Abstract
Background. Previous analyses of TOP2A and HER2 in the Danish Breast Cancer Coopererative Group (DBCG) trial 89D suggested that TOP2A amplifications and possible also deletions are predictive markers for the effect of adjuvant epirubicin in patients with primary breast cancer. We present an updated and extended statistical analysis, requested for IVD-labeling of TOP2A testing. Material and methods. In the DBCG trial 89D 980 Danish patients were randomly assigned to nine cycles of intravenous CMF (cyclophosphamide, methotrexate, and fluorouracil) or CEF (cyclophosphamide, epirubicin, and fluorouracil). Archival tumor tissue was collected retrospectively from 806 of these patients in a prospectively designed, biological sub-study, and was successfully analyzed for TOP2A aberrations and HER2 status in 773 samples (96%). Recurrence-free survival (RFS) was the primary endpoint. Results. TOP2A aberrations (amplifications and deletions) were significantly associated with shorter RFS (p<0.0001) and overall survival (OS) (p<0.0001). Deleted cases had worse prognosis than amplified cases. In a Cox proportional hazard model TOP2A was an independent prognostic marker for RFS and OS. Patients with amplifications had a 61% reduction in the risk of an event (p=0.002) and a 51% reduction in the risk of death (p=0.01) if allocated to CEF compared to 6% and 10% in TOP2A normal patients. A similar but non-significant trend (p=0.08) was shown in patients with TOP2A deletions. Clear statistical evidence of a differential benefit, favoring CEF among patients with TOP2A aberrations was found for RFS (p=0.02 for interaction) but not for OS (p=0.14 for interaction). Conclusion. In conclusion, this updated analysis of TOP2A aberrations in DBCG trial 89D suggests a differential benefit of adjuvant chemotherapy in patients with primary breast cancer, favoring treatment with epirubicin in patients with TOP2A amplifications, and perhaps deletions. Additional studies are needed to clarify the exact importance of TOP2A deletions on outcome, but deletions have proven to be associated with a very poor prognosis.
The TOP2A gene codes for the enzyme topoisomerase IIα (topo IIα), that catalyzes the breakage and reunion of double-stranded DNA leading to relaxation of DNA supercoils. Type II topoisomerases are essential enzymes that interconvert topological forms of DNA by making transient double-stranded breaks in the DNA backbone. These enzymes play important roles in a number of fundamental nuclear processes including DNA replication, transcription, chromosome structure, condensation and segregation. The topoisomerase IIα gene, TOP2A, is present in two copies in all normal diploid cells. The TOP2A gene spans an area on chromosome 17q21 of approximately 27.5 kb and contains 35 exons encoding a 170 kDa protein Citation[1].
The topo IIα protein has been recognized as a proliferation marker and expression of topo IIα varies during cell cycle both in normal and cancerous cells Citation[2]. The expression of topo IIα in breast tumors correlates with Ki-67 expression Citation[3]. Based on a limited number of in vitro studies of different cancer types, it was initially hypothesized that sensitivity to topo IIα inhibitors was dependent on the expression level of the topo IIα protein as cell cultures with low levels were less sensitive to the drugs than cell cultures with high levels Citation[4]. Based on data from five breast cancer cell lines it was thus concluded that protein expression correlated with gene copy numbers and thus with sensitivity Citation[4]. However, subsequent studies of patient samples have failed to confirm such simple relationship for topo IIα at the protein and gene level Citation[3]. Only 20% of the topoisomerase IIα protein overexpressed cases have TOP2A gene amplification but among the TOP2A gene amplified cases 93% had overexpression of topo IIα protein Citation[5]. Topo IIα overexpression seems to be composed of several contributing factors, both the cancer-specific amplification and the elevated cell proliferation rate. The Ki-67 and topo IIα proteins are expressed in parallel, which can be interpreted as a confirmation of the influence of cell proliferation rate on topo IIα expression, even in cases with TOP2A amplification Citation[6].
