Abstract
In addition to nationwide standardized pathology forms for operable primary invasive breast cancer, the Danish Breast Cancer cooperative Group (DBCG) in 1982 introduced pathology forms for breast cancer in situ (CIS). The histological reporting form was used primarily for ductal cancer in situ (DCIS) treated with wide local excision. The form however, also provided information on lobular carcinoma in situ (LCIS), atypia and benign lesions. In 1989 the reporting form for DCIS was extended and now provided information on histological subtype, malignancy grade, growth pattern and both Estrogen receptor (ER) and Progesteron receptor (PR) status. Also mastectomy specimens were included. In 2004 the previous malignancy grading was replaced by the Van Nuys classification, and information on microcalcifications was introduced. The axillary status now included the sentinel node technique only. In 2006 the pleomorphic subtype of LCIS was added to histological subtypes.
The present work reviews the DBCG guidelines and recommendations concerning CIS adding a brief characterization of the Danish CIS population. It also refers to the introduction of modern molecular pathology and distinction between low-risk and high-risk CIS lesions. A major point is that without the thirty years of outstanding efforts by the DBCG, future research would not be able to meet expectations.
Definition
Ductal carcinoma in situ (DCIS) and lobular carcinoma in situ (LCIS) represent a non-invasive proliferation of malignant epithelial cells of the breast.
History
LCIS
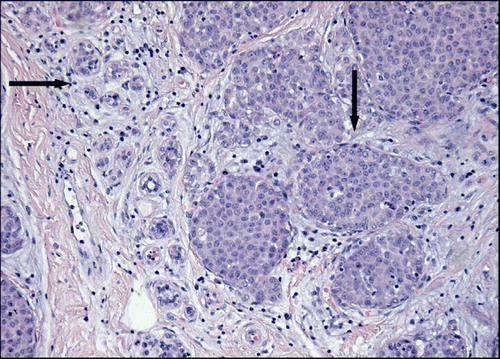
LCIS has been regarded as an incidental microscopic finding (). There are no specific clinical or mammographic findings associated with LCIS, and a true incidence of LCIS is not known. In 1984 a hospital based autopsy study performed by Maja Nielsen et al. discovered a prevalence of occult LCIS as high as 6% Citation[1]. It is difficult to predict the time to appearance of invasive cancer after a diagnosis of LCIS. An average interval of 20 years from time of biopsy has been shown in a separate study and this extended time span has significant implications for planning patient follow-up Citation[2], Citation[3]. The rate of breast cancer related death after a primary diagnosis of LCIS is exceedingly low; 1.1% after 12 years Citation[4].
DCIS
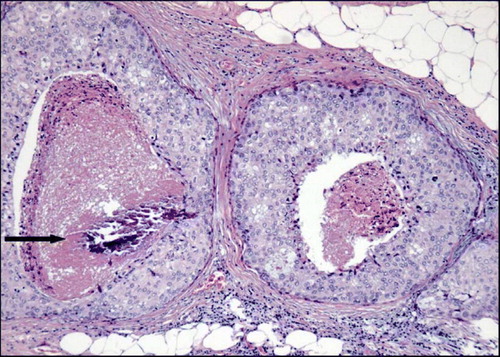
Before the introduction of mammography, most cases of DCIS were detected accidentally or by clinical symptoms such as a palpable tumour mass, secretion from the nipple or Pagets Disease of the Nipple (PDN). At mammography, DCIS is usually detected by microcalcifications (). DCIS represents 3–5% of all clinically detected breast malignancies, but accounts for up to 20% of screen-detected breast malignancies Citation[5]. It is assumed that invasive ductal carcinoma of the breast is preceded by DCIS and it is indicated that approximately 50% of untreated DCIS lesions proceed to invasive carcinoma during a period of up to 30 years Citation[6]. Due to current diagnostic and therapeutic strategies, the rate of breast cancer related death is low; 1.6% at 8 years Citation[7].
