Abstract
Introduction. Since 30 years DBCG (Danish Breast Cancer Coperative Group) has maintained, on a nation-wide basis, a clinical database of diagnostic procedures, therapeutic interventions, and clinical outcome in patients with primary breast cancer. The present analysis was undertaken to evaluate the development of the prognosis since 1977, and to analyse factors potentially contributing to the change of the prognosis. Material and Methods. All cases of invasive breast cancer reported to DBCG during the period 1977–2006 were included in the present analysis. Results. A total of close to 80 000 patients were registered in the DBCG Database. Since 1977 the prognosis has improved significantly, thus 5 year survival for the total population of patients with primary breast cancer has increased from 65 to 81%. Discussion. According to the present analysis diagnosis at an earlier stage in the natural course of the disease and especially the development of more active systemic treatment modalities have contributed to the improved prognosis.
DBCG (Danish Breast Cancer Cooperative Group) is a multidisciplinary organisation of all disciplines involved in diagnosis, treatment and research in breast cancer Citation[1]. Since the establishment in 1977, almost all new breast cancer patients have been registered in the DBCG database, including reporting of primary tumour characteristics, local therapy (surgery, radiotherapy), systemic therapy (chemotherapy, endocrine therapy, trastuzumab) and follow-up. Previously we demonstrated a significant improvement in the survival in breast cancer during the period 1948–1987 based upon more than 70 000 cases reported to the Danish Cancer Registry Citation[2].
The aim of the present study is to analyse the development of the prognosis of invasive breast cancer based upon cases reported to DBCG during the period 1977–2006.
Material and methods
All cases of primary invasive breast cancer reported to DBCG during the period August 1977 to January 1, 2007 are included in the analysis. The date of last follow-up of the patient cohort is August 1, 2007.
The vast majority of the breast cancer cases were clinically detected, but from 1991 and 1993 respectively, screening by mammography was offered to women aged 50–69 years living in two regions, the municipality of Copenhagen and the county of Funen. The population of these two regions is approximately 20% of the total Danish population.
Surgery, radiotherapy and systemic therapy were given as part of consecutive national treatment programmes set up by DBCG (DBCG 77, 82, 89, 99, 01, 04). Briefly, the first three programmes (77, 82, 89) were set up as nationwide trials with different research questions, whereas the later programmes (99, 01, 04) were nationwide guidelines for standard care but also included participation in international trials primarily within the frames of SBG (Scandinavian Breast Cancer Group) or BIG (Breast International Group). Systemic therapies were offered to patients at high risk of recurrence according to criteria described below. Since the DBCG 89 programme the oestrogen receptor status of the primary tumour was used to select patients for endocrine therapy. Briefly the DBCG 77 programme primarily analysed the benefit of adjuvant systemic therapy (chemotherapy with CMF (cyclophosphamide, metotrexate, fluororacil) or cyclophosphamide alone in pre-menopausal women, tamoxifen one year in post-menopausal women) versus controls. The DBCG 82 programme included three trial questions; a: breast conserving therapy + irradiation against the residual breast versus mastectomy; b: the benefit of adding tamoxifen one year to CMF in pre-menopausal patients and adding CMF to tamoxifen one year to post-menopausal patients; c: the benefit of postoperative radiotherapy in addition to systemic therapy. The DBCG 89 programme included three research questions of adjuvant systemic therapy; a: in pre-menopausal patients with receptor positive tumours, ovarian ablation versus CMF; b: in post-menopausal patients with receptor positive tumours, the duration of tamoxifen; c: pre- and post-menopausal patients with receptor negative tumours, chemotherapy with and without anthracyclines (CMF versus CEF (cyclophosphamide, epirubicin, fluororacil)), and the benefit of adding bisphosphonates to chemotherapy. The DBCG 99, 01 and 04 programmes included national guidelines for endocrine therapy (tamoxifen 5 years) and chemotherapy, with a gradual shift from CMF to CEF. These programmes also included participation in international trials of adjuvant aromatase inhibitors, anthracycline escalation, taxanes and trastuzumab. Details about the national programmes and the clinical trials are presented elsewhere Citation[3].
All diagnostic procedures, histopathological examinations and therapeutic interventions (surgery, radiotherapy, systemic therapy) were defined according to national guidelines prepared by DBCG Citation[4] and were reported to the DBCG database.