Type II topoisomerases are the primary targets for anthracyclines such as doxorubicin and epirubicin, which are also termed topoisomerase inhibitors Citation[7], Citation[8]. Increasing evidence indicates that TOP2A gene aberrations rather than the topo IIα protein amount is the determining factor for the efficacy of the topoisomerase inhibitors. In contrast to HER2 where the gene dosage correlates with mRNA and protein level, such simple relationship has not been shown for TOP2A Citation[9]. In the DBCG 89D adjuvant study Citation[10] patients were randomized CEF (cyclophosphamide, epirubicin, and fluorouracil) versus CMF (cyclophosphamide, methotrexate, and fluorouracil) and this trial is well suited for answering the question about TOP2A and anthracyclines. A biological sub-study Citation[11] was designed to investigate the prognostic and predictive value of HER2 and TOP2A status. Here we present an update and supportive statistical analysis, performed in connection with the IVD-labeling of the TOP2A FISH pharmDx™ Kit and previously presented orally at ASCO Citation[12].
Material and methods
The design of the original clinical trial and the biological sub-study has been described previously in detail Citation[10], Citation[11]. DBCG trial 89D was an open-label randomized, phase III, trial comparing CEF (cyclophosphamide, epirubicin, and fluorouracil) against CMF (cyclophosphamide, methotrexate, and fluorouracil). Trials were run in parallel in node positive patients with hormone receptor positive tumors, and eligible for the 89D trial were patients who were node positive or had tumor size ≥5 cm and hormone receptor negative breast cancer, and premenopausal patients with malignancy grade II or III. The Danish Breast Cancer Cooperative Group prepared the original protocol (DBCG trial 89D) as well as the biomarker sub-study protocol (DBCG 89D/TOP2A) and The Danish National Committee on Biomedical Research Ethics approved both before activation.
Retrospective collection of tumor tissue for the DBCG 89D biomarker study
From January 1990 to January 1998, 1 224 patients were randomized in DBCG trial 89D and 980 of these were recruited in Denmark. Last follow-up was December 31, 2004. Archival paraffin embedded tissue blocks from 806 Danish patients enrolled in the trial were collected between September 2001 and August 2002 from the study sites and stored centrally (, ).
Figure 1. The study population consisted of 980 Danish patients randomized in the DBCG-89D protocol. A total of 767 patients were available for multivariate analysis. Numbers in brackets gives number of patients in the 2 treatment arms (CEF/CMF).
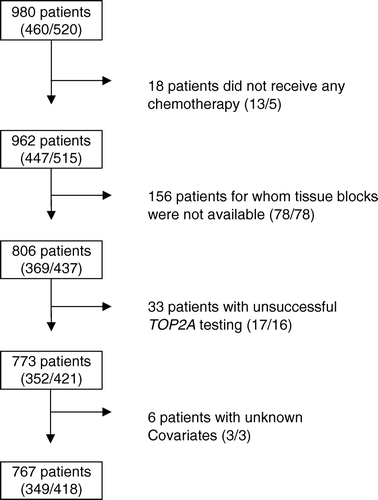
Table I. HER2 and TOP2A results (N = 773) in the 2 treatment arms of the DBCG 89D/TOP2A study.
Assessment of HER2 and TOP2A
TOP2A and HER2 gene aberrations were identified by FISH (K5333 TOP2A FISH pharmDx™ Kit and K5331 HER2 FISH pharmDx™ Kit, Dako, Denmark) and HER2 expression was visualized by immohistochemistry (K5201, HercepTest™, Dako, Denmark) according to the manufactures instructions. The FISH probes are based on combined DNA/PNA techniques Citation[13]. With respect to TOP2A and HER2 gene aberrations the ratio was calculated as the number of signals for the gene probes divided by the number of signals for the centromere 17. Cases were scored as HER2 or TOP2A FISH amplified when the ratio was ≥2. A TOP2A deletion was considered present when the ratio was <0.8. For the HercepTest™ all 1+, 2+ and 3+ positive specimens were subject to HER2 FISH analysis. The scoring of HER2 positivity was defined as HercepTest = 3+, or HercepTest = 2+ and FISH HER2 ratio ≥2.0.
Two different counting methods Citation[14] leads to identical results: Either the signals were counted in 60 nuclei or a total of 60 red signals were counted along with the green signals in the same nuclei. The latter method was used in the DBCG 89D/TOP2A study and has the advantage, that the highest number of cells will be counted in the deleted and normal cases, while the lowest number of cells will be counted in the amplified cases. Amplified cases are often obvious to identify just by looking in the microscope, but are more time demanding to evaluate if 60 nuclei should be scored. The counting methods are described in details in the package insert of the TOP2A FISH pharmDx™ Kit (download at www.dako.com).