The Danish Breast Cancer Cooperative Group (DBCG) 1977–1982
From 1974–1977 the Danish pathologist Johan A. Andersen produced several publications on LCIS. His doctoral dissertation from 1977 had a major impact on the perception of treatment strategies of LCIS in Denmark Citation[3], Citation[8], Citation[9]. The study included 52 patients diagnosed with LCIS with a median follow-up of 16 years. An interval of 18 years from LCIS diagnosis to development of invasive carcinoma was observed, and only 5% of the patients died from breast cancer during a 10 year follow-up Citation[3]. Andersen concluded that the current treatment strategy, which at the time was mastectomy, should be replaced by long-term clinical and mammography follow-up Citation[8]. From 1977 to 1982, DCIS and LCIS were reported in the DBCG mastectomy pathology form for invasive breast cancer.
DBCG 82-IS
In 1982 DBCG initiated a nationwide in situ protocol, DBCG 82-IS, implicating registration of patients with a histologically verified diagnosis of DCIS and/or LCIS and treated with breast conserving surgery (BCS). Patients treated by mastectomy were not included in the protocol. For patients with a diagnosis of LCIS, long-term observation was initiated after diagnostic excision biopsy. All patients were followed by two clinical examinations and one mammography annually.
In the primary registration charts the focus was based on registration of LCIS and DCIS, as well as atypical lesions of lobular and ductal type and furthermore on the registration of both non-proliferative and proliferative benign diseases. The registration of benign lesions was however omitted in the following DBCG-IS protocols since these lesions proved not to be risk factors for invasive carcinoma. The risk factors were limited to include atypical hyperplastic lesions of ductal or lobular type along with a family history of breast cancer Citation[10].
Supervised by Mogens Blichert-Toft and Johan A Andersen, protocol DBCG 82-IS has been thoroughly investigated Citation[11–14].
Gyda Ottesen et al. performed a major follow-up study of the DBCG82-IS registered patients 1982–1990 Citation[14]. The study showed that the main factors for predicting recurrent disease or invasive carcinoma in an univariate analysis were high nuclear grade, presence of comedo necrosis and size of the lesion, thus supporting the data published by Silverstein et al. Citation[15].
As for LCIS the study added the new insight to the natural history that the majority of recurrences were ipsilateral Citation[3], Citation[13]. Furthermore the study showed similar behaviour of low grade DCIS and LCIS with regard to recurrence rates, confirming the assumption that these lesions may arise via common pathways of tumorigenesis Citation[16]. In case of recurrence as invasive cancer after a primary diagnosis of LCIS the majority appeared as invasive ductal carcinoma Citation[17].
DBCG 1989-IS
In 1989 a revised CIS pathology form introduced growth pattern, subtypes for DCIS and included PDN. Patients treated by mastectomy were now included.
During the DBCG 89-IS, malignancy grading of DCIS according to recommendations by Holland et al. was added to previous characteristics Citation[18]. The DBCG 89-IS protocol also included information on resection margin (RM) since Silverstein et al. documented a reduction of recurrence rate when free RM was achieved after BCS Citation[15], Citation[19].
Both Ottesen and Blichert-Toft investigated the significance of growth pattern Citation[14], Citation[20]. It was hypothesized that a distinction between microfocal, tumour forming and diffuse growth pattern was related to biology rather than the histogenetic subtypes, but eventually growth pattern and histological subtypes except for the papilliferous type proved not to be significantly associated with recurrence.
Convincing studies published in 1986 indicated a significant reduction in recurrence rate by additional radiotherapy (RT) after BCS Citation[21] and consequently, RT was introduced as supplementary treatment to BCS in the DBCG 89-IS protocol. In 2004, RT finally became a fully integrated part of the treatment strategy for DCIS.
Introduction of mammography screening
In the 1990′s two events had a major impact on the diagnosis and treatment of CIS; the first event was the establishment of two centers for organized mammography screening for breast cancer in Copenhagen and in the county of Funen (Odense). The second major event was the introduction of Guidelines for Quality Assurance in Breast Cancer by the European Commission in accordance with the UK National Health Service Breast Screening Programme publications Citation[22]. The two Danish screening centers and DBCG adopted the guidelines including the diagnostic categories for non-operative needle biopsy procedures. The diagnosis of non-palpable lesions was now possible mainly through mammography detected microcalcifications and ultra-sonography or stereotactic guided Fine Needle Aspiration Cytology and Needle Core Biopsy.
DBCG 2004-IS
In 2004, a new CIS pathology form was launched, DBCG 2004-IS, with the Van Nuys classification of DCIS, including registration of microcalcifications. The information on the axillary status now included the Sentinel Lymph Node Biopsy (SLNB) only.