The histopathological examinations included histological type according to WHO, tumour size measured by the pathologist, except for 26% of the cases before 1982 measured by the surgeon only, malignancy grade (ductal carcinoma only) according to Bloom and Richardson Citation[5], number of nodes examined, hereof tumour positive, ER and/or PgR status by extraction method or (from 1990) by IHC Citation[6], and HER-2 status (from 2002) by IHC and/or (if HER-2 + + by IHC) FISH (Dako, Denmark). Positive hormone receptor status was defined by ≥10 fmol/mg cytosol protein or by ≥10% stained cells. Positive HER-2 status was defined by HER-2 + ++ by IHC or by FISH ratio >2.0. Additional analyses are described in detail elsewhere Citation[7].
The primary surgery included mastectomy or breast conserving surgery (BCS) + lymph node dissection which should include level I-II. The requirement for the number of retrieved axillary lymph nodes became strengthened during the period from at least one node in 1977 to at least 4 but preferably 10 nodes in 1990 and by 1994 DBCG stated that at least 10 removed lymph nodes should be the national standard of level I-II dissection of the axilla Citation[1]. The sentinel node technique was introduced as an experimental procedure in 1998 and nationwide from 2002 Citation[8]. Since then axillary dissection was limited to cases non-eligible for this technique and to sentinel node positive cases.
Postoperative radiotherapy was offered to all patients following BCS (target residual breast + chest wall, 48 Gy, 5 fr/w + boost, 10 Gy, 5 fr/w, 2 Gy/fr), and to patients with positive nodes or invasion through the deep fascia or into the skin (target chest wall + regional nodes, 48 Gy, 5 fr/w) with some modifications during the period as described in details elsewhere Citation[3], Citation[9].
Following the primary surgery and histopathological examinations the patients were allocated to risk groups according to the current St. Gallen criteria Citation[10], a low risk group eligible for local therapy only (surgery +/− radiotherapy) and a high risk group (=St. Gallen intermediate and high risk group) eligible for systemic adjuvant therapy in addition to the local therapy. The definition of risk groups varied according to time. Essentially, from 1977–1999 the low risk group was defined by node negativity, and a tumour ≤5cm, irrespective of grade, age and hormone receptor status, whereas patients with node positive tumours or tumours >5cm were allocated to the high risk group. From 1989 pre-menopausal patients with ductal carcinomas malignancy grade II-III were allocated to the high risk group irrespective of nodal status and tumour size. From 1999 patients with node negative tumours were allocated to the high risk group if the tumour was >2cm or ≤2cm and receptor negative or ductal grade II-III. From 2002 age <35 years was an additional criteria to be allocated to the high risk group. Retrospectively we could therefore isolate a group of patients with node negative tumours ≤5cm, who were either <35 years of age or had tumours either >2cm or ≤2cm and grade II-III or receptor negative. These patients were initially allocated to the low risk group (i.e. who received no adjuvant systemic therapy) and later allocated to the high risk group (i.e. who received adjuvant systemic therapy). In the following this group is termed the retrospective low → high risk group. More detailed description of the modifications of risk definitions during time is given elsewhere Citation[3].
High risk patients were allocated to adjuvant systemic therapy, chemotherapy (from 2005 with the addition of trastuzumab if positive HER-2 status) and/or endocrine therapy. The systemic therapies were given as part of national treatment programmes or as part of prospective clinical trials. Details about the national programmes and the clinical trials are presented elsewhere Citation[3].
For patients who had surgery and postoperative adjuvant therapy or control according to the DBCG guidelines (enrolled patients) clinical follow-up was recommended to be continued as long as they remained disease-free (see definition below) or up to 10 years following surgery. Follow-up for survival was complete by linkage to the Danish Civil Registration System. This included all patients, i.e. enrolled patients and non-enrolled patients. The latter group consisted of patients, who according to the guidelines were excluded from the standard treatment programmes due to high age or poor physical condition, bilateral breast cancer, distant metastases at time of primary diagnosis or prior other malignant disease.
The estimates of disease-free survival (DFS) and overall survival (OS) were calculated according to the Kaplan-Meier method. Overall survival was defined as the time from the primary surgery to death, irrespective of cause, and DFS as the time from primary surgery to loco-regional and/or distant recurrence, contra-lateral breast cancer, other new malignant disease or intercurrent death, whichever occurred first.
Relative survival rates were estimated as the ratio of the observed survival to the expected survival according to the Hakulinen's method Citation[11]. Expected survival was estimated from national figures from the general population Citation[12] matched on gender, age and calendar time. For the total material DFS and OS were calculated according to 5 years cohorts from 1977–2006. Subsequently, for enrolled patients <70 years, DFS and OS were analysed according to risk groups and according to the single risk factors determining the risk group (nodal status, tumour size, malignancy grade, hormone receptor status and age) and the distribution of these variables according to time are presented. In addition we analysed the prognostic implications of screening and treatment. The reason why these analyses were limited to patients <70 years is the variation, according to time, in the upper age limit for patients ≥70 years to be included in the treatment programmes Citation[3].