Statistical analysis
The primary endpoint for the study was RFS, defined as the time from randomization to an event or censoring. An event is defined as relapse (local or distant), second malignancy or death, whichever comes first. A censoring is defined as ‘lost to follow-up’, ‘patient will no longer participate’ or ‘alive without disease at end of follow-up’. The secondary endpoint was OS, defined as the time from randomization to death or censoring. Because of the linkage to the Danish CPR-register, no patients are lost to follow-up in relation to OS. The effect of treatment with CEF over CMF on RFS and OS was quantified in terms of the hazard ratio, estimated using the Cox proportional hazards model. The Cox proportional hazards model was adjusted according to the results of the goodness-of-fit procedures and the investigation of interaction, defining the basic multivariate Cox model for analysis of RFS and OS. The hazards ratio (HR), the 95% confidence interval and the p-value of the Wald test was given for each covariate and interaction-term in the Cox model. The HR for treatment with CEF vs. treatment with CMF in each TOP2A-subgroup (and HER2-subgroup) was inferred from the results, and shown by a Forrest-plot. After the Cox proportional hazards model on RFS and OS was adjusted according to the results of the goodness-of-fit procedures, and interaction investigations, 3 types of secondary analyses were carried out: 1) Multivariate estimation in the 3 TOP2A-subgroups: Deleted, Normal, Amplified. 2) Multivariate estimation in the 2 HER2-subgroups: Negative, Positive. 3) Unified multivariate estimation for all patients including TOP2A and HER2 interaction. Correlations between TOP2A status and clinical and pathological variables including HER2-status were tested by χ2 test. Follow-up time was quantified in terms of a Kaplan-Meier estimate of potential follow-up. Analyses were performed for possible selection bias using the χ2 test and log-rank test. Patients with missing values, except for receptor status, were excluded from the multivariate analyses in the Cox proportional hazard model. P-values are two-tailed. Statistical analyses were done with the SAS 8.2 program package.
Results
Comparison of patients with and without material for TOP2A analysis
The assessable 767 patients differed significantly from patients not assessable due to unavailability of tumor tissue (n = 156), unsuccessfulness of TOP2A test (n = 33) or unknown covariates (n = 6) with respect to several patient and tumor characteristics (). The assessable patients had a significant larger tumor size, higher grade, and were more often postmenopausal women and had HER2-negative tumors. Age, positive lymph nodes, TOP2A status, hormone receptor status, treatment, the number of events, RFS and OS showed no significant differences between assessable and non-assessable patients.
TOP2A assessment
The TOP2A FISH analysis was successful on 773 of the 806 (96%) breast cancer samples available ().There were no significant differences between the two treatments arms and the tests results (). Amplification of TOP2A was seen in 92 (11.9%) of the 773 patients and deletion in 87 (11.3%). Totally, 179 (23.2%) of the patients had abnormal TOP2A status (). The occurrence of TOP2A aberrations were significantly associated with baseline patient and tumor characteristics, including age, menopausal status, tumor size, nodal status, estrogen receptor status, and HER2 status. TOP2A aberrations were seen in 139 (56.5%) of the 246 HER2 positive tumors and in 40 (7.6%) of the 527 HER2 negative tumors (). Thus, 40 (22.3%) patients with TOP2A aberrations would have been overlooked, if only the HER2 positive samples had been analyzed. For patients with TOP2A test results available the potential follow-up time for RFS was 9.4 years and the corresponding numbers of events were 371. The median of the potential follow-up time for OS was 11.1 years, and the numbers of events were 336.
Table II. Distribution of HercepTest, HER2 FISH and TOP2A FISH tests in the 806 available tumor samples.
