline the randomization offer, 54 patients (N = 39 + 15) randomly assigned to BCS in fact ended up with M, and 33 patients allocated at random to M in fact had BCS. Consequently, 87% (N = 350) of the BCS subset had the treatment to which they were initially allocated at random, while the corresponding figure of the M subset reached 92% (N = 356).
Figure 3. Algorithm showing patient distribution and patient flow of the randomized series (N = 793 patients) enrolled in DBCG-82TM protocol according to intent to treat and treatment in fact given. (BCS = Breast conserving surgery. Mast.=Mastectomy).
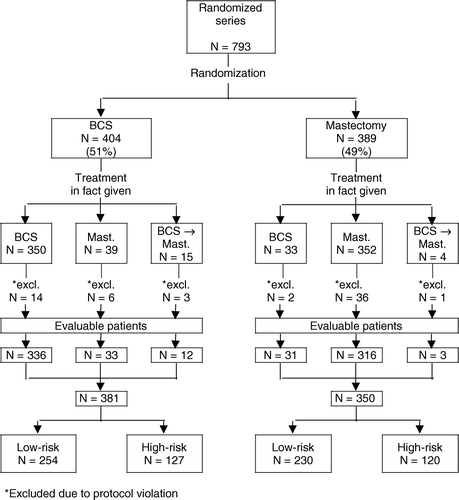
Further, the algorithm reveals that protocol violation happened in 8% of cases, N = 62, i.e., 23 patients belonging to the “intent to treat” BCS subset vs. 39 patients to the “intent to treat” M subset. The most important causes of protocol violation comprised erroneous allocation for schedules of adjuvant systemic therapy, misclassification to risk groups due to deficient pathological work-up, and surgical divergences from protocol requirements. Hereafter, in accordance with the intent to treat principle, the study included a total of 731 evaluable patients treated according to initial protocol requirements, i.e., 381 patients belonging to the BCS subset and 350 patients allocated to the M subset. Low-risk patients constituted 67% of the evaluable BCS series and 66% of evaluable M series, ().
In certain patient- and tumour characteristics of prognostic significance were analyzed in order to examine the similarity of patient distribution between the two option series based on intent to treat principle, (N = 793). The findings did not reveal statistically significant differences for patient age, menopausal status, tumour size, number of axillary nodes retrieved, and number of axillary metastatic nodes.
Table II. Comparison of patient- and tumour characteristics of 793 patients based on intent to treat in the randomized DBCG-82TM protocol.
Recurrence pattern
Specifications of recurrence as a first event are confined to the subset composed of randomized evaluable patients (N = 731, ) and related to the treatment in fact given. shows the distribution of recurrence according to site of appearance. In total, 250 cases of first event recurrence occurred, viz. 133 cases among BCS treated patients and 117 among the mastectomized. Distant spread as a first event prevails the pattern. The distribution pattern of recurrences diagnosed in BCS patients compared with that in M patients did not disclose significant difference (p = 0.27, χ2).
Table III. Distribution pattern of recurrences as a first event in 731 evaluable patients based on treatment in fact given in the DBCG-82TM protocol. (M = Mastectomy. BCS = Breast conserving surgery. DM = Distant metastasis. Contra lateral = Contralateral breast).
Local recurrence was registered in 47 patients as a first event. Forty-one patients had local relapse alone and six patients experienced local relapse in addition to regional relapse (). Twenty-two cases occurred in the BCS series and 25 cases in patients who underwent M. Revision of original histological slides classified 15 local failures as new primary tumours and 28 events as true recurrences. In four cases it was not possible to distinguish between the two histological features (). Occurrence of new primary tumours dominated strongly in the BCS series, while in the M series true recurrences prevailed. Statistical analysis corroborated a significant dependence on surgical procedure regarding histological type of local failures (p < 0.001, χ2). In the BCS series new primaries were mostly located in a quadrant different from the quadrant harbouring the original tumour (10 of 13 cases), whereas in case of a true recurrence the predominant location was confined to the same quadrant as that of the original tumour (5 of 8 cases). The crude mortality of patients with new primaries amounted 53% compared with 82% of patients with true recurrences.
