Abstract
Introduction. Estrogen receptor (ER) is a prognostic and predictive biomarker, which has been known for 40 years. The detection method has developed over the years from different biochemical assays (BCA) to immunohistochemistry (IHC) on paraffin embedded tissue. The aim of the present study is to describe the development in ER analysis in the Danish Breast Cancer cooperative Group (DBCG), in the period of 1977 to 2006, regarding quantity and method of analyses. To compare BCA with IHC, and to report the prognosis for low-risk breast cancer patients. Patients and methods. In the period of 1991–1993, BCA and IHC were both performed on 2 364 tumours from breast cancer patients in Denmark. Three central laboratories in Copenhagen, Aarhus and Aalborg, respectively, performed BCA, while IHC was done in each of the pathology departments participating in the study. Data on ER status, clinical variables and prognostic factors were obtained from the DBCG database. Prognosis is calculated from the DBCG protocol 89a, regarding recurrence free survival (RFS) and overall survival (OS). Results. We find an increasing frequency of ER positive tumours over time, with correlation to patient age. There is a better RFS and OS for tumours positive in both ER determinations. However, BCA is more sensitive than IHC. We find a significant correlation between positive ER status and other low risk factors, except lymph node status. Discussion. Immunohistochemistry has several advantages compared with BCA; it is decentralised, only requiring small amounts of tumour tissue, with direct light microscopic interpretation of invasive tumour cells. It is less expensive and more rapid than BCA.
Results in this study show the same RFS in both ER determinations. We conclude that IHC in analysing ER is a rapid, reliable and easy method, and we recommend the use of external quality control programme.
Estrogen receptor (ER) is a nuclear protein present in normal breast epithelial cells, as well as in a major part of breast cancers. The receptor is both a prognostic and a predictive marker, and has been known for 40 years Citation[1]. Studies have shown that patients with ER positive breast cancer tumours have a better prognosis than patients with ER-negative tumours, but in recent studies, this advantage seems to disappear after 5–15 years Citation[2], Citation[3]. The predictive value of ER positive tumours is related to the benefit from endocrine treatment Citation[4], Citation[5] as well as chemotherapy Citation[6]. The methods used to analyse the presence of ER has changed over the years, starting with biochemical assays (BCA), and was later, with the development of highly specific monoclonal antibodies, replaced by immunohistochemistry (IHC).
Analysis for ER was introduced in Denmark in 1977 and was performed in 3 central laboratories: Biochemical laboratory in Aalborg and the Danish cancer society's laboratories in Copenhagen (Fibiger laboratory) and Aarhus. All laboratories used the biochemical method, initially based on dextran coated charcoal (DCC), and later replaced by an Enzyme Linked Immuno Sorbent Assay, (ELISA) in the laboratories in Copenhagen and Aarhus. Both methods used fresh, crushed, tumour tissue. The DCC method is ligand based, and can be difficult to standardise Citation[7], Citation[8]. Studies have shown that the charcoal absorbs 10–20% of ER, with the risk of increased rate of false negative tumours Citation[7]. The ELISA method is based on antibodies, detecting ER irrespective of the ability to bind Estrogen or not. In Denmark, the BCA were standardized in 1981, with some improvement of inter-laboratory reproducibility Citation[9].
These two biochemical methods were replaced by IHC on frozen tissue sections, and finally on formalin fixed, paraffin embedded tumour tissue. With the introduction of IHC, it appeared that the ER was located in the nucleus instead of, as previously assumed, in the cytoplasm Citation[10].
The IHC has the advantage that determination can be decentralized and performed in any laboratory familiar with the technique. The analysis is suitable even for very small tumours, in contrast to BCA. It is less expensive and less time consuming. Using paraffin embedded tissue; it is also suitable for retrospective studies. The immunohistochemical stained glass slides are examined by light-microscope, facilitating the pathologist interpretation of the result, distinguishing between normal/benign epithelial cells, invasive tumour cells, and in situ lesions. According to the Danish Breast Cancer cooperative Group (DBCG) guidelines, a tumour is considered ER positive when at least 10% of the invasive tumour cells show a detectable nuclear staining Citation[11]. The result is reported as the percentage positive tumour cells, without taking the staining intensity into account.
Since 1977, the DBCG database has collected nationwide information regarding ER status from patients undergoing breast cancer surgery.
