Abstract
A significant reduction in the risk of recurrence and death was achieved three decades ago with adjuvant chemotherapy in patients with operable breast. The major pivotal trials used oral cyclophosphamide (C) days 1–14 with intravenous methotrexate (M) and fluorouracil (F) on days 1 and 8, repeated every 28 days. The classical CMF has later been modified as concerns dose and schedule, without formal comparisons in randomised trials between the classical CMF and the modifications. Material and methods. Classical CMF was used in the first adjuvant chemotherapy trial performed by the Danish Breast Cancer Cooperative Group (DBCG), and two succeeding randomised trials in premenopausal patients with node positive breast cancer used three-weekly or four-weekly intravenous CMF in one of the treatment arms. Results. Between November 1977 and January 2001 these trials included 2 213 patients who in addition to surgery and radiotherapy received CMF. Ten-year disease-free survival (DFS) rates were 48% following classical CMF, 45% following four-weekly and 47% following three-weekly CMF. Major differences in patient characteristics were observed across these three cohorts, and a multivariate analysis was performed adjusting for the known prognostic factors. In the adjusted analysis a 30% increase in the risk of recurrence was observed for two the intravenous regimens as compared to classical CMF. As concerns survival a significant 40% increase in the risk of death was observed with the four-weekly regimen, while a similar risk of death was observed with the three-weekly intravenous. Classical CMF was associated with a higher risk of amenorrhoea, and this may at least in part explain an observed interaction between age and efficacy. Discussion. This cross trial comparison suggests a detrimental effect in premenopausal patients with node positive breast cancer when shifting from classical CMF to intravenous regimens with lower dose-intensity. Caution is required in the interpretation of these results due to the non-experimental study design.
Combination chemotherapy was exploited in metastatic breast cancer following its successful introduction in the treatment of Hodgkin's disease and other haematological diseases. Early phase II reports suggested that response rates of 80 to 90% could be obtained with multi-drug regimens Citation[1], Citation[2]. Superiority of combination chemotherapy over single agent therapy was later confirmed by randomised trials, with response rates of 50–60% compared to 20–25% Citation[3], Citation[4].
Four-weekly oral cylophosphamide 100 mg/m2 days one to fourteen in combination with intravenous methotrexate 40 mg/m2 and 5-fluorouracil 600 mg/m2 days one and eight was the first efficacious adjuvant combination regimen used in early breast cancer and was later named classical CMF Citation[5]. In non-randomised comparisons the Milan group observed no detrimental effects switching from classical CMF to twelve cycles of three-weekly intravenous CMF [600 mg/m2, 40 mg/m2, 600 mg/m2) Citation[6]. Based on indirect comparisons others have however hypothesized that classical CMF might be superior to intravenous CMF in the adjuvant setting Citation[7], but a direct comparison in a randomised trial has never been undertaken. In advanced breast cancer, a single small phase III trial has demonstrated superiority of classical CMF over three-weekly intravenous CMF Citation[8]. The meta-analyses performed by the Early Breast Cancer Trialists’ Collaborative Group have confirmed that CMF improves disease-free survival and overall survival in patients with operable breast cancer, but have not explored the possible differences between classical and intravenous CMF Citation[9].
The randomised trials attached to DBCG programs 77, 82 and 89 all included a treatment arm for premenopausal patients consisting exclusively of CMF combined with radiotherapy. Classical CMF used for 12 months in the 77 program was replaced by four-weekly intravenous low-density CMF in the 82 program, followed by three-weekly intravenous and intermittent dose-intensity CMF in the 89 program. Patients in the three programs were included population based and nationwide, were treated according to standardized flow sheets and reported prospectively to the DBCG Registry. In absence of randomised trials, we have undertaken an indirect comparison of the three different CMF regimens used in the early DBCG programs.
Material and methods
The Danish Breast Cancer Cooperative Group (DBCG) founded a population-based registry in 1977 and nation-wide diagnostic, therapeutic, and follow-up data has since been reported to the DBCG registry by the use of standardized forms Citation[10]. Virtually all involved Danish departments mutually implemented these uniform guidelines for diagnostic procedures, surgery, radiotherapy, systemic therapy, and follow-up for early breast cancer.