Table IV. Type of 47 local recurrences as a first event according to treatment in fact given in 731 evaluable patients enrolled in the DBCG-82TM protocol. (M = Mastectomy. BCS = Breast conserving surgery. True rec.=True recurrence).
In the BCS group 64% of local failures occurred within 5 years after primary treatment, while the figure reached 76% in the M group.
Recurrence free (RFS) and overall survival (OS)
In the randomized evaluable patient group (N = 731 patients, intent to treat principle) the10-year RFS curves comparing the outcome of BCT (breast conserving treatment) vs. M did not differ significantly (p = 0.57), (). At 10 years of observation, the probability of RFS amounted 59.5%, (95% CI: 54.5–64.4) in the BCT group vs. 61.1%, (95% CI: 55.9–66.3) in the M group. For the same patient group the probability of 20-year OS among patients having BCT equalled that of M (p = 0.20), respectively 57.8%, (95% CI: 52.7–62.9) vs. 50.6%, (95% CI: 45.0–56.2), ().
Figure 4. Recurrence free survival (RFS) according to intent to treat in 731 evaluable patients enrolled in the DBCG-82TM protocol. (M = Mastectomy. BCT = Breast conserving therapy).
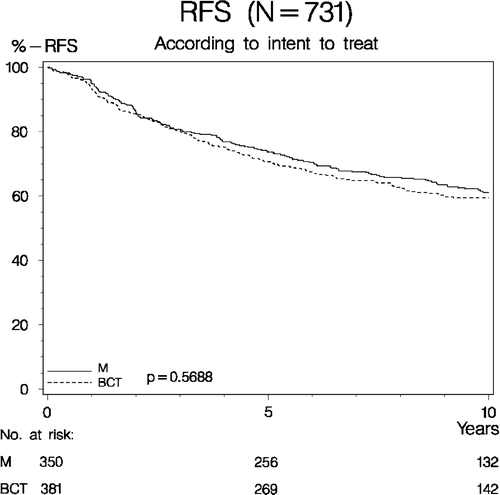
Figure 5. Overall survival (OS) according to intent to treat in 731 evaluable patients enrolled in the DBCG-82TM protocol. (M = Mastectomy. BCT = Breast conserving therapy). “Population” refers to cumulated deaths over 20 years in the background female population matched according to age and calendar years. (Denmark's Statistical Registry).
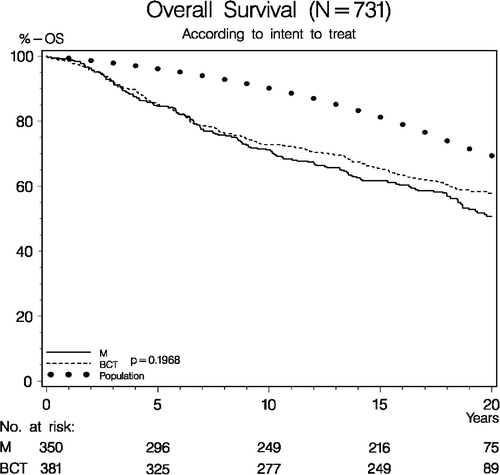
The total number of deaths registered among the randomized evaluable patients (N = 731) reached 327, viz., 162 deaths among patients receiving BCT vs. 165 deaths in the M group based on treatment in fact given.
Looking into probabilities of 10-year RFS and 20-year OS including the complete randomized patient series (N = 793), (), according to intent to treat principle no significant difference in outcome between treatment options could be traced, respective p-values p = 0.95 and p = 0.10. Similar results were achieved when entering data based on treatment in fact given (Data not shown). The results indicate that protocol violation did not significantly influence the final treatment results.