Univariate analysis
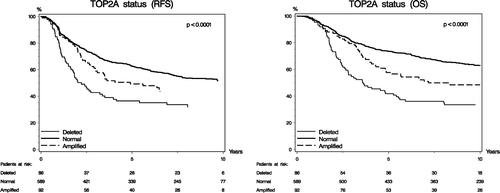
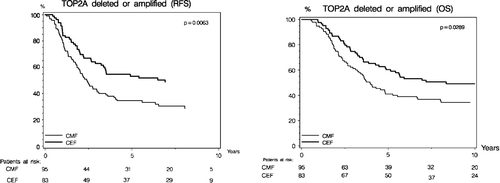
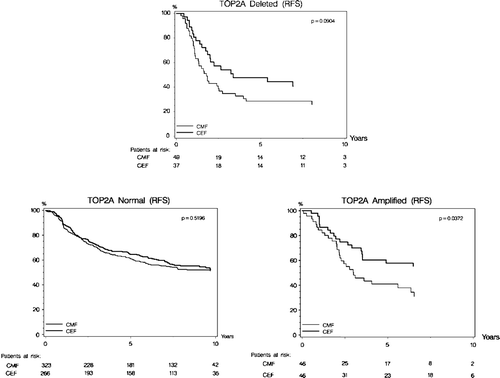
Recurrence-free survival was statistically different according to TOP2A status (p < 0.0001; ). Kaplan-Meier estimates of RFS shows that patients with TOP2A normal tumors have a significantly longer RFS as compared to patients with TOP2A amplifications or deletions. The prognostic implications of TOP2A aberrations concerning RFS differed according to treatment allocation, and RFS was significantly longer in patients randomized to CEF compared to patients randomized to CMF (p = 0.006, ). The Kaplan-Meier plots for RFS with respect to treatment allocation in each of the 3 TOP2A subgroups are shown in . Patients with TOP2A normal tumors had a significantly longer survival (p < 0.0001, ) compared to patients with TOP2A aberrations, and among patients with TOP2A aberrations those randomized to CEF had a significantly longer survival (p = 0.03, ).
Multivariate analysis
The Cox proportional hazards regression analysis of RFS was defined as the primary analysis in the original DBCG 89D/TOP2A sub-study protocol. Adjustments were made in the Cox model according to the results of the goodness-of-fit procedures and the investigation of interaction, defining the basic multivariate Cox model for analysis of RFS and OS.
TOP2A aberrations had an independent prognostic value. Using the Cox proportional hazard model it was demonstrated that a TOP2A gene aberration was associated with a significant worse prognosis both with respect to RFS (p = 0.02) and OS (p = 0.01). The HR and the 95% confidence limits based on Cox model for RFS and OS are shown in .
Table III. Interaction between TOP2A aberration and treatment with CEF.
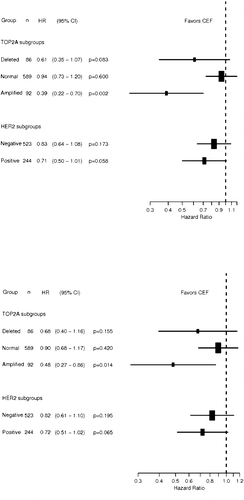
RFS differed significantly according to treatment and TOP2A status (p = 0.02 for interaction), favoring CEF among patients with TOP2A aberrations. A similar statistical evidence of a differential OS according to treatment and TOP2A status was not established (p = 0.14 for interaction). The relative effect of CEF in each of the TOP2A and HER2 subgroups are shown in with respect to RFS and OS. The group of patients with TOP2A amplifications had a 61% relative risk reduction in RFS when treated with CEF compared to CMF (HR = 0.39, p = 0.002). A non-significant trend was found for patients with TOP2A deletions (HR = 0.61, p = 0.08). Similar results were found for OS among patients with TOP2A amplifications (HR = 0.48, p = 0.01) and TOP2A deletions (HR = 0.68, p = 0.16). The predictive value of the HER2 status was also investigated and the data showed no significant effect with respect to treatment interactions, neither for RFS nor OS ().
Discussion
Our results indicate a strong impact of TOP2A copy number variation (amplifications and deletions) on recurrence-free and overall survival in patients receiving chemotherapy for early breast cancer. An independent prognostic impact of TOP2A aberrations was demonstrated in a multivariate Cox model were only lymph node status surpassed the prognostic importance of TOP2A. Less decisive but still significant independent prognostic factors were tumor size and menopausal status, while HER2 only reached borderline significance. The data also demonstrated that patients with TOP2A deletions have a worse prognosis those patients with TOP2A amplifications. For recurrence-free survival there was a clear statistical significant evidence of a differential treatment effect according to TOP2A status, and a significant interaction was established in the Cox model. Patients with TOP2A amplifications had a significant better recurrence-free survival when treated with CEF compared to CMF, and the risk of recurrence was reduced by 61% compared to 6% in patients with TOP2A normal tumors. For overall survival the estimates concerning interaction were similar, but did not reach statistical significance. One possible explanation for this difference could be that upon relapse, patients from the CMF arm received anthracycline containing treatment. No significant interaction was demonstrated between HER2 status and recurrence-free or overall survival.