Results
Information about overall survival (OS) and disease-free survival (DFS) is available for 77 284 and 53 869 patients respectively hereof for patients <70 years of age for 56 384 and 47 359 respectively. The exclusion of the non-enrolled patients from the DFS analyses explains the lower numbers.
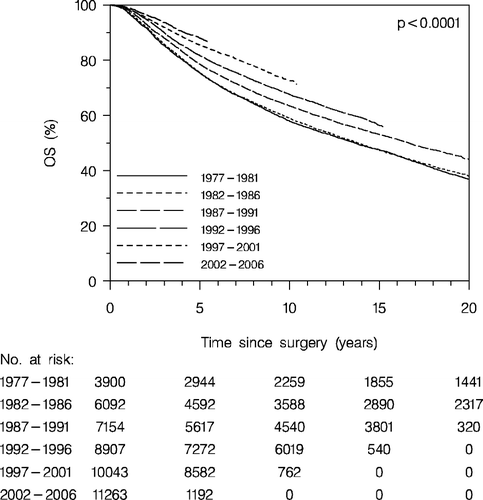
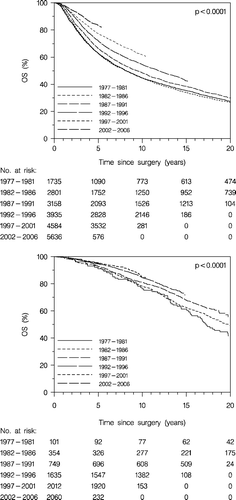
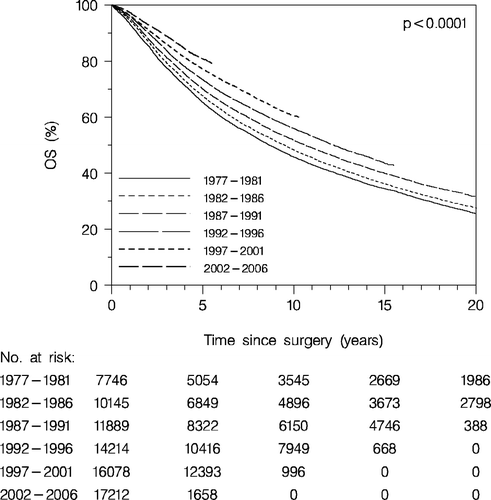
Overall survival of the total group of patients (enrolled and non-enrolled patients) is presented in . A constant improvement of the prognosis according to period of the primary diagnosis is apparent. Thus the 5 years OS increases from 65% for patients diagnosed with breast cancer in the period 1977–1981 to 81% for those diagnosed in the period 2002–2006.
Figure 1. Overall survival (OS) according to diagnosis period. All patients (enrolled and non-enrolled patients).
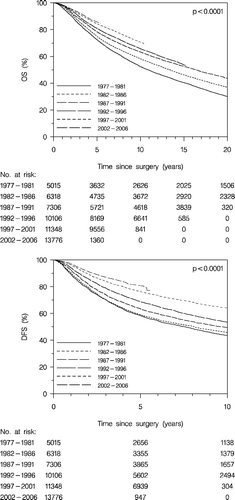
For enrolled patients the development in OS (, upper panel) and DFS (, lower panel) according to time is similar. Five years survival for patients diagnosed in 1977–1981 is 72% and 85% for those diagnosed 2002–2006. The corresponding figures for DFS are 58 and 81%, respectively. It is also apparent that the improved early prognosis is maintained throughout the period of observation.
Figure 2. Overall survival (OS) (upper panel), and disease-free survival (DFS) (lower panel) according to diagnosis period. Enrolled patients.
The 5 years survival of the background population has increased from 88% in the 1977–1981 cohort to 89% in the 1997–2001 cohort, hereof for women <70 years from 95 to 96% and the 10 years survival has increased from 75% in the 1977–1981 cohort to 77% in the 1992–1996 cohort, hereof for women <70 years from 88 to 89%.
In the following we will describe the prognosis in enrolled patients in terms of OS according to risk groups and according to the variables defining the prognostic risk groups, their development over time, and the potential influence on the prognosis by treatment and screening. During the period 1977–2006 the treatment and follow-up strategy in the age group ≥70 years has varied. Therefore we chose to limit the following analyses to patients less than 70 years at time of diagnosis. The improvement of prognosis of this group in terms of DFS (data not shown) and OS over time () was consistent with the total group of patients.