Finally, including all 1 133 patients eligible for the protocol () in 10-year RFS and 20-year OS analyses according to treatment in fact given no difference in outcome appeared. The respective p-values were p = 0.94 and p = 0.24. The probability of RFS at 10 years attained 57.4%, (95% CI: 53.1–61.7) in the BCT group vs. 58.4%, (95% CI: 54.5–62.4) in M group. The 20-year probability of OS reached 53.7%, (95% CI: 49.3–58.2) in BCT vs. 49.1%, (95% CI: 44.9–53.3) in M treated patients.
Discussion
Including the present study the long-term results are published from all four major randomized trials conducted during the 1970s and 1980s comparing BCS with mastectomy. Our study in addition to the previously published trials Citation[5], Citation[7], Citation[8] confirm consistently that overall survival does not exhibit significant differences irrespective of surgical procedure, provided that the approved eligibility criteria are fulfilled. It appears mandatory obtaining long-term results due to excess mortality from breast cancer during at least 20 years of follow-up Citation[28], Citation[29]. Although the four trials use the same design, they differ to some extent from one another regarding patients- and tumour characteristics as well as surgical procedure (). Such diversities may explain dissimilar findings regarding local tumour control in BCS vs. mastectomy. So, explicit comparison of our results with those from other studies appears less appropriate.
In a randomized trial, preferentially all eligible patients should be recorded in order to avoid the possibility of selection bias in patient recruitment Citation[30]. The study design in the present paper complied with the requirement of complete recording. From it appears that 18% of eligible probands denied randomization and 12% did not pass the scrutiny of randomization procedure. Nonetheless, all candidates irrespective of randomization status merged into the final analysis in compliance with treatment in fact given in order to refute any suspicion of skewness in patient allocation. A similar attitude to population based recruitment of potential eligible candidates into the randomized studies was not complied with in the three international studies Citation[5], Citation[7], Citation[8].
The limited number of local recurrences in our study impedes an elaborate analysis of the phenomenon. However, two items of major importance can be clarified – promoted by the random design. Firstly, our data demonstrate that the distribution pattern of recurrences within 10 years of observation does not differ significantly in BCS vs. mastectomy. Further, local tumour control after BCS equals that of mastectomy (crude percentages 4.5 vs. 6.9, respectively, p = 0.16, χ2). In comparison with the three other large randomized trials Citation[5], Citation[7], Citation[8] quite the opposite result came up. However, an explicit comparison is hampered by the fact that age distribution, surgical methods, boost/no boost, tumour size, and the definition of a local recurrence vary between studies. On the other hand, in all the studies local tumour control was consistent with approved level of good surgical quality Citation[31].
Another interesting point in BCS deals with the histological type of local failures, viz., new primaries vs. true recurrences. In the present study it appeared that new primaries were significantly associated with BCS as opposed to mastectomy and, further, that new primaries seem to present a less aggressive event compared with true recurrences. Moreover, new primaries seem to turn up in a quadrant different from the one of the original tumour. These observations are quite consistent with the findings from a retrospective, albeit rather comprehensive American study comprising 1 152 patient undergoing BCS and radiotherapy. A total of 130 local failures could be classified, crude 11.3% at 10 years of follow-up, viz., 70 new primaries and 60 true recurrences Citation[32]. Among the randomized studies Citation[5], Citation[7], Citation[8], only Veronesi et al. Citation[5] looked into the phenomenon of the subtypes of local failures. They found 30 cases of local failures (crude 8.5%) of which 20 were classified as new primaries located in other quadrants.
The study of cosmetic and functional outcome in BCS has not been the scope of the present review. On the other hand, the object was addressed in a recent paper involving a subgroup of 266 BCS patients derived from the present series after a median follow-up time of 6.6 years Citation[33]. The main findings revealed that the cosmetic outcome was reported as excellent/good by 73% of the patients (self-assessment) vs. 47% as assessed by the oncologist (p < 0.001). Further, the treatment with tangential photons came out cosmetically superior to an anterior electron field (p = 0.002). Moreover, the anterior electron field caused significantly more grade 2 and grade 3 late radiation reactions compared with tangential photons. Constant or frequent breast pain occurred in 13% of patients, although only one patient required analgesics and then merely occasionally. The study had consequences in favour of the choice of photons in the future national planning of radiation following BCS.