Patients
The current analysis included women who were treated with CMF and radiotherapy according to DBCG 77, 82 or 89 program guidelines, had a completely resected unilateral invasive carcinoma of the breast and no signs of distant metastasis as determined by routine examinations (physical examination, clinical chemistry, chest radiography, and other examinations as indicated). Patients included were premenopausal (normal menstrual period within 2 months, normal menstrual period within 12 months and FSH in the premenopausal range, or aged 50 years or younger with at least one ovary preserved at hysterectomy) with node positive tumors, defined as metastasis to at least one lymph node. Axillary sampling or clearance (level I and part of level II) in combination with breast-conserving surgery or mastectomy was required. Patients receiving endocrine therapies, e.g. tamoxifen or ovarian suppression, were not included.
Pathological procedures
Classification of histological type and grade (ductal carcinomas) according to WHO, examination of tumour margins, invasion into skin or deep fascia, measurement of gross tumour size, total number of lymph nodes identified and number of metastatic nodes was reported prospectively.
Treatment
Patients in the three cohorts were treated according to the formerly active treatment programs, whether enrolled or not enrolled in the randomized trial attached to each of the programs. Data from all patients in the three cohorts was collected prospectively and accumulated centrally by the DBCG Registry by the use of standardized forms. Apart from randomization, the DBCG Data Center undertook the same procedures in all patients, including centrally review, querying, and analysis of data. The randomised trials attached to DBCG program 77, 82 and 89 are described briefly:
DBCG trial 77-B
Premenopausal patients were randomised to radiotherapy alone, or radiotherapy combined with CMF, single agent cyclophosphamide or levamisole Citation[11]. Patients assigned to radiotherapy plus CMF received twelve cycles of CMF (cyclophosphamide 80 mg/m2 orally days 1–14, methotrexate 30 mg/m2 intravenously day 1 and 8, and 5-fluorouracil 500 mg/m2 intravenously day 1 and 8) every 4 weeks concomitant with radiotherapy. The radiotherapy was given to the chest wall and regional lymph nodes with an intended total dose equivalent to1345 ret (NSD). Two different schedules were used (40.92 Gy in 22 fractions, 5 fractions per week, and 36.60 Gy in 12 fractions, 2 fractions per week).
DBCG trial 82-B
Premenopausal patients were randomised to radiotherapy plus CMF, CMF alone, or CMF plus tamoxifen Citation[12]. In patients assigned to radiotherapy plus CMF the timing was the following: One week after the first cycle of intravenous CMF (600, 40, and 600 mg/m2) patients received radiotherapy for 5 weeks with 48–50 Gy in 22–25 fractions to the chest wall and regional lymph nodes. Chemotherapy was resumed 1–2 weeks after completion of radiotherapy with seven further cycles of CMF given intravenously with 4 weeks intervals.
DBCG trial 89-B and trial 89-D
Premenopausal patients with hormone receptor positive tumours were in trial 89-B randomised to radiotherapy plus ovarian ablation versus radiotherapy plus CMF Citation[13], and premenopausal patients with hormone receptor negative tumors were in trial 89-D randomised to radiotherapy plus CMF or radiotherapy plus CEF Citation[14]. Trial 89-D permitted secondary randomisation to pamidronate 150 mg given orally twice daily for 4 years against control. In both trials patients assigned to CMF plus radiotherapy received one or two cycles of CMF (600, 40, and 600 mg/m2) before radiotherapy and one or two cycles of single agent cyclophosphamide (850 mg/m2) concomitant with radiotherapy followed by CMF to a total of nine cycles of chemotherapy. Radiotherapy was given against the residual breast following lumpectomy (48 Gy with a 10 Gy boost) or chest wall following mastectomy (48 Gy) and regional nodes (48 Gy), all in 2 Gy fractions and 5 fractions per week.
Follow-up
Treatment related adverse events and findings on clinical examination were recorded every three months during the first year, then every six months during the second through the fifth year, and thereafter annually for a total of 10 years. A complete follow-up on vital status was obtained for all patients through linkage to the Danish Central Population Registry. Haemoglobin, white blood cell count, and platelet count, were examined on day one of each chemotherapy cycle. Additional biochemical tests and imaging examinations were done when indicated by existing symptoms or signs.