The aim of the present study is:
To describe the development in the analysis of ER, regarding the quantity, type and method of analyses, in DBCG in the period of 1977 to 2006;
To compare BCA and IHC ER analyses, in the period of 1991–1993, from a previous unpublished study;
To report the prognosis for breast cancer patients in the low-risk group, who underwent surgery in the period of 1989–1998.
Patients and methods
Patients
In the years 1991–1993, 5 816 patients underwent breast cancer surgery, and were reported to the DBCG database. One thousand four hundred and seventy four (25%) were treated in centres not included in the study, leaving 4 342 (75%) patients. Among these 4 342 patients, BCA and IHC were both performed in the analysis for ER in 2 364 (54%) while the remaining 1 978, (46%) were only analysed with one method, or not at all. The main reason for failure of performing both analyses was in case of a small tumour without sufficient amount of tumour tissue.
Data on ER determination and other clinical variables were obtained from the DBCG database.
Methods
The BCA were performed in three central laboratories; biochemical laboratory in Aalborg, Danish cancer society's laboratories in Copenhagen (Fibiger laboratory) and Aarhus. The laboratory in Aalborg used DCC. A positive tumour was defined as ≥10 fmol receptor/mg protein. The laboratories in Aarhus and Copenhagen used Abbott's ELISA. A positive tumour also being defined as ≥10 fmol receptor/mg protein. All three laboratories participated in a national and an international quality control programme regarding receptor analyses.
The IHC analyses were performed in each of the pathology departments participating in the study, using Abbott's ER immunohistochemical assay (ER-IHC). Receptor positive tumours were defined as ≥10% positive tumour cells, with a detectable nuclear staining in the invasive component of the tumour.
The study compares the BCA with frozen-section IHC, and the latter with paraffin-section IHC.
Prognosis is calculated from the DBCG protocol 89a (1989–1998), which included low risk patients, not receiving adjuvant therapy. Including ER negative (ER − ) tumours in post menopausal women aged 70–74.
Statistical analyses
Categorical data are shown by their frequencies. Test for independence are performed using the χ2 test. Comparisons of binary outcome of the three assay methods are done using McNemar's test and the degree of agreement given by the kappa statistic including its 95% confidence limits. Survival probabilities for both recurrence free survival (RFS) as well as overall survival (OS) are estimated by the Kaplan-Meier method. Survival data are analyzed for DBCG program DBCG89 stratified by protocol a. Comparison between categories is tested by the log rank test as well as the Wilcoxon test. The latter test is more sensitive to differences in the early part of the follow-up period. In addition, the hazard ratio with 95% confidence limits is given based on the Cox proportional hazards model. The proportional hazards assumption is assessed by graphical methods. The calculations are done using SAS (SAS Institute, v 9.1, Cary, N.C. USA). P-values less than 5% are considered significant.
Results
shows the frequency of ER analyses and ER positive (ER + ) tumours in the protocols 1977, −82 and −89.
Table I. Frequency of ER analyses and positivity in 3 DBCG protocols.
There is an increase in ER+ over time. In the protocols from 1977 and 1982, all tumours were analysed with BCA; however, in the protocol from 1989 both BCA and IHC were used, laboratory dependent. The DCC analysis turned out to be more sensitive than the ELISA, resulting in a higher frequency of ER+ in the laboratory using the DCC method (Aalborg) (test for independence p = 0.0005, data not shown).
The comparison of the results of BCA and IHC (frozen tissue) analyses is shown in .
Table II. Biochemical (BCA) and immunohistochemical (IHC) ER analysis in 2 364 tumours.
The frequency of ER positive tumours detected with BCA (BCA + ) is 81%, versus 68.0% in ICH (IHC + ) (p < 0.0001, Kappa = 0.58).
A similar comparison between the BCA and IHC on paraffin embedded tissue (215 tumours) shows a frequency of 82% ER+ in BCA versus 62% in IHC, (p < 0.0001, Kappa = 0.47). Today, the frequency of ER+ tumours in the DBCG database is 77% Citation[11].
When comparing IHC analyses in frozen tumour tissue with formalin fixed, paraffin embedded tissue, on 343 tumours there was a very close correlation, with a kappa value of 0.759 (p = 0.87 McNemars test), (data not shown).
