Abstract
Purpose. To examine p53 and BCL2 expression in high-risk breast cancer patients randomized to postmastectomy radiotherapy (PMRT). Patients and methods. The present analysis included 1 000 of 3 083 high-risk breast cancer patients randomly assigned to PMRT in the DBCG82 b&c studies. Tissue microarray sections were stained with immunohistochemistry for p53 and BCL2. Median potential follow-up was 17 years. Clinical endpoints were locoregional recurrence (LRR), distant metastases (DM), overall mortality, and overall survival (OS). Statistical analyses included Kappa statistics, χ2 or exact tests, Kaplan-Meier probability plots, Log-rank test, and Cox univariate and multivariate regression analyses. Results. p53 accumulation was not significantly associated with increased overall mortality, DM or LRR probability in univariate or multivariate Cox regression analyses. Kaplan-Meier probability plots showed reduced OS and improved DM and LRR probabilities after PMRT within subgroups of both p53 negative and p53 positive patients. Negative BCL2 expression was significantly associated with increased overall mortality, DM and LRR probability in multivariate Cox regression analyses. Kaplan-Meier probability plots showed a significantly improved overall survival after PMRT for the BCL2 positive subgroup, whereas practically no survival improvement was seen after PMRT for the BCL2 negative subgroup. In multivariate analysis of OS, however, no significant interaction was found between BCL2 and randomization status. Significant reductions in LRR probability after PMRT were recorded within both the BCL2 positive and BCL2 negative subgroups. Conclusion. p53 was not associated with survival after radiotherapy in high-risk breast cancer, but BCL2 might be.
Most cell killing after radiotherapy is probably due to mitotic cell death Citation[1]. However, some of the cell killing is due to apoptosis, which seems commonly to be a p53-dependent process Citation[2]. In agreement herewith p53 mutated cells have in some experimental studies been associated with radioresistance Citation[3], Citation[4] or with a time delay for the radiation to induce apoptosis Citation[5]. Within a clinical setting associations between potential markers and radioresistance may be exposed most optimally examining loco-regional recurrence (LRR) probability among patients randomized to receive or not receive radiotherapy radiotherapy. Until now, such as study has never been published for p53 and breast cancer. Askmalm et al. have examined p53 alterations in 266 mainly node positive patients randomized to CMF or radiotherapy. A significant reduction in LRR probability after radiotherapy as compared with CMF was found in the group of patients without p53 alterations, whereas no benefit from radiotherapy was found for the group of patients showing p53 alterations Citation[6]. One of the first cell death-regulating genes to be identified was BCL2, an anti-apoptotic gene that among others appears to block a distal step in the p53 pathway crucial to apoptosis. Only few studies have dealt with BCL2 and radioresistance, of which two cell culture studies have suggested a relationship between BCL2 and radioresistance Citation[7], Citation[8]. As for p53, no published studies have to our knowledge examined BCL2 in breast cancer patients randomized to receive or not receive radiotherapy radiotherapy. We hypothesize that immunohistochemical p53 positive and BCL2 positive tumors, when examined as single markers, will be associated with significantly smaller reductions in LRR probability after PMRT, but that patients with a combined variable of both p53 positive and BCL2 positive tumors experience a notably smaller reduction in LRR probability after PMRT.
P53 positive and BCL2 negative tumors have been significantly associated with reduced survival from breast cancer in several studies Citation[9–20] although not consistently Citation[21–28]. Tumors characterized by biological markers of reduced survival may have a particularly aggressive tumor subtype. Such a tumor subtype may already at time of diagnosis have developed distant micrometastases. These distant micrometastases may, if it is a very aggressive subtype, be unresponsive to the systemic therapy applied. As a consequence these patients may not experience a survival improvement after locoregional PMRT as compared with patients having biologically less aggressive tumors responding to the systemic therapy applied. Based on the presented assumption, a second hypothesis was formulated. In case, p53 positive and BCL2 negative tumors are significantly associated with reduced survival, we hypothesize that no survival improvement after PMRT will be seen within these poor prognostic subgroups of patients.
Materials and methods
Patients
For a small method study, in which stainings of TMA biopsies were compared with stainings of whole sections, 27 patients with at least two paraffin blocks from the same primary tumor were selected. For details, see Kyndi et al. Citation[29] and Offersen et al. Citation[30].