Statistical analysis
The DBCG Data Center undertook central review, querying, and analysis of data. Follow-up time was quantified in terms of a Kaplan-Meier estimate of potential follow-up. OS was calculated as the elapsed time from the date of definitive surgery until death, irrespective of cause of death. DFS was defined as the duration of survival without invasive loco-regional recurrence, distant metastases, contralateral invasive breast cancer, second primary nonbreast invasive cancer or death irrespective of cause. OS and DFS were analysed unadjusted and treatment regimens were compared using the log-rank test. For multivariate analysis the Cox proportional hazards regression model was applied to assess the adjusted hazard ratio of treatment regimen, and to explore interactions. Factors included in the multivariate analyses were treatment cohort (77, 82, and 89), age (≤40, 41–45, 46–50, 51–59), tumour size (<21 mm, 21–50 mm, >50 mm), nodal status (1–3, 4–9, ≥10 positive combined with 1–3, 4–9, ≥10 examined), histological type and grade (ductal grade I and unknowns, ductal grade II, ductal grade III, other histological types), and hormone receptor status (ER or PgR positive, both negative or one negative and the other unknown, both unknown). Type of surgery had no significant impact and was excluded. Interactions between treatment cohort and the covariates age, positive lymph nodes and tumour size were investigated in categories and in separate models. The assumptions of proportional hazards were assessed by Schoenfeld residuals, and by including in the model a time-dependent component for each covariate. The hazard rate of histological type and grade as well as hormone receptor status was not proportional and therefore stratification was used. Associations between regimen and other characteristics (excluding unknowns) were analysed by χ2 test. P-values are two-tailed. Statistical analyses were done with the SAS 8.2 program package.
Results
A total of 5 652 premenopausal and node positive breast cancer patients were registered onto the DBCG Registry between November 1977 and January 2001, and 2 213 of these patients received CMF in addition to loco-regional radiotherapy. Among the 2 213 patients 323, 982 and 908 had surgery according to the DBCG 77, DBCG 82, and DBCG 89 program and postoperatively they exclusively received CMF and radiotherapy according to the treatment guidelines within each of the three DBCG programs. A complete follow-up for survival was achieved for all 2 213 patients. All patients were included in the analyses of DFS and OS. Significant differences (p < 0.05) were however identified within the three cohorts with reference to age, type of surgery, excised and positive lymph nodes, tumour size, malignancy grade and hormone receptor status ().
Table I. Patient characteristics.
Study outcome
This analysis was conducted for both DFS and OS with a cut-off date 10 years after the date of the definitive surgery. The total number of first events during the 10-year period is in displayed in . The 10-year DFS rates were 47.6% (95% CI, 41.6% to 53.6%) with CMF in the 77 cohort, 45.4% (95% CI, 41.9% to 48.9%) in the 82 cohort, and 47.1% (95% CI, 43.3% to 50.9%) in the 89 cohort. No statistically significant difference was detected in the pair wise comparisons of DFS, and the unadjusted hazard ratios were 1.06 (95% CI, 0.88 to 1.27, p = 0.55) for the 82 cohort, and 1.07 (95% CI, 0.89 to 1.29, p = 0.48) for the 89 cohort as compared to the 77 cohort, respectively ().
Figure 1. Disease-free survival (DFS) comparison between the 77 and 82 programs (Panel A) and the 77 and 89 programs (Panel B).
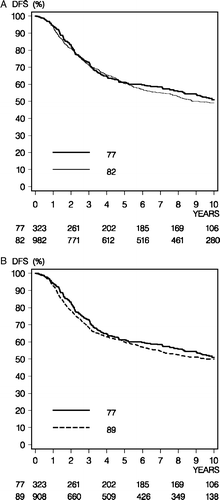
Table II. End-points.