Risk group
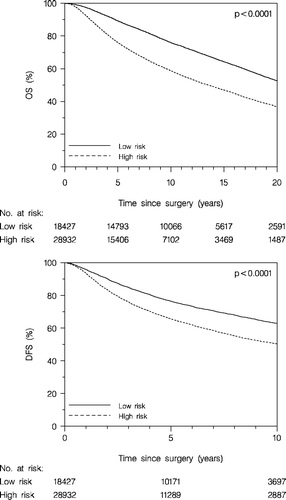
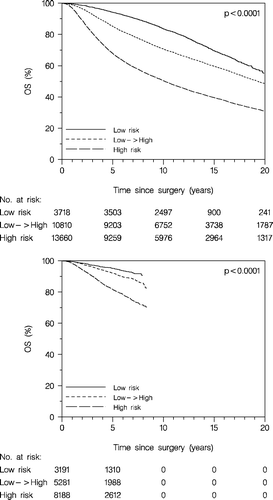
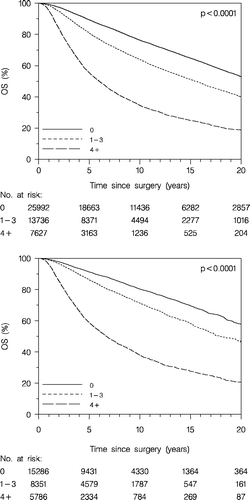
The patients, at any time allocated to the high risk group, according to the DBCG guidelines, did significantly worse compared to the patients at any time allocated to the low risk group, as concerns OS (, upper panel) and DFS (, lower panel). From 1977 to 1999 the low risk group included approximately 50% of the enrolled patients. Subsequently (with the new definitions of the risk groups) the proportion of the low risk group decreased to approximately 20%.
Nodal status
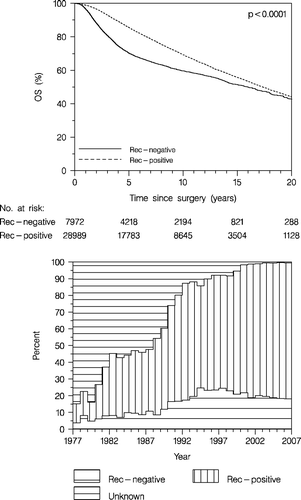
According to time more lymph nodes were examined and previous studies indicated that the risk of false negative nodal status increased with decreasing number of lymph nodes examined Citation[13]. Thus, prior to the sentinel node technique era it was indicated that at least 10–15 nodes should be examined to ensure ‘true’ negative nodal status. We therefore analysed OS according to nodal status (node negative (0), 1–3 positive nodes, and 4+ positive nodes) for the whole series (, upper panel) of patients and for those with at least 10 examined nodes, or sentinel node technique applied (, lower panel). After the introduction of the sentinel node technique 10 examined nodes was required only if the tumour was node positive, or if the inclusion criteria for the sentinel node method Citation[8] was not fulfilled. As appears, nodal status is a strong prognostic factor and there is a significantly inferior prognosis with increasing number of positive nodes. It is also apparent that the difference in short-term prognosis (5 years survival) is translated to the long-term prognosis.
Figure 5. Overall survival (OS) according to nodal status in all patients with lymph node assessment (upper panel), and in patients with at least 10 examined nodes or sentinel node technique applied (lower panel). Node-negative (0), 1-3 positive nodes, and 4+ positive nodes. Enrolled patients <70 years.
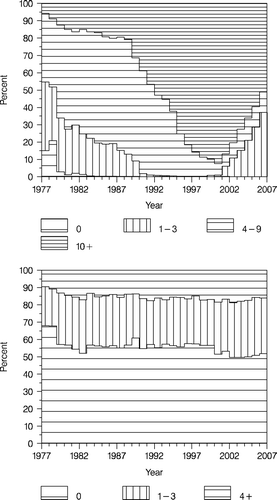
, upper panel shows the distribution of patients according to number of lymph nodes removed over time. Since 1990, when more radical axillary dissection was recommended, there has been a dramatic change in the proportion of operations with at least 10 lymph nodes removed, from approximately 20 to 90%. Since the introduction of the sentinel node technique this proportion has decreased again. However, looking only at cases not eligible for the sentinel node technique and sentinel node positive cases (in whom axillary dissection is recommended) the proportion remains high (data not shown). The distribution according to nodal status (, lower panel) has remained quite constant until 2000 when the sentinel node technique was introduced. Since then there has been a slight decrease in the proportion of node negative patients. This can be explained by the diagnosis of more cases with micrometastatic disease Citation[7].