Analysis of time to recurrence and time to death of any cause has been done in the systematically untreated patients accrued in the 89a protocol (n = 746). Kaplan-Meier estimates of survival of patients stratified by the result of the combination of the BCA and IHC, i.e. ER − /−, ER + /−, ER − /+ and ER + /+ are shown in a and b (RFS and OS respectively). The log rank statistic cannot demonstrate a significant difference; however, comparing the BCA − /IHC− group with the BCA + /IHC+ does demonstrate significance using the Wilcoxon test (p = 0.02). Restricting analysis of RFS to the first 5 years shows a hazard ratio of 0.67 (ER+ versus ER−, 95%CI: 0.46–0.96, p = 0.03; log rank test) between the BCA − /IHC− group and the BCA + /IHC+ group. Patients with negative IHC determination but positive BCA have a RFS very similar to patients positive in both determinations, and better RFS than patients negative in both determinations, however, this is not significant (p = 0.26). A significant difference is shown for OS, the hazard ratio between the positive and negative groups is 0.31 (95% CI: 0.21–0.45, p < 0.0001). Patients with positive BCA and negative IHC have OS almost equivalent to patients positive for both BCA and IHC, and this group of patients have significantly better OS than patients with negative BCA and IHC determinations (p = 0.01). The number of patients in the discordant groups is too small for statistical inference.
Figure 1. Kaplan-Meier estimates of recurrence free survival (A) and overall survival (B) for patients in 89A. The strata are BCA − /IHC−, BCA + /IHC−, BCA − /IHC+ and BCA + /IHC+. Patients at risk for each stratum are shown below the axis at times 0 (from operation), 24, 48, 72 and 96 months. The number of events is shown to the left of each stratum.
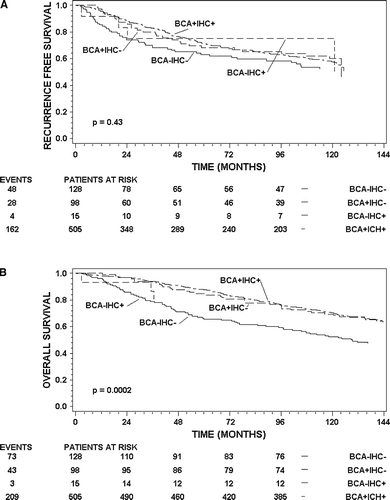
shows other prognostic factors, clinical variables and ER status according to method of analyses in the patients treated in the period of 1991–1993. There is an increasing frequency of ER+ tumours with increasing patient age in both BCA and IHC. The number of grade I tumours is significantly higher in the ER+ group, compared with the ER−, in both determinations, but it is more pronounced in the BCA. The other prognostic factors show that ER+ tumours and low risk factors are correlated, with the exception of axillary lymph node status, where no difference between node positive and node negative tumours and ER status is observed for BCA. The BCA + /IHC− have a better prognosis than BCA − /IHC−, and the same prognoses as BCA + /IHC+.
Table III. Prognostic factors and ER receptor status in 2 364 patients tested with both biochemical and immunohistochemical analyses.
Multivariate Cox regression analysis including covariates from cannot demonstrate significant prognostic impact for RFS similar to the univariate analysis, however the hazard ratio for BCA + /IHC+ and BCA + /IHC− are almost equal (0.87 and 0.81). The same analysis for OS shows a significant value for BCA+ versus BCA− (p = 0.04, HR = 0.70, 95% CI 0.50–0.99) and a near significant result for IHC+ versus IHC− (p = 0.05, HR = 0.75, 95% CI: 0.55–1.00).
Discussion
There are several advantages in using IHC compared with BCA; it can be performed decentralized in the pathology departments, hereby saving time. It is possible to test even small tumours, and needle biopsies, thus reducing the number of tumours with unknown ER status. It has the ability to distinguish between normal/benign epithelial cells, invasive tumour cells and in situ lesions, giving a more precise result, and it is less expensive. With the introduction of IHC analyses for ER, BCA was soon replaced, and IHC is now solely performed on formalin fixed, paraffin embedded tissue in Danish laboratories.