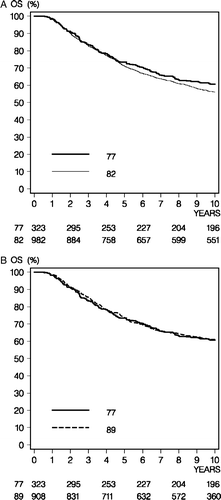
Ten-year OS rates were 56.9% (95% CI, 51.1% to 62.7%) in the 77 cohort, 52.3% (95% CI, 49.0% to 55.7%) in the 82 cohort, and 57.1% (95% CI, 53.6% to 60.6%) in the 89 cohort. No statistically significant difference was detected in the pair wise comparisons of OS, and the unadjusted hazard ratios were 1.14 (95% CI, 0.93 to 1.39, p = 0.20) for the 82 cohort, and 0.99 (95% CI, 0.81 to 1.21, p = 0.90) for 89 cohort as compared to the 77 cohort, respectively ().
Figure 2. Overall survival (OS) comparison between the 77 and 82 programs (Panel A) and between the 77 and 89 programs (Panel B).
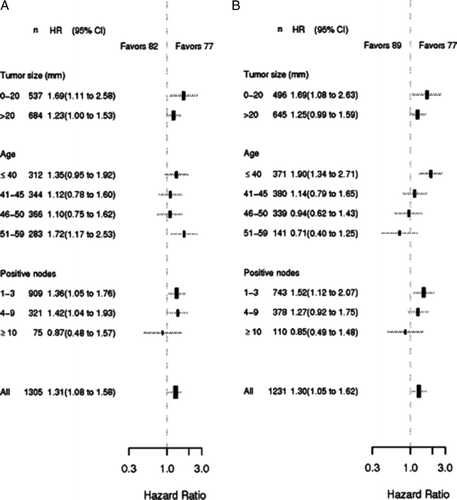
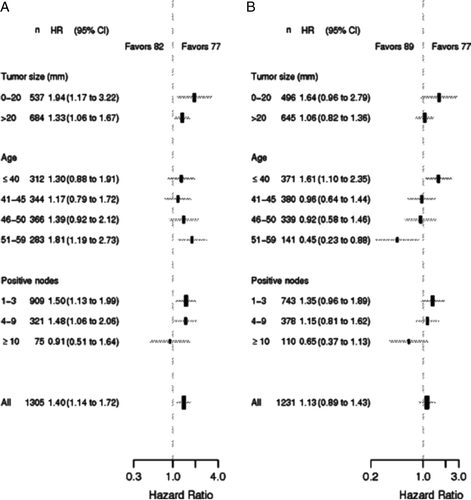
Major differences were observed in patient characteristics across the three cohorts, and multivariate analysis evaluating the 82 and 89 cohorts against the 77 cohort was carried out for DFS () and OS (). The prognostic factors included in the Cox model were treatment cohort, age, nodal status, tumour size, hormone receptor status, and histological type and grade. Type of surgery had no significant impact and was excluded (data not shown). In the multivariate model DFS was significantly longer in the 77 cohort as compared to the 82 and 89 cohorts, and the adjusted hazard ratios was 1.31 (95% CI, 1.08 to 1.58, p < 0.01) for the 82 cohort, and 1.30 (95% CI, 1.05 to 1.62, p = 0.02) for the 89 cohort as compared to the 77 cohort, respectively. A significant difference was found in OS between the 82 and 77 cohorts, adjusted hazard ratio 1.40 (95% CI, 1.14 to 1.72, p < 0.01), but not between the 89 and 77 cohorts, adjusted hazard ratio 1.13 (95% CI, 0.89 to 1.43, p = 0.32). Only minor and non-significant differences were observed in hazard ratios for DFS and OS according to age, tumour size and no. of positive nodes when comparing the 82 cohort to the 77 cohort (A and 4A). B however shows a qualitative interaction between age and DFS (p < 0.01). Patients 40 years or younger have a significant better DFS in the 77 cohort in contrast to patients older than 50 years who have a significant better DFS in the 89 cohort. Likewise a significant survival advantage is observed for patients 40 years or younger in the 77 cohort and for patients older than 50 years in the 89 cohort (p < 0.01).