Figure 6. The distribution of patients according to number of lymph nodes examined (upper panel), and the distribution of nodal status according to time (lower panel). Enrolled patients <70 years.
In the two counties with screening since 1991 and 1993, respectively, the distribution following the prevalence round among node negative, 1–3 and 4 + positive nodes was 63, 24 and 13%, respectively, compared to 53, 29 and 18%, respectively, in patients with similar age and diagnosed during the same period but outside the screening counties.
Tumour size
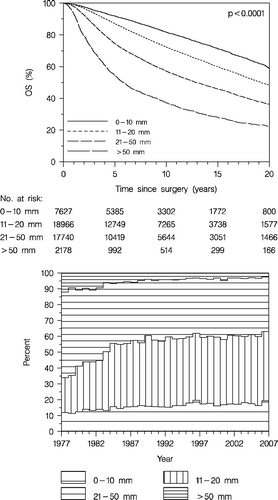
Tumour size is a prognostic factor (, upper panel). As shown in , lower panel, there is a trend since 1982, when size was measured in all cases by the pathologist, towards a smaller proportion of tumours > 50 mm, and a greater proportion of tumours up to 10 mm in size, whereas the other groups remained fairly constant. The median (mean) tumour size was 20 mm (mean 25.3 mm) in 1985 compared to 18 mm (mean 20.9 mm) in 2005. Following the prevalence round tumour size in the screening population was smaller than in the corresponding non-screening population: 28% ≤10 mm and 74% ≤20 mm compared with 15 and 56%, respectively.
Grade
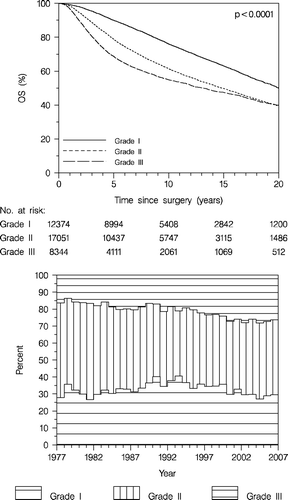
Overall survival according to grade is shown in , upper panel. Throughout the follow-up time patients with malignancy grade I ductal tumours do better than patients with grade II-III tumours. The annual risk rates differ in grades I, II, and III tumours. They remain fairly constant in the grade I tumours. As concerns grade II annual risk rates are higher than in grade I tumours up to 10 years following diagnosis but subsequently mimic the grade I tumours. In grade III tumours, however, the annual risk rates are even higher during the first 5 years, but then level off and get lower than in the grade I and II tumours. The superior prognosis in grade I tumours and the similar prognosis in grade II-III tumours, evident from year 15, persists even up to 28 years (data year 21–28 year not shown). According to time there is a trend towards a greater proportion of grade III tumours and a smaller proportion of grade II tumours, but a constant proportion of grade I tumours (, lower panel). Thus the distribution among ductal carcinomas malignancy grade I versus grade II + III remains fairly constant.
Hormone receptor status
, upper panel presents OS according to receptor status, positive (ER or PgR or both positive) and negative (ER and PgR both negative, or one negative, the other unknown). It is apparent that positive status is associated with superior prognosis up to 15 years following primary diagnosis. Indeed during the first 5 years the annual risk rates are significantly higher in the receptor negative tumours but subsequently get lower than in the receptor positive tumours. The very similar prognosis in receptor negative and receptor positive tumours from 15 years following primary diagnosis persists up to 28 years (data years 21–28 not shown). The distribution of receptor status changes over time (, lower panel). Early on a large proportion had tumours with unknown status. Since the analysis was conducted in the vast majority of cases (from 1992) there has been a trend towards an increase of the ER positive population, from 74% in the period 1992–1996 to 81% during the period 2002–2006, among those with known receptor status.
Age
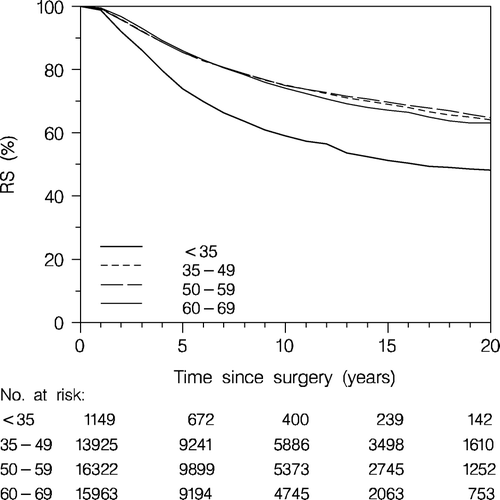
Overall survival according to age is increasingly influenced by non-breast cancer deaths with increasing age. To compensate for this the relative survival was calculated, which reflects the survival of the patients in the hypothetical situation in which breast cancer is the only cause of death. As seen in patients < 35 years do significantly worse than the older age groups up to 70 years, which share a fairly similar prognosis. The annual number of patients < 35 years increases from 125 in 1977–1981 to 230 in 2001–2006. The proportion of patients < 35 years of age, however, decreases from 3.2 to 2.0% whereas the distribution among the other age groups remained fairly constant.