SLNB was recommended in cases of DCIS with large solid tumours, diffuse microcalcifications, high nuclear grade or suspicion of microinvasion Citation[23].
A variety of histopathological classification systems for DCIS had been applied in order to define the invasive potential and risk of recurrence. However, reproducibility was poor with the exception of the Van Nuys classification Citation[24]. The Van Nuys classification combines nuclear grade and the presence of necrosis in three distinct groups. Silverstein et al. showed that the classification significantly separated the three groups according to local recurrence free survival Citation[15], Citation[19].
Finally BCS followed by RT was now applied as a fully integrated part of the treatment strategies for DCIS. The benefit of RT had been clearly demonstrated in both the NSABP B-17 trial and the EORTC 10653 trial with a reduction of the recurrence rate from approximately 30 to 15% during a 10 year follow-up Citation[25].
Consequently, at present the main known factors determining risk of local recurrence and progression to invasive carcinoma are extent of disease, status of the resection margins, cyto-nuclear differentiation, presence of necrosis, young age at primary diagnosis and a family history of breast cancer.
DBCG 2006-IS-and molecular pathology
In the DBCG 2006-IS form, pleomorphic LCIS (PLCIS) was inserted and mammographic /sonographic findings were added to the clinical findings.
LCIS
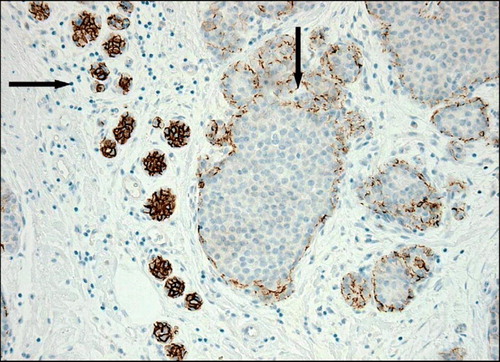
Molecular studies including comparative genome hybridization support the perception of LCIS being a true precursor lesion since loss of 16q has been demonstrated in both LCIS and invasive lobular carcinoma. The candidate gene being CDH1, which maps to 16q22.1 the locus for the adhesion molecule E-cadherin Citation[26]. Loss of E-cadherin is a characteristic feature for both LCIS and invasive lobular carcinoma. The expression of E-cadherin can be determined by immunohistochemistry (IHC) (). All subtypes of LCIS are associated with ER expression. Recently a subgroup of LCIS described as PLCIS has been characterized. PLCIS is more aggressive, may show over-expression of growth factor receptor HER2/neu (HER2) and is often seen in association with pleomorphic invasive lobular carcinoma Citation[27]. Consequently, patients with a diagnosis of PLCIS are treated in accordance to DCIS in the DBCG 2006-IS protocol.
DCIS
Immunohistochemical staining for ER and PR in DCIS has demonstrated a positive reaction in approximately 50% of the lesions Citation[28]. Clinical trials of tamoxifen treatment have shown some or no benefit in terms of reducing recurrence and invasive cancer development but such trials have been hampered by failure to assess ER prior to entry. A subsequent study of the NSABP trial demonstrated a significant effect of tamoxifen only in ER positive DCIS Citation[29]. Tamoxifen is however not in widespread use for DCIS and has not been applied in the DBCG-IS protocols.
Over-expression of HER2 has been detected in more than 50% of DCIS lesions and has been associated with higher risk of recurrence Citation[28]. Similarly, investigations of the expression of type 1 tyrosine kinase receptors (RTK) HER1–4 indicate that co-expression of HER4 and HER2 reduces the risk of recurrence and is associated with both higher ER expression and lower rates of cellular proliferation Citation[30].
In conclusion specific genetic alterations in both DCIS and LCIS seem to be associated with disease outcome and there is clearly a need for a better understanding of the significance of these alterations.
Characterization of the Danish CIS population
In the next passage a brief characterization of the Danish CIS population will be presented. The introduction of mammography screening in the two regions in Denmark resulted in an overall increase in the number of DBCG registered patients with a diagnosis of CIS. From 1982–1989 only patients with BCS were registered in DBCG, hence the small number of CIS in that period. The rate rose from 88 cases in 1983 to 118 cases in 1989. One hundred forty-two cases were diagnosed in 1990 increasing to 212 cases in 2004. Presently, CIS accounts for approximately 5% of diagnosed breast malignancies in Denmark. The screen-related increase in the number of CIS was mainly restricted to the histological subtype of pure DCIS. The rate of DCIS increased from 108 cases in 1990 to 188 cases in 2004. The number of LCIS has been fairly constant since 1996 with a median of 16 cases annually (range 10–20) consistent with LCIS being a rare screen-detected lesion.