As stated in the introduction, risk factors of applicability should be determined to refine patient selection for BCS in order to safeguard local tumour control. Detailed analysis of such efforts is, however, beyond objectives of the present trial. On the other hand, some information is available. The four randomized studies [5,7,8, present study] shared a set of precautions only allowing patients with low aggressiveness to enter the trials. Consequently, our experience with BCS based on a randomized design is limited to patients with low risk of local recurrence. The majority of tumours belonged to the T1 category, except for the EORTC trial. Axillary nodal status revealed a low percentage of patients with four or more involved nodes (Milan I, 5.1%; NSABP-06, 11.0%; EORTC, 11.1%; DBCG-82TM, 6.7%). Nonetheless, crude cumulative incidence of local/locoregional failures varied from 4.5% (DBCG-82TM) to 17.0% (EORTC) within 10–20 years of observation.
Although beyond the scope of the present study, it appeared from the remaining three randomized trials Citation[5], Citation[7], Citation[8] that patient age, tumour size, nodal status, surgical radicality, and +/- radiation of residual breast tissue composed a significant bearing on local tumour control. However, some of the data presented exposed inconsistent findings. More recent investigations based on pooled data from randomized trials or cohort studies maintained that significant risk factors for local tumour control were associated with young age, extensive intraductal component of tumour, and narrow free margins in BCS with radiotherapy vs. mastectomy Citation[12], Citation[13], Citation[15].
In conclusion, over the long term our data indicate that BCS in eligible patients proves to be as effective as mastectomy both regarding local tumour control, RFS, and OS. Local recurrence as a first event consistent with new primaries is strongly associated with BCS, whereas true recurrence predominates in the lapse of mastectomy.
References
- Keynes G. Conservative treatment of breast cancer. Br J Surg 1932; 19: 415–80
- Atkins H, Hayward JL, Klugman DJ, Wayte AB. Treatment of early breast cancer: A report after ten years of a clinical trial. Br Med J 1972; 2: 423–9
- Mustakallio S. Conservative treatment of breast carcinoma- Review of 25 years follow up. Clin Radiol 1972; 23: 110–6
- Hayward JL. The Guy′s trial of treatments of early breast cancer. World J Surg 1977; 1: 314–6
- Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002; 347: 1227–32
- Sarrazin D, Le M, Rouesse J, Contesso J, Petit JY, Lacour J, et al. Conservative treatment versus mastectomy in breast cancer tumors with macroscopic diameter of 20 millimeters or less. The experience of the Institut Gustave-Roussy. Cancer 1984; 53: 1209–13
- Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347: 1233–41
- Dongen JA van, Voogd AC, Fentiman IS, Legrand C, Sylvester J, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 Trial. J Natl Cancer Inst 2000; 92: 1143–50
- Straus K, Lichter A, Lippman M, Danforth D, Swain S, Cowan K, et al. Results of the National Cancer Institute Early Breast Cancer Trial. J Natl Cancer Inst Monogr 1992; 11: 27–32
- Blichert-Toft M, Rose C, Andersen JA, Overgaard M, Axelsson CK, Andersen KW, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: Six years of life-table analysis. J Natl Cancer Inst Monogr 1992; 11: 19–25
- NIH Consensus Conference. Treatment of early-stage breast cancer. JAMA 1991;265:391–5.