The strengths of the current design were primarily that a specific hypothesis, endpoints and statistical methods were defined prospectively, as were the biomarkers. In addition, the evaluation of biomarkers were done centrally, independently and blinded. The current design has some limitations, primarily related to the retrospective collection of tissue samples. The study cohort represents 78% of the Danish patients randomized in DBCG trial 89D, and was not entirely typical of trial population. Patients with tissue not available for the study had an overall better prognosis and were less likely to have lymph nodes metastasis, large tumors or high grade (data not shown). We adjusted for these differences in the multivariate analysis, but residual confounding cannot be excluded.
The present analyses confirm our earlier primary core analysis Citation[11] of an association between TOP2A aberrations and benefit from epirubicin containing adjuvant chemotherapy. With more prolonged follow-up statistical evidence is provided of a differential effect on RFS from substituting methotrexate with epirubicin in CMF based chemotherapy according to TOP2A status.
The DBCG 89D/TOP2A study has demonstrated significant predictive and prognostic value of TOP2A gene aberrations. Deletions and amplifications showed the same negative impact on outcome and in contrast to the initial cell culture based hypothesis Citation[2], Citation[4], Citation[15], Citation[16] both patients with deletions and amplifications have benefit from anthracycline-based chemotherapy. Instead of dividing the patients into 3 groups according to TOP2A status, it seems more appropriate only to consider 2 groups: TOP2A normal and abnormal. The topo IIα protein level in the TOP2A deleted patients was initially assumed to be low based on cell line data Citation[4], but when studied in patient samples neither mRNA nor protein levels were decreased among patients with TOP2A deletion Citation[9]. This study also reports data showing that polysomy of chromosome 17 does not affect the HER2 expression. Similar data for topo IIα expression is not available, but polysomy of chromosome 17 is a highly debated subject and is currently under investigation in many studies including the DBCG89/TOP2A study.
Based on the comparisons to HER2 status it can be concluded that the HER2 status and TOP2A status are not interchangeable neither for the predictive or the prognostic value. While HER2 is the molecular target of Herceptin, topo IIα is the molecular target for the pharmacological action of epirubicin and the study has demonstrated that a TOP2A gene aberration, especially amplifications, is useful as a predictive marker for the response to epirubicin. Anthracycline-based chemotherapy with doxorubicin or epirubicin is among the most active regimens in breast cancer Citation[17]. However, these compounds possess significant acute and long-term serious side effects, such as cardiotoxicity and leukemia.
The DBCG 89D/TOP2A study Citation[11], Citation[12] reports TOP2A gene amplification in 12% of breast cancers and deletions with approximately equal frequency when both the HER2 positive and negative tumors are included in the studies. The Canadian NCIC CTG MA.5 study Citation[19] with a design very similar to DBCG 89D, confirmed the 12% TOP2A amplifications while only 7% deletions were found. Some of this discrepancy could be due to variations in the selection of cells for FISH signal counting. The Canadian and the Danish studies are among the few that allows assessment of the clinical importance of TOP2A deletions and in contrast to the initial hypothesis, both studies shows that patients with TOP2A deletion have a very poor prognosis and seems to benefit from anthracycline-based therapy. Initially, it was assumed that TOP2A gene copy variants, as a result of amplification or deletion, were restricted to HER2 amplified tumors Citation[4], Citation[15] but copy number variants of the TOP2A gene have been detected in tumor samples with normal HER2 gene status Citation[11], Citation[12], Citation[14], Citation[18], Citation[19]. Furthermore, a number of studies only include cases “co-amplified” with HER2 Citation[16], Citation[20–24]. Also, three of the studies were preformed with single color CISH which does not allow the detection of deletions Citation[20], Citation[22], Citation[24].