In the county of Funen organized mammography screening for breast cancer was introduced in 1993 and CIS constituted 14% of the detected cases in the first round Citation[31].
Presently CIS accounts for 12% of screen-detected breast malignancies. None of the screen-detected lesions represent LCIS.
Since 1983 a total number of 3 256 CIS patients from the whole country have been registered by the DBCG. Of these, 367 are screen-detected by the two screening centers. A total number of 387 patients with a primary diagnosis of CIS experienced invasive recurrence. Forty-eight of these patients had a primary screen-detected CIS. summarizes the histopathological findings for the entire CIS population, sub-classified in two groups; non screen-detected CIS and screen-detected CIS. Each group is further divided according to appearance of invasive recurrence (IR). The number of unknown cases is high for some parameters, since they were not recorded in the early DBCG-IS pathology forms. A significant difference (χ2 p < 0.01) for all parameters (exclusive unknown) was shown between the non screen-detected CIS group and the screen-detected CIS group (). Furthermore, the screen-detected patients with subsequent IR are characterized by CIS lesions <20mm, high nuclear grade (2–3) and presence of necrosis. In general, higher nuclear grade and necrosis are characteristic findings in the screen-detected lesions probably reflecting the diagnostic approach of CIS by mammography identifying primarily calcifications, which are often localized in the necrotic area of CIS.
Table I. Histopathological parameters; Lesion size, resection margin, nuclear grade and necrosis related to screen-detection status and invasive recurrence (IR) among 3 256 Danish women diagnosed with carcinoma in situ of the breast 1983–2005
Although the majority of lesions are smaller than 20 mm the non-screen detected group of patients with subsequent IR also includes lesions >20mm. The lesions are likewise characterized by high nuclear grade but the presence of necrosis is not a dominant feature as compared to the screen-detected lesions.
The relationship between the histopathological subtype of IR and the original CIS lesion is presented in . The majority of invasive recurrences represent invasive ductal carcinoma regardless of the subtype of the original CIS. Only 12/59 (20%) of the primary LCIS lesions later presented as invasive lobular carcinoma (exclusive unknown). This phenomenon may relate to the presumption that LCIS and DCIS share common pathways of tumorigenesis Citation[16] and furthermore, low grade DCIS and LCIS show morphological identical features resulting in diagnostic difficulties, which however presently can be overcome by IHC for E-cadherin. The localization of IR presented in shows no difference between the screen-detected group and the non screen-detected group; 193/323 (60%) of invasive recurrences in the non screen-detected group presented in the ipsilateral breast (exclusive unknown) compared to 26/46 (57%) in the screen-detected group (exclusive unknown).
Table II. Type of carcinoma and localization in the breast among 387 cases of invasive recurrence (IR) treated in Denmark 1983–2005.
Over-all survival (OS) for DBCG 89-IS registered patients is presented in . Log rank test shows no significant difference (p = 0.67) between the screen-detected and non screen-detected groups of patients. The 10-year OS rates are 87.1% (95 CI, 82.8–91.3%) and 86.9% (95 CI, 85.1–88.7%) for the screen-detected and non screen-detected groups respectively. Likewise as presented in , log rank test shows no significant difference (p = 0.06) between the screen-detected and non screen-detected groups of patients in overall 10-year invasive recurrence (IR) free survival. The 10-year IR free survival rates are 82.9% (95 CI, 78.0–87.8%) and 87.1% (95 CI, 85.7–88.5%) for the screen-detected and non screen-detected groups respectively.
Figure 4. Kaplan–Meier estimates of overall survival (OS) for a total number of 2 651 Danish women diagnosed with carcinoma in situ of the breast and registered in DBCG 89-IS protocols sub-classified according to screen detection status. Screen-detected CIS (+), non screen-detected CIS (−).