Toxicity
No treatment related deaths were reported. Patients in the 89 cohort had less nausea and vomiting compared to patients in the 77 and 82 cohorts (p < 0.01), while complete alopecia were more frequent among patients in the 77 cohort (23%), compared to the 82 cohort (9%), and the 89 cohort (6%) (p < 0.01) (). Only 8% of the patients in the 77 cohort had regular menses throughout chemotherapy compared to 16% in the 82 cohort and 23% in the 89 cohort. The discrepancy was most pronounced in patients 40 years or younger were 15% of the patients in the 77 cohort had regular menses throughout chemotherapy compared to 37% in the 82 cohort and 47% in the 89 cohort. The mean dose intensities (mg/m2/week) of cyclophosphamide, methotrexate and fluorouracil are illustrated in and . Radiotherapy was in all three cohorts initiated after completion of the first cause of chemotherapy. Chemotherapy was continued during radiotherapy in the 77 cohort, while chemotherapy was suspended during radiotherapy in 82 cohort and CMF was substituted with single agent cyclophosphamide (850 mg/m2) in the 89 cohort. The mean relative dose intensities (actual/planned mg/m2 per time unit) were 67%, 87% and 88%, respectively, in the 77, 82 and 89 cohorts. The mean actual dose intensity (mg/m2/week) of cyclophosphamide was 195.3 (95% CI, 192.1 to 198.6) during the first 24 weeks in 77 cohort compared to 136.2 (95% CI, 135.2 to 137.1) in the 82 cohort and 194.3 (95% CI, 193.1 to 195.6) in 89 cohort (A). The mean of the total dose of cyclophosphamide was 8 408 mg/m2 in the 77 cohort, compared to 4 234 mg/m2 in the 82 and 5 316 mg/m2 in the 89 cohorts.
Figure 5. Delivered dose-intensity of cyclophosphamide (Panel A), methotrexate (Panel B) and fluorouracil (Panel C) (weekly dose in mg/m2) in the 77 (•) 82 (▪) and 89 (▴) programs.
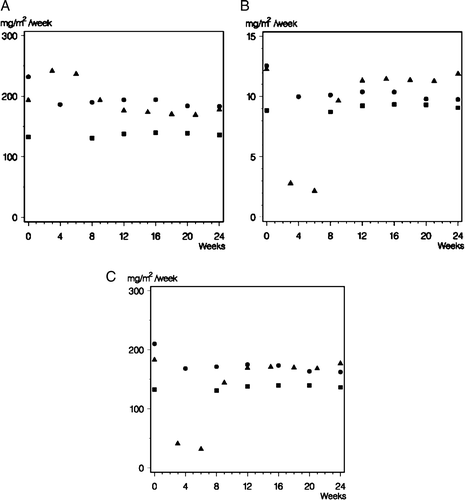
Table III. Treatment-related adverse effects (Modified WHO criteria).
Table IV. Planned dose-intensity.
Discussion
The results of this retrospective and unplanned cross trial comparison demonstrated superior efficacy of classical CMF containing oral cyclophosphamide when compared to intravenous CMF regimens with lower dose intensity and in particular when compared to a four weekly, low dose-density CMF regimen. A shift from classical CMF to intravenous and less dose-intensive 3-weekly or 4-weekly CMF regimens was accompanied by 30% increase in the risk of recurrence in the adjusted analysis. In addition the four-weekly CMF was associated with a 40% increase in the risk of death, while the risk of death was similar for three-weekly intravenous and classical CMF. The doses of CMF were delivered without major deviation from the prescription in the 82 protocol, while drug doses were modified in the 77 and 89 cohorts mainly according to toxicities as shown in . The prescribed drug doses were higher in the 77 protocol as compared to the 89 protocol, but the delivered dose-intensity was almost similar. The total dose delivered was however considerable higher of all three drugs in the 77 cohort as compared to the 82 and 89 cohorts.
A qualitative interaction was observed between age and efficacy of classical CMF contra 3-weekly intravenous CMF. This may be mediated by amenorrhea occurring more frequently especially in younger patients following classical CMF as compared to 3-weekly intravenous CMF, and the benefits of classical CMF may therefore in part be ascribed to an endocrine effect Citation[15].