For optimal treatment with doxorubicin and epirubicin it is important to have tools available that can identify patients who are most likely to benefit from the therapy. HER2 status is a marker for treatment with anthracyclines Citation[25] but the biological basis for this link is lacking. Further, it has been discussed if HER2 rather has to be viewed as a surrogate marker or “pseudo-marker” for the real anthracycline target, topoisomerase IIα Citation[4], Citation[11], Citation[15], Citation[16]. The copy number variants of TOP2A have in a number of trials been shown to influence the sensitivity of the tumor towards topoisomerase inhibitors Citation[11], Citation[12], Citation[15], Citation[16], Citation[18–24]. These trials share many of the same methodological shortcomings, but although variable results are found, they all point in the same direction. A direct comparison between the data of the different studies is hampered by the fact that most reports omit a detailed description of the counting method used. Reporting the cut-off values and the probe manufacture is not enough to make transparency of the data. The description must also include evaluation of the slide to select the nuclei to be scored, criteria for omitting nuclei from scoring, and any inclusions of rules for percentage of nuclei with a certain number of signals. E.g. the cut-off criteria of “5 or more signals in at least 50% of the nuclei” may lead to exclusion of 50% of the amplified cases scored by a cut-off of “TOP2A/CEN-17 ratio ≥2.0”. Further, applying the HER2 scoring guideline to other genes does not allow detection of all deleted cases. Future reports should preferentially include a detailed description of the scoring or use one from an IVD (CE or FDA) approved kit. A meta-analysis of TOP2A results from randomized trials comparing CMF with anthracycline-based regimens has been initiated and the results are expected to be published soon.
Acknowledgements
We thank Vinni Bredahl and Birgit Hansen for excellent technical assistance and MD Eva Balslev and MD, PhD Birgitte Bruun Rasmussen for supporting the pathological evaluation of the FISH analysis of the original DBCG 89D/TOP2A study. Kirsten Vang Nielsen and Jan Trøst Jørgensen are employed by Dako A/S. Susanne Møller and Henning Mouridsen have received honoraria from Dako A/S.
References
- Sng JH, Heaton VJ, Bell M, Maini P, Austin CA, Fisher LM. Molecular cloning and characterization of the human topoisomerase IIalpha and IIbeta genes: Evidence for isoform evolution through gene duplication. Biochim Biophys Acta 1999; 1444: 395–406
- Smith K, Houlbrook S, Greenall M, Carmichael J, Harris AL. Topoisomerase II alpha co-amplification with erbB2 in human primary breast cancer and breast cancer cell lines: Relationship to m-AMSA and mitoxantrone sensitivity. Oncogene 1993; 8: 933–8
- Mueller RE, Parkes RK, Andrulis I, O'Malley FP. Amplification of the TOP2A gene does not predict high levels of topoisomerase II alpha protein in human breast tumor samples. Genes Chromosomes Cancer 2004; 39: 288–97
- Järvinen TA, Tanner M, Rantanen V, Barlund M, Borg A, Grenman S, et al. Amplification and deletion of topoisomerase II? associate with ErbB-2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. Am J Pathol 2000; 156: 839–47
- Callagy G, Pharoah P, Chin SF, Sangan T, Daigo Y, Jackson L, et al. Identification and validation of prognostic markers in breast cancer with the complementary use of array-CGH and tissue microarrays. J Pathol 2005; 205: 388–96
- Cardoso F, Durbecq V, Larsimont D, Paesmans M, Leroy JY, Rouas G, et al. Correlation between complete response to anthracycline-based chemotherapy and topoisomerase II-alpha gene amplification and protein overexpression in locally advanced/metastatic breast cancer. Int J Oncol 2004; 24: 201–9
- Hortobagyi GN. Anthracyclines in the treatment of cancer. An overview. Drugs 1997; 54(Suppl 4)1–7
- Mouridsen HT, Alfthan C, Bastholt L, Bergh J, Dalmark M, Eksborg S, et al. Current status of epirubicin (Farmorubicin) in the treatment of solid tumours. Acta Oncol 1990; 29: 257–85
- Corzo C, Bellosillo B, Corominas JM, Salido M, Coll MD, Serrano S, et al. Does polysomy of chromosome 17 have a role in ERBB2 and topoisomerase IIalpha expression? Gene, mRNA and protein expression: A comprehensive analysis. Tumour Biol 2007; 28: 221–8