Screening
Screening programmes started in two Danish regions in 1991 (Copenhagen) and in 1993 (The county of Funen), respectively. Women aged 50–69 years were invited. So far survival data only from Copenhagen have been analysed. Using historical, national and historical national control groups, breast cancer mortality was reduced by 25% over a 10 year follow-up period Citation[14].
Treatment
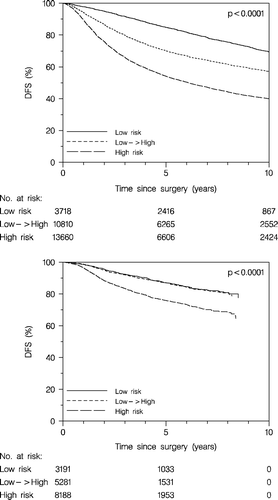
To get information about the potential impact of systemic therapy on the prognosis we analysed DFS and OS for patients belonging to the retrospective low → high risk group, which was considered low risk early on (receiving no adjuvant systemic therapy) and high risk later on (receiving chemotherapy). As seen in this group had an intermediate prognosis as concerns DFS when untreated systemically (upper panel) but when treated systemically (lower panel) a DFS very similar to a retrospective low risk group with similar criteria throughout the period (i.e. tumour node negative, ≤2cm and grade I, and receptor positive, and age ≥35 years). This difference was also translated to survival difference (). In addition, we observed that the improvement of the prognosis according to time was more pronounced in the retrospective high risk patients (, upper panel), who all received systemic therapy, compared to the retrospective low risk patients (, lower panel), none of whom received systemic therapy. In these analyses the low → high risk patients were excluded, i.e. the tumour characteristics of the primary tumour stayed stable within the risk groups. However, some stage migration had occurred as a result of the more extensive axillary dissection and the introduction of the sentinel node technique.
Figure 11. Disease-free survival (DFS) according to retrospective risk groups, the low → high risk group untreated systemically (upper panel), and treated systemically (lower panel). Enrolled patients <70 years.
Discussion
The data presented clearly demonstrate a significant improvement of the prognosis in primary breast cancer diagnosed and reported to DBCG during the period 1977–2006. Thus 5 year OS increased from 65 to 81%, and this early improvement also translated into the long-term outcome.
To extrapolate this conclusion to all new cases of breast cancer would require knowledge about any potential selection of newly diagnosed patients reported to DBCG. A past comparison Citation[15] of cases reported during the period 1979–1994 to DBCG and to The Danish Cancer Registry, which is considered to have a close to complete reporting of all cases, demonstrated a missing reporting to DBCG of approximately 20% of cases in the beginning of the period decreasing to approximately 10% of the cases in 1994. In addition, the vast majority of missing cases were elderly women over 70 years. The prognostic improvement for patients lower than 70 years in the present report was similar to that for the total patient population. Thus selection bias is unlikely to contribute to the observed improvement of the survival.
Theoretically the observed improved prognosis could be ascribed to alteration in the general health condition, to alterations in the biology of the disease, to earlier diagnosis and to treatment.
As shown a general improvement of the health condition of the female population could explain only a negligible part of the observed improvement of the survival in breast cancer patients.
Grade and hormone receptor status may be considered as characteristics of the biology of the breast cancer. As concerning malignancy grade it was apparent that grade I tumours fared better compared with grade II and III tumours. The latter two groups had a similar prognosis from 15 years following primary diagnosis. There was an unexplained trend towards larger proportion of malignancy grade III tumours according to time. However, the distribution between grade I and higher grade tumours remained fairly constant. Positive hormone receptor status was a prognostic factor for the early outcome of disease and remained so until 15 years following the primary diagnosis when the prognosis for patients with receptor positive and receptor negative tumours was similar. Among those with known hormone receptor status the distribution among receptor positive and receptor negative cases remained fairly constant, except for the last 10 years when the proportion of receptor positive cases increased from 74 to 81%. Thus changes in the biology of breast cancer could hardly explain the observed improvement of the prognosis.