Classical CMF has not been compared in randomised adjuvant trials to intravenous regimens with lower dose-intensity. Increased dose-intensity or total dose of intravenous cyclophosphamide was not associated with a prolongation of disease-free or overall survival in NSABP trials B-22 or B-25 Citation[16], Citation[17]. Different dose-intensities of anthracycline containing regimens have likewise been compared directly in randomised trials. Outcome was worse in a trial comparing low dose (30 or 40 mg/m2) to standard dose (60 mg/m2) doxorubicin while escalation from 60 to 75 or 90 mg/m2 was associated with no benefits Citation[18], Citation[19]. Standard dose of epirubicin (100 mg/m2) was likewise superior to lower dose (50 mg/m2) in a French and small Belgian trial Citation[20], Citation[21].
The efficacy of adjuvant chemotherapy with CMF has most clearly been demonstrated in premenopausal patients with node positive breast cancer Citation[9], who we accordingly selected for this retrospective analysis. Ovarian ablation and tamoxifen may modify the effect of chemotherapy in patients with endocrine responsive tumours, and as a consequence we excluded patients who received combined endocrine- and chemotherapy. Another possible source of bias could emerge from improvements in loco-regional control following refinements in surgical procedures. To counteract bias from loco-regional therapies we restricted this comparison to patients in three consecutive cohorts, in whom surgical and radiotherapy was given according to DBCG guidelines. Despite our efforts to minimize bias indirect comparisons, including the current, are prone to bias and major differences were observed between patient characteristics in the three groups of patients selected for the current analysis. We used multivariate modelling to adjust for these differences but residual confounding cannot be excluded.
The current design is, despite the shortcomings, more robust than the design in several previous reports, were patients prescribed identical therapy were sub-grouped according to delivered drug doses Citation[22], Citation[23]. This analysis provides additional support to the importance of chemotherapy dose and schedule.
Acknowledgements
The following institutions participated in the study: DBCG Registry, Copenhagen, Denmark: Maj-Britt Jensen, M.Sc; Susanne Möller, M.Sc; Aalborg Hospital, Aarhus University Hospital, Aalborg, Denmark: Marianne Ewertz, MD; Aarhus University Hospital, Aarhus, Denmark, Jorn Andersen, MD; Esbjerg County Hospital, Esbjerg, Denmark: Brita Bjerregaard, MD; M.Sc; Herlev University Hospital, Herlev, Denmark: Claus Kamby, MD; Herning County Hospital, Herning, Denmark: Knud Aage Moller, MD; Naestved County Hospital, Naestved, Denmark: Preben Philip, MD; Odense University Hospital, Odense, Denmark: Soren Cold, MD; Rigshospitalet, Copenhagen, Denmark: Henning T. Mouridsen, MD; Roskilde County Hospital, Roskilde, Denmark: Peter Grundtvig, MD; Sonderborg County Hospital, Sonderborg, Denmark: Ebbe Lindegaard-Madsen, MD; Vejle County Hospital, Vejle, Denmark: Erik H. Jakobsen, MD; and Viborg County Hospital, Viborg, Denmark: Vera Haahr, MD.
References
- Greenspan EM, Fieber M, Lesnick G, Edelman S. Response of advanced breast carcinoma to the combination of the antimetabolite, Methotrexate, and the alkylating agent, thio-TEPA. J Mt Sinai Hosp N Y 1963; 30: 246–67
- Cooper, RG. Combination chemotherapy in hormone resistant breast cancer. Proc Am Ass Cancer Res 1969;10( abstr 15).
- Canellos GP, Pocock SJ, Taylor SG, III, Sears ME, Klaasen DJ, Band PR. Combination chemotherapy for metastatic breast carcinoma. Prospective comparison of multiple drug therapy with L-phenylalanine mustard. Cancer 1976; 38: 1882–6
- Mouridsen HT, Palshof T, Brahm M, Rahbek I. Evaluation of single-drug versus multiple-drug chemotherapy in the treatment of advanced breast cancer. Cancer Treat Rep 1977; 61: 47–50
- Bonadonna G, Brusamolino E, Valagussa P, Rossi A, Brugnatelli L, Brambilla C, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 1976; 294: 405–10
- Bonadonna G, Moliterni A, Zambetti M, Daidone MG, Pilotti S, Gianni L, et al. 30 years’ follow up of randomised studies of adjuvant CMF in operable breast cancer: Cohort study. BMJ 2005; 330: 217–22
- Goldhirsch A, Colleoni M, Coates AS, Castiglione-Gertsch M, Gelber RD. Adding adjuvant CMF chemotherapy to either radiotherapy or tamoxifen: Are all CMFs alike? The International Breast Cancer Study Group (IBCSG). Ann Oncol 1998; 9: 489–93
- Engelsman E, Klijn JC, Rubens RD, Wildiers J, Beex LV, Nooij MA, et al. “Classical” CMF versus a 3-weekly intravenous CMF schedule in postmenopausal patients with advanced breast cancer. An EORTC Breast Cancer Co-operative Group Phase III Trial (10808). Eur J Cancer 1991; 27: 966–70
- Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;365:1687–717.