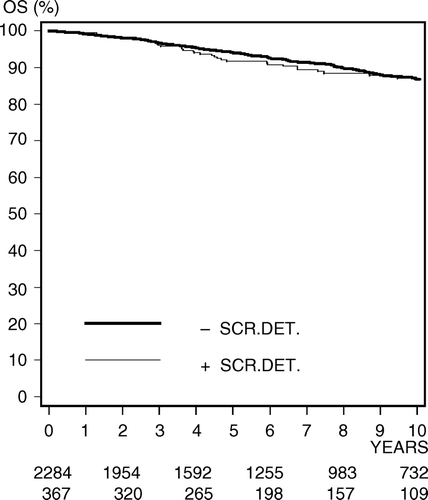
Figure 5. Kaplan-Meier estimates of invasive recurrence free survival among a total of 3 256 Danish women diagnosed with carcinoma in situ of the breast 1983–2005. Interval from primary diagnosis of CIS to invasive recurrence (IR). Screen-detected CIS (+), non screen-detected CIS (−).
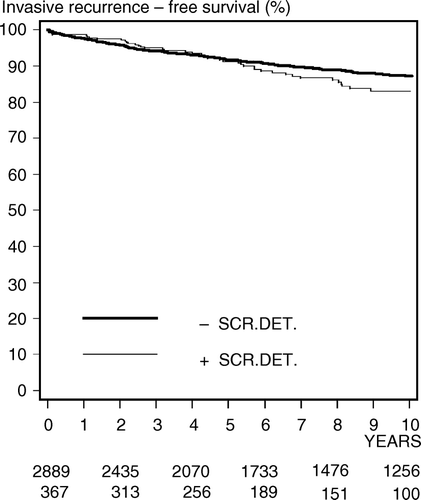
Finally, when the patients are grouped according to whether they experienced invasive recurrence or not (+/− IR), log rank test shows not unexpectedly a significant difference (p < 0.01) between the two groups. The 10-year OS rates are 82.8% (95 CI, 78.0–87.8%) and 87.8% (95 CI, 86.1–89.6%) for the +IR and −IR groups respectively.
Conclusion
In conclusion, as a consequence of the 30 years of outstanding efforts by the DBCG it has been possible to implement nation wide treatment regimens for this very heterogeneous patient population. In view of the evolving insight into modern molecular pathology, individualized treatment stratification with respect to optimization of the distinction between high and low risk patients might be possible in the future.
References
- Nielsen M, Jensen J, Andersen J. Precancerous and cancerous breast lesions during lifetime and at autopsy: A study of 83 women. Cancer 1984; 54: 612–5
- Rosen PP, Kosloff C, Lieberman PH, Adair F, Braun DW, Jr. Lobular carcinoma in situ of the breast: Detailed analysis of 99 patients with average follow-up of 24 years. Am J Surg Pathol 1978; 2: 225–51
- Andersen JA. Lobular carcinoma in situ: A long-term follow-up in 52 cases. Acta Pathol Microbiol Scand Sect A 1974; 82: 519–33
- Fisher ER, Land SR, Fisher B, Mamounas E, Gilarski L, Wolmark N. Pathological findings from the National Surgical Adjuvant Breast and Bowel Project. Twelve-year observations concerning lobular carcinoma in situ. Cancer 2004; 100: 238–44
- McCann J, Treasure P, Duffy T. Modelling the impact of detecting and treating carcinoma in situ in a breast screening programme. J Med Screen 2004; 11: 117–25
- Page DL, Dupont WD, Rogers LW, Jensen RA, Schuyler PA. Continued local recurrence of carcinoma 15–25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer 1995; 76: 1197–200
- Fisher ER, Dignam J, Tan-Chiu E, Costantino J, Fisher B, Paik S, et al. Pathological findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17 Intraductal carcinoma. Cancer 1999; 86: 429–38
- Andersen JA. Lobular carcinoma in situ of the breast. An approach to rational treatment. Cancer 1977; 39: 2597–602
- Andersen JA. Lobular carcinoma in situ. A histological study of 52 cases. Acta Pathol Microbiol Scand A 1974; 82: 735–41
- Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer 1985; 55: 2698–708
- Graversen HP, Blichert-Toft M, Dyreborg U, Andersen JA. In situ carcinomas of the female breast. Incidence, clinical findings and DBCG proposals for management. Acta Oncol 1988; 27: 679–82
- Ottesen GL, Graversen HP, Blichert-Toft M, Zedeler K, Andersen JA. Ductal carcinoma in situ of the female breast. Short term results of a prospective nationwide study. Am J Surg Pathol 1992; 16: 1183–96
- Ottesen GL, Graversen HP, Blichert-Toft M, Zedeler K, Andersen JA. Lobular carcinoma in situ of the female breast. Short term results of a prospective nationwide study. Am J Surg Pathol 1993; 17: 14–21