Nodal status and tumour size reflect time of diagnosis during the natural course of the disease. As concerns nodal status previous analyses from DBCG Citation[13] indicated, that the risk of false negative nodal status is decreased with an increased number of lymph nodes being examined. During the period 1990 to 2000 the proportion of node negative cases remained fairly constant. However, assuming a decrease in the proportion of patients falsely categorized as having node negative tumours, this indicates a trend towards an increase in truly node negative tumours. Hereafter a small decrease in the proportion of node negative cases was seen, because more patients with micrometastatic disease were diagnosed in connection with the introduction of the sentinel node technique. As concerns tumour size it was apparent from the present analysis that there was a trend towards a decreasing tumour size at time of diagnosis from median 20 mm in 1985 to 18 mm in 2005. Tumour size in the population offered screening, following the first prevalence round was, as expected, even smaller than in the non-screened population. Thus according to time the patients were diagnosed with smaller tumours and with a more favourable nodal status, indicating diagnosis at an earlier stage. This was even more pronounced in regions with mammography screening programmes. In agreement herewith these cases were observed to have a prognosis significantly superior to the cases detected clinically, with a relative reduction in the risk of breast cancer death by 25%. The alterations in the tumour size according to time in non-screened population could probably be ascribed to the increasing awareness about breast cancer as a result of the public discussion about screening.
As concerns the potential prognostic impact of treatment the data of the different treatment approaches can be considered in relation to the existing hypotheses about the biology of breast cancer Citation[16]. Until approximately 40 years ago breast cancer was considered, according to the Halsted paradigm for the disease, to remain localised to the breast during the early course, later progressing by direct extension, through the lymphatics to the lymph nodes and then to distant metastatic sites. An alternate hypothesis was raised in the ninety-forties to -fifties and stated by Bernard Fisher in 1980 that breast cancer is a systemic disease early on, putting emphasis on the use of adjuvant systemic antineoplastic therapy. Later Hellman argued for the so-called spectrum concept. Accordingly breast cancer is to be considered a heterogeneous disease extending from a disease that remains local throughout its cause to one that is systemic when first diagnosed. According to this model residual local disease serves as a source of distant metastases. Thus both effective local control as well as systemic approaches is important.
As concerns the primary surgery a shift towards a higher proportion of patients having breast conserving surgery (BCS) rather than mastectomy was observed from 1% in the late seventies to 52% in 2006. However, a randomised DBCG trial of mastectomy versus BCS in patients eligible for the latter confirmed the conclusions from Italian and American studies, that the two surgical procedures were associated with a similar long-term survival Citation[17]. These data agree with all the hypotheses of the biology of breast cancer in that local control, although achieved with different approaches, is important. As concerns radiotherapy similar guidelines have been used during the period except for the DBCG 82 programme, which randomised patients, otherwise eligible for radiotherapy according to the general guidelines, to either radiotherapy or to no radiotherapy. The study demonstrated the beneficial prognostic achievements with radiotherapy added to systemic therapy as concerns rate of recurrence, especially locally, and survival as well Citation[18], Citation[19]. These results agree with the spectrum hypotheses of the biology of breast cancer. Several trials in early breast cancer conducted by DBCG, nationally or in international collaboration, have demonstrated the superiority of chemotherapy and endocrine therapy over control Citation[20–22], in agreement with the Fisher hypothesis, and in addition the superiority of anthracycline combinations over CMF Citation[22], Citation[23], of aromatase inhibitors over tamoxifen Citation[24], and of trastuzumab over control Citation[25]. These have all included patients belonging to the DBCG high risk group. In agreement herewith the observed significant improvement of the prognosis of breast cancer was especially pronounced in the high risk group of patients whereas in the low risk patients, who were not offered systemic treatments, the improvement was much smaller. That the systemic therapy is responsible for the prognostic improvement is further indicated by the significant difference in the prognosis in the retrospective low → risk patient group when going from no systemic therapy to systemic therapy.
In conclusion we observe a significant improvement of the prognosis of primary breast cancer during the period 1977 to 2006. According to the present analysis diagnosis at an earlier stage in the natural course of the disease and especially the development of more active systemic treatment modalities have contributed although this analysis is unable to score the relative role of each of them. Similar conclusions have been drawn as concerns mortality trends observed in other western countries Citation[26].
References
- Blichert-Toft, M, Christiansen, P, Mouridsen, HT. Danish Breast Cancer Cooperative Group–DBCG: History, organization, and status of scientific achievements at 30-year anniversary. Acta Oncol 2008; 47:497–505.