- Andersen KW, Mouridsen HT. Danish Breast Cancer Cooperative Group (DBCG). A description of the register of the nation-wide programme for primary breast cancer. Acta Oncol 1988; 27: 627–47
- Dombernowsky P, Andersen JA, Andersen KW, Axelsson CK, Blichert-Toft M, Hansen M, et al. [Adjuvant chemotherapy in premenopausal and menopausal high-risk patients with breast cancer. 4.] Results of the DBCG (Danish Breast Cancer Cooperative Group) 77B study. Ugeskr Laeger 1991; 153: 2280–3
- Andersson M, Kamby C, Jensen MB, Mouridsen H, Ejlertsen B, Dombernowsky P, et al. Tamoxifen in high-risk premenopausal women with primary breast cancer receiving adjuvant chemotherapy. Report from the Danish Breast Cancer Co-Operative Group DBCG 82B trial. Eur J Cancer 1999; 35: 1659–66
- Ejlertsen B, Mouridsen HT, Jensen MB, Bengtsson NO, Bergh J, Cold S, et al. Similar efficacy for ovarian ablation compared with cyclophosphamide, methotrexate, and fluorouracil: From a randomized comparison of premenopausal patients with node-positive, hormone receptor-positive breast cancer. J Clin Oncol 2006; 24: 4956–62
- Ejlertsen B, Mouridsen HT, Jensen MB, Andersen J, Cold S, Edlund P, et al. Improved outcome from substituting methotrexate with epirubicin: Results from a randomised comparison of CMF versus CEF in patients with primary breast cancer. Eur J Cancer 2007; 43: 877–84
- Brincker H, Rose C, Rank F, Mouridsen HT, Jakobsen A, Dombernowsky P, et al. Evidence of a castration-mediated effect of adjuvant cytotoxic chemotherapy in premenopausal breast cancer. J Clin Oncol 1987; 5: 1771–8
- Fisher B, Anderson S, Wickerham DL, DeCillis A, Dimitrov N, Mamounas E, et al. Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-22. J Clin Oncol 1997; 15: 1858–69
- Fisher B, Anderson S, DeCillis A, Dimitrov N, Atkins JN, Fehrenbacher L, et al. Further evaluation of intensified and increased total dose of cyclophosphamide for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-25. J Clin Oncol 1999; 17: 3374–88
- Budman DR, Berry DA, Cirrincione CT, Henderson IC, Wood WC, Weiss RB, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst 1998; 90: 1205–11
- Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 2003; 21: 976–83
- Piccart MJ, Di Leo A, Beauduin M, Vindevoghel A, Michel J, Focan C, et al. Phase III trial comparing two dose levels of epirubicin combined with cyclophosphamide with cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer. J Clin Oncol 2001; 19: 3103–10
- Benefit of a high-dose epirubicin regimen in adjuvant chemotherapy for node-positive breast cancer patients with poor prognostic factors: 5-year follow-up results of French Adjuvant Study Group 05 randomized trial. J Clin Oncol 2001;19:602–11.
- Colleoni M, Price K, Castiglione-Gertsch M, Goldhirsch A, Coates A, Lindtner J, et al. Dose-response effect of adjuvant cyclophosphamide, methotrexate, 5-fluorouracil (CMF) in node-positive breast cancer. International Breast Cancer Study Group. Eur J Cancer 1998; 34: 1693–700
- Cameron DA, Massie C, Kerr G, Leonard RC. Moderate neutropenia with adjuvant CMF confers improved survival in early breast cancer. Br J Cancer 2003; 89: 1837–42