- Ottesen GL, Graversen HP, Blichert-Toft M, Christensen IJ, Andersen JA. Carcinoma in situ of the female breast. 10 year follow-up results of a prospective nationwide study. Breast Cancer Res Treat 2000; 62: 197–210
- Silverstein MJ, Poller DN, Waisman JR, Colburn WJ, Barth A, Gierson ED, et al. Prognostic classification of ductal carcinoma-in-situ. Lancet 1995; 345: 154–7
- Simpson PT, Reis-Filho JS, Gale T, Lakhani SR. Molecular evolution of breast cancer. J Pathol 2005; 205: 248–54
- Ottosen GL. Carcinoma in situ of the female breast. APMIS 2003; 111(Suppl.108)1–67
- Holland R, Peterse JL, Millis RR, Eusebi V, Faverly D, van de Vijver MJ, et al. Ductal carcinoma in situ-a proposal for a new classification. Semin Diagn Pathol 1994;11:167–80.
- Silverstein MJ. The University of Southern California/Van Nuys prognostic index for ductal cancinoma in situ of the breast. Am J Surg 2003; 186: 337–43
- Blichert-Toft M, Graversen HP, Andersen J, Dyreborg U, Green A. In situ carcinomas: A population based study on frequency, growth pattern and clinical aspects. World J Surg 1988; 2: 845–51
- Zafrani B, Fourguet A, Vilcoq JR, Legal M, Calle R. Conservative management of intraductal breast carcinoma with tumorectomy and radiation therapy. Cancer 1986; 57: 1299–301
- Perry N, Broeders M, Wolf C, Tornberg S, Holland R, Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis4th ed. European Communities. 2006
- Veronesi P, Intra M, Vento AR, Naninato P, Caldarella P, Paganelli G, et al. Sentinel lymph node biopsy for localised ductal carcinoma in situ?. Breast 2005; 14: 520–2
- Sloane JP, Amendoeira I, Apostolikas N, Bellocq JP, Bianchi S, Boecker W, et al. Consistency achieved by 23 European pathologists in categorizing ductal carcinoma in situ of the breast using five classifications. European Commission Working Group on Breast Screening Pathology. Hum Pathol 1998; 29: 1056–62
- Breast Cancer Cooperative Group EORTC, Radiotherapy Group EORTC, Bijker N, Meijnen P, Peterse JL, Bogaerts J, Van Hoorebeeck I, Julien JP, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: Ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853–a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol 2006; 24: 3381–7
- Shelley Hwang E, Nyante SJ, Chen YY, Moore D, DeVries S, Korkola JE, et al. Clonality of lobular carcinoma in situ and synchronous invasive lobular carcinoma. Cancer 2004; 100: 2562–72
- Reis-Filho JS, Simpson PT, Jones C, Steele D, Mackay A, Iravani M, et al. Pleomorphic lobular carcinoma of the breast: Role of comprehensive molecular pathology in characterization of an entity. J Pathol 2005; 207: 1–13
- Kepple J, Henry-Tillman RS, Klimberg VS, Layeeque R, Siegel E, Westbrook K, et al. The receptor expression pattern in ductal carcinoma in situ predicts recurrence. Am J Surg 2006; 192: 68–71
- Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the national surgical adjuvant breast and bowel project experience. Semin Oncol 2001; 4: 400–18
- Barnes NLP, Khavari S, Boland GP, Cramer A, Knox F, Bundred NJ. Absence of HER4 expression predicts recurrence of ductal carcinoma in situ of the breast. Clin Cancer Res 2005; 11: 2163–68
- Njor SH, Olsen AH, Bellstrøm T, Dyreborg U, Bak M, Axelsson C, et al. Mammography screening in the county of Fyn. November 1993–December 1999. APMIS 2003; Suppl ; 110(111)1–33