- Andreasen AH, Mouridsen HT, Andersen KW, Madsen M, Olesen KP. Prognose-forbedring for brystkræft. Ugeskr læger 1994; 156: 6512–6
- Møller, S, Jensen, M-B, Eljertsen, B, Bjerre, KD, Larsen, M, Hansen, HB, et al. The clinical database and the treatment guidelines of the Danish Breast Cancer Cooperative Group (DBCG); its 30-years experience and future promise. Acta Oncol 2008; 47:506–24.
- http://www.dbcg.dk, 14.11.07.
- Bloom J, Richardson WW. Histological grading and prognosis in breast cancer. A study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer 1957; 11: 359–77
- Talman MM-L, Rasmussen, BB, Andersen, J, Christensen, IJ. Estrogen receptor analyses in the Danish Breast cancer Cooperative Group. Acta Oncol 2008; 47:789–94.
- Kier, HV, Nielsen, BB, Bjerre, KD, Lænkholm, AV. Conventional pathological parameters recorded in the Danish Breast Cancer Cooperative Group's register 1978–2006. Acta Oncol 2008; 47:778–83.
- Christiansen, P, Friis, E, Balslev, E, Jensen, D, Møller, S. Sentinel node biopsy in breast cancer: Five years experience from Denmark. Acta Oncol 2008; 47:561–68.
- Thomsen, MS, Beca, M, Nielsen, HM, Pedersen, AN, Overgaard, M, Ewertz, M, et al. Post-mastectomy radiotherapy in Denmark: From 2D to 3D treatment planning guidelines of The Danish Breast Cancer Cooperative Group. Acta Oncol 2008; 47:654–61.
- Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thürlimann B, Senn H-J, et al. Progress and promise: Highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 2007; 18: 1133–44
- Hakulinen T. Cancer survival corrected for heterogeneity in patient withdrawal. Biometrics 1982; 38: 933–42
- Statistics Denmark – http://www.statbank.dk, , 2009.07.
- Axelsson, CK, Mouridsen, HT, Zedeler, K, on behalf of the Danish Breast Cancer Cooperative Group (DBCG). Axillary dissection of level I and II lymph nodes is important in breast cancer classification. Eur J Cancer 1992;28A:1415–8.
- Olsen AH, Njor SH, Vejborg I, Schwartz W, Dalgaard P, Jensen M-B, et al. Breast cancer mortality in Copenhagen after introduction of mammography screening: cohort study. BMJ 2005; 330: 220–2
- Rostgård K, Holst H, Mouridsen HT, Lynge E. Do clinical databases render population-based cancer registers obsolete. The example of breast cancer in Denmark. Cancer Chemother Control 2000; 11: 669–74
- Hellman S. Natural history of small breast cancers. J Clin Oncol 1994; 12: 2229–34
- Blichert-Toft, M, Nielsen, M, Düring, M, Møller, S, Rank, F, Overgaard, M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol 2008; 47:672–81.
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. for the Danish Breast Cancer Cooperative Group 82b Trial. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med 1997; 337: 945–55
- Overgaard M, Jensen M-B, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999; 353: 1641–8
- Dombernowsky P, Brincker H, Hansen M, Mouridsen HT, Overgaard M, Panduro J, et al. Adjuvant therapy of premenopausal and menopausal high-risk breast cancer patients. Present status of the Danish Breast Cancer Cooperative Group Trials 77-B and 82-B. Acta Oncol 1988; 27: 691–7
- Mouridsen HT, Rose C, Overgaard M, Dombernowsky P, Panduro J, Thorpe S, et al. Adjuvant treatment of postmenopausal patients with high risk primary breast cancer. Results from the Danish adjuvant trials. DBCG 77 C and DBCG 82 C. Acta Oncol 1988; 27: 699–705
- et al. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomized trials. Lancet 2005;365:1687–717.
- Ejlertsen B, Mouridsen HT, Jensen M-B, Andersen J, Jensen BB, Cold S, et al. Improved outcome from substituting methotrexate with epirubicin: Results from a randomised comparison of CMF versus CEF in patients with primary breast cancer. Eur J Cancer 2007; 43: 877–84
- Thürlimann B, Keshaviah A, Coates AS, Mouridsen HT, Mauriac L, Forbes JF, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 2005; 350: 2747–57
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Traztuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005; 353: 1659–72
- Jatoi, I, Chen, BE, Anderson, F, Rosenberg, PS. Breast cancer mortality in the United States according to estrogen receptor status and age at diagnosis. J Clin Oncol 2007; 25 APUD.