- Ejlertsen B, Mouridsen HT, Jensen MB, Andersen J, Cold S, Edlund P, et al. Improved outcome from substituting methotrexate with epirubicin: Results from a randomised comparison of CMF versus CEF in patients with primary breast cancer. Eur J Cancer 2007; 43: 877–84
- Knoop AS, Knudsen H, Balslev E, Rasmussen BB, Overgaard J, Nielsen KV, et al. Retrospective analysis of topoisomerase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, methotrexate, and fluorouracil or cyclophosphamide, epirubicin, and fluorouracil: Danish Breast Cancer Cooperative Group. J Clin Oncol 2005; 23: 7483–90
- Knoop A, Knudsen H, Balslev E, Rasmussen B, Overgaard J, During M, et al. TOP2A aberrations as predictive and prognostic marker in high-risk breast cancer patients. A randomized DBCG Trial (DBCG89D). ASCO Annual Meeting Proceedings Part I. J Clin Oncol 2006; 24(18S)532
- Nielsen KV, Müller S, Poulsen TS, Gabs S, Schonau A. Combined use of PNA and DNA for Fluorescence In Situ Hybridization (FISH). Peptide nucleic acids: Protocols and applications. 2nd ed, PE Nielsen. Horizon Bioscience, Norfolk 2004; 227–260
- Olsen KE, Knudsen H, Rasmussen BB, Balslev E, Knoop A, Ejlertsen B, et al. Amplification of HER2 and TOP2A and deletion of TOP2A genes in breast cancer investigated by new FISH probes. Acta Oncol 2004; 43: 35–42
- Järvinen TAH, Tanner M, Bärlund M, Borg Å, Isola J. Characterization of topoisomerase II( gene amplification and deletion in breast Cancer. Genes Chromosomes Cancer 1999; 26: 142–50
- Di Leo A, Gancberg D, Larsimont D, Tanner M, Jarvinen T, Rouas G, et al. HER-2 amplification and topoisomerase IIalpha gene aberrations as predictive markers in node-positive breast cancer patients randomly treated either with an anthracycline-based therapy or with cyclophosphamide, methotrexate, and 5-fluorouracil. Clin Cancer Res 2002; 8: 1107–16
- EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005; 365(9472)1687–717
- Harris L, Dressler L, Cowan D, Berry D, Cirrincione C, Broadwater G, et al. The role of HER-2 + Topo IIa Amplification in predicting benefit form CAF dose escalation CALGB 8541. ASCO Annual Meeting 2004(Abstract no. 9505).
- O'Malley F, Chia S, Tu D, Shepherd L, Levine M, Huntsman D, et al. Prognostic and predictive value of topoisomerase II alpha in a randomized trial comparing CMF to CEF in premenopausal women with node positive breast cancer (NCIC CTG MA.5). ASCO Annual Meeting Proceedings Part I. J Clin Oncol 2006; 24(18S)533
- Hannemann J, Kristel P, van Tinteren H, Bontenbal M, van Hoesel QG, Smit WM, et al. Molecular subtypes of breast cancer and amplification of topoisomerase II alpha: Predictive role in dose intensive adjuvant chemotherapy. Br J Cancer 2006; 95: 1334–41
- Press MF , Mass RD , Zhou JY , Sullivan-Halley J , Villalobos IE , Lieberman G , et al . Association of topoisomerase II-alpha (TOP2A) gene amplification with responsiveness to anthracycline-containing chemotherapy among women with metastatic breast cancer entered in the Herceptin H0648g pivotal clinical trial. ASCO Annual Meeting; 2005. Abstract No. 9543.
- Tanner M, Isola J, Wiklund T, Erikstein B, Kellokumpu-Lehtinen P, Malmstrom P, et al. Topoisomerase IIalpha gene amplification predicts favorable treatment response to tailored and dose-escalated anthracycline-based adjuvant chemotherapy in HER-2/neu-amplified breast cancer: Scandinavian Breast Group Trial 9401. J Clin Oncol 2006; 24: 2428–36
- Press M , Sauter G , Buyse M , Bernstein L , Eiermann T , Pienkowski V , et al . Alteration of topoisomerase II-alpha gene in human breast cancer and its association with responsiveness to anthracycline-based chemotherapy. ASCO Annual Meeting Proceedings. J Clin Oncol 2007;25, (Suppl)Abstract no. 524.
- Arriola E, Rodriguez-Pinilla SM, Lambros MB, Jones RL, James M, Savage K, et al. Topoisomerase II alpha amplification may predict benefit from adjuvant anthracyclines in HER2 positive early breast cancer. Breast Cancer Res Treat 2007; 106: 181–9
- Pritchard KI, Messersmith H, Elavathil L, Trudeau M, O'Malley F, Dhesy-Thind B. HER-2 and topoisomerase II as predictors of response to chemotherapy. J Clin Oncol 2008; 26: 736–44