- Voogd A, Nielsen M, Peterse JL, Blichert-Toft M, Bartelink H, Overgaard M, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: Pooled results of two European randomized trials. J Clin Oncol 2001; 19: 1688–97
- Kroman N, Holtveg H, Wohlfahrt J, Jensen M-B, Mouridsen HT, Blichert-Toft M, et al. Effect of breast-conserving therapy versus radical mastectomy on prognosis for young women with breast carcinoma. Cancer 2004; 100: 688–93
- Kurtz JM, Spitalier J-M, Amalric R, Brandone H, Ayme Y, Bressac C, et al. Mammary recurrence in women younger than forty. Int J Radiat Oncol Biol Phys 1988; 15: 271–6
- Freedman G, Fowble B, Hanlon A, Nicolaou N, Fein D, Hoffman J, et al. Patients with early stage invasive cancer with close or positive margins treated with conservative surgery and radiation have an increased risk of breast recurrence that is delayed by adjuvant systemic therapy. Int J Radiat Oncol Biol Phys 1999; 44: 1005–15
- Malmström P, Holmberg L, Anderson H, Mattsson J, Jönsson P-E, Tennvall-Nittby L, et al. Breast conservation surgery, with and without radiotherapy, in women with lymph node-negative breast cancer: A randomised clinical trial in a population with access to public mammography screening. Eur J Cancer 2003; 39: 1690–7
- Harris JR, Connolly JL, Schnitt SJ, Cady B, Love S, Osteen RT, et al. The use of pathologic features in selecting the extent of surgical resection necessary for breast cancer patients treated by primary radiation therapy. Ann Surg 1985; 2001: 164–9
- Locker AP, Ellis IO, Morgant DAL, Elston CW, Mitchell A, Blamey RW, et al. Factors influencing local recurrence after excision and radiotherapy for primary breast cancer. Br J Surg 1989; 76: 890–4
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med 1997; 337: 949–55
- Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 1997; 337: 956–62
- Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast cancer patients given adjuvant tamoxifen. Danish Breast Cancer Cooperative Group DBCG 82c randomized trial. Lancet 1999; 353: 1641–8
- Zelen M. A new design for randomized clinical trials. N Engl J Med 1979; 300: 1242–5
- Danish Breast Cancer Cooperative Group, DBCG (eds). The structure and organization of DBCG. www.dbcg.dk Copenhagen, 2004.
- Andersen D, Hilsted J, Riis P, Walter S. The DBCG case. Annual Report, The Danish Committee on Scientific Dishonesty, DCSD, Copenhagen 1995; 79–81
- Danish Breast Cancer Cooperative Group (eds). Protocol collection of DBCG manuals. DBCG Secretariat, Copenhagen 1982;2:1–121. ( In Danish.)
- Ottesen G, Andersen JA, Blichert-Toft M, Axelsson C. Frequency and types of chest wall recurrences among node negative breast cancer patients. Acta Oncol 1988; 27: 601–4
- Zedeler K. Assessment and presentation of survival experience in the Danish Breast Cancer Cooperative Group. Acta Oncol 1988; 27: 649–62
- Langlands AO, Pocock SJ, Kerr GR, Gore SM. Long-term survival of patients with breast cancer: a study of the curability of the disease. Br Med J 1979; 2: 1247–51
- Rutqvist LE, Wallgren A, Nilsson B. Is breast cancer a curable disease? A study of 14731 women with breast cancer from the cancer registry of Norway. Cancer 1984; 53: 1793–800
- Blichert-Toft M, Mouridsen HT, Andersen KW. Clinical trials. Seminars Surg Oncol 1996; 12: 32–8
- Rutgers EJTh for the EUSOMA Consensus Group. Quality control in the locoregional treatment of breast cancer. EJC 2001;37:447–53.
- Smith TE, Lee D, Turner BC, Carter D, Haffty BG. True recurrence vs. new primary ipsilateral breast tumor relapse: An analysis of clinical and pathological differences and their implications in natural history, progress, and therapeutic management. Int J Radiat Oncol Biol Phys 2000; 48: 1281–9
- Johansen J, Overgaard J, Rose C, Engelholm SAa, Gadeberg CC, Kjaer M, On behalf of DBCG., et al. Cosmetic outcome and breast morbidity in breast conserving treatment. Results from the DBCG-82TM national randomized trial in breast cancer. Acta Oncol 2002; 41: 369–80