The data shows that the BCA is more sensitive than IHC in detecting ER positivity. We found that the DCC method was more sensitive than the ELISA method, in contrast to Andersen et al., who found the ELISA to be more sensitive than DCC Citation[7]. The difference between the BCA and IHC is not statistically significant regarding RFS, but it is statistically different regarding OS. Studies by Harvey et al. and Regan et al. shows a discordance of 10–30% when comparing BCA with IHC Citation[4], Citation[6], depending on the cut-off level. In the study by Regan et al., comparing BCA with IHC in analysing ER on tumours from 1547 patients, results similar to ours were found Citation[6].
Our study shows increasing frequency of ER+ tumours over time, a result confirmed by Benzon et al. Citation[3]. They also demonstrated a correlation with patient age, with more frequent ER+ tumours among older patients, and they found that the positive prognosis of the ER+ tumours is diminished after 5 years, a finding we can also detect. Likewise, we also found increasing frequency with increasing age. With regard to the relation between ER-positivity and other prognostic factors, we found somewhat surprising that there was only a week significant relation between ER and a positive lymph-node status in the IHC analysis and none with the BCA.
Axillary lymph node status is a strong prognostic marker, and we would expect a correlation between ER+ tumours and negative lymph node status. Estrogen receptor has been regarded as a surrogate marker for differentiation and grade of malignancy, in contrast to lymph node status, which is not a differentiation factor. This might explain our findings.
Patients are allocated to endocrine therapy on the basis of the results of the ER analyses. It is important to distinguish between patients who will benefit from a specific treatment, from patients who will not benefit, to avoid useless treatment, perhaps with adverse side effects. It is therefore important, that these analyses are reliable. Thus there is a need for external quality control. There are several options for a laboratory to participate in a quality control programme, e.g. the British UK National External Quality Assessment Scheme (UK-NEQAS) Citation[5] and Nordic Immunohistochemical Quality Control (NordiQC) Citation[12], based in the Nordic countries.
In conclusion we find that IHC in determining ER status is a reliable method of analysis, with results that correlates well with the BCA. However, we do find a small population of patients with discordant determinations in the two methods; BCA + /IHC− and BCA − /IHC+, the latter being very small, but we do not believe that this difference justifies not using IHC. We highly recommend laboratories performing ER analyses to participate in an external quality control programme.
Acknowledgements
We wish to thank The Danish Cancer Society for financial support.
References
- Jensen EV, Suzuki T, Kawashima T, Stumpf WE, Jungblut PW, DeSombre ER. A two step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci USA 1968; 59: 632–8
- Dowset M. Estrogen receptor; methodology matters. J Clin Oncol 2006; 24: 5626–8
- Benzon N, Düring M, Rasmussen BB, Mouridsen H, Kroman N. Prognostic effect of estrogen receptor status across age in primary breast cancer. Int J Cancer 2008; 122: 1089–94
- Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 1999; 17: 1474–81
- Rhodes A, Jasani B, Balaton AJ, Barnes DM, Miller KD. Frequency of oestrogen and progesterone receptor positivity by immunohistochemical analysis in 7016 breast carcinomas: Correlation with patient age, assay sensitivity, threshold value, and mammographic screening. J Clin Pathol 2000; 53: 688–96
- Regan MM, Viale G, Mastropasqua MG, Maiorano E, Golouh R, Carbone A, et al. Re-evaluating adjuvant breast cancer trials: Assessing hormone receptor status by immunohistochemical versus extraction assays. JNCI 2006; 98: 1571–81
- Andersen J, Bentzen SM, Poulsen HS. Relationship between radioligand binding assay, immunoenzyme assay and immunohistochemical assay for estrogen receptors in human breast cancer and association with tumor differentiation. Eur J Cancer Clin Oncol 1988; 24: 377–84
- Poulsen HS. Oestrogen receptor assay–Limitation of the method. Eur J Cancer 1981; 17: 495–501
- Thorpe SM, Poulsen HS, Pedersen KO, Rose C. Impact of standardization of estrogen and progesteron receptor assays of breast cancer biopsies in Denmark. Eur J Cancer Clin Oncol 1988; 24: 1263–9
- Poulsen HS, Ozzello L, King WJ, Greene GL. The use of monoclonal antibodies to estrogen receptors (ER) for immunoperoxidase detection of ER in paraffin sections of human breast cancer tissue. J Histochem Cytochem 1985; 33: 87–92
- www.dbcg.dk.
- www.NordiQC.org.