In addition, a subgroup of the 3 083 high-risk Danish breast cancer patients included in the DBCG82 b&c studies in the period 1982–1990 was selected for biological analyses. The 3 083 patients have been described in detail in previous publications Citation[31], Citation[32]. In brief, high-risk was defined as either positive lymph nodes and/or tumor size larger than 5 cm and/or invasion of tumor to surrounding skin or pectoral fascia. All women had a total mastectomy and a partial axillary dissection. A median of 7 lymph nodes was removed from the axilla. The pre-menopausal women were enrolled in the DBCG82 b protocol and were randomized to either radiotherapy + CMF chemotherapy (8 cycles) or to CMF chemotherapy alone (9 cycles) Citation[31]. The post-menopausal women were enrolled in the DBCG82 c protocol and were randomized to either radiotherapy + tamoxifen (30 mg daily/one year) or to tamoxifen alone Citation[32]. Recently a long-term clinical follow-up has been performed for the patients Citation[33]. Median potential follow-up time was 17 years. The subgroup, selected for biological analyses consisted of 1 241 patients with at least 8 lymph nodes surgically removed. Of these patients, paraffin embedded tumors blocks were available from 1 078 patients.
Methods
For the small method study at least two paraffin embedded tumor blocks were collected, sectioned, and hematoxylin-eosin (HE) stained from each of the 27 patients and for the DBCG82 b&c study at least one paraffin block from each of the 1 078 patients. Invasive tumor was verified and marked on HE-sections from all 54 paraffin blocks in the method study and on sections from 1 000 of the DBCG82 b&c paraffin blocks. A central and a peripheral 1mm TMA core including invasive tumor were transferred to tissue microarrays (TMA)s from each of the 54 paraffin blocks and a central 1mm core from each of the 1 000 paraffin blocks. All TMAs and the 54 paraffin blocks were sectioned and sections were stored at 4°C until manual staining for p53 and BCL2. For details regarding handling of the biological material, including construction of the TMAs and immunohistochemical (IHC) stainings for estrogen receptor (ER), and progesterone receptor (PgR), and IHC stainings and FISH analyses for the HER2 receptor, see Kyndi et al. Citation[29]. Prior to IHC staining for p53 and BCL2, deparaffinization with 99% ethanol, blocking of the endogen peroxidase with H2O2, and epitope retrieval with microwaves was performed. Sections were IHC stained for p53 with the monoclonal antibody DO-7 from Dako, Glostrup, Denmark (K4001) at a dilution of 1:600 and for BCL2 with the monoclonal antibody Anti-Human BCL2 Oncoprotein also from Dako, Glostrup, Denmark (M0887) at a dilution of 1:400. The sections were incubated over night at a temperature of 4°C. On day two the sections were incubated with a peroxidase conjugated to goat antimouse immunoglobulins (DAKO Envision Mouse, K4001, Dako, Glostrup, Denmark) for 60 minutes at room temperature. Sections stained for p53 and BCL2 and were then stained with Novared (Vector, SK-4800) and DAB (Dako, K3468), respectively, and afterwards all counterstained with haematoxylin. Sections from tumors previously defined as positive when stained with the respective antibodies were additionally stained with and without the primary antibody and served as controls.
For both stainings, percentage of invasive tumor with nuclear p53 staining and cytoplasmic BCL2 staining was recorded, as was intensity (on a scale from 0 to 3). All sections were scored semi-quantitatively by one observer. If any doubt occurred during scoring of the DBCG82 sections a second observer was consulted and agreement was reached with consensus diagnosis. TMA cores were scored if they contained at least 10 invasive tumor cells. In the DBCG82 b&c study p53 was recorded as positive if either no nuclear staining at all was found (0%) or if more than 49% of invasive tumor nuclei stained with strong intensity (score 3). The p53 cutpoint was chosen with reference to the study by Alsner et al. showing a high proportion of null-mutations (producing no detectable p53 protein) among tumors with no nuclear IHC staining for p53 as well as the highest proportion of missence mutations among tumors with more than 49% of invasive tumor nuclei staining for p53 Citation[34].
BCL2 was recorded as positive when cytoplasmic staining at any intensity was found in more than 10% of invasive tumor.
Statistics
The small method study was analyzed using Kappa statistics. In the DBCG82 b&c study the χ2 or exact tests were used for testing relationships between variables. Kaplan-Meier probability curves were made and tested for differences by a log-rank test. Prognostic value of p53 and BCL2 was analyzed using Cox univariate and multivariate regression analyses. Tests of interactions were applied to Cox multivariate regression analyses, as well. Hazard ratios (HR)s provided on Kaplan-Meier overall survival (OS) probability plots were HRs of overall mortality. Level of significance was set to 5% and all estimated p-values were two-tailed. Statistical endpoints were isolated locoregional recurrence (LRR), distant metastases (DM), and OS. Statistical calculations were performed using the statistical program STATA version 8.2.
Results
Agreement between p53 and BCL2 stainings of TMAs and full sections
Using a cutpoint of 10% invasive tumor staining determining p53 positivity, a poor agreement was seen between TMA cores and full sections (κ = 0.4). However, using a cutpoint determining tumor positivity of either 0% or at least 50% of invasive tumor staining with a strong intensity, the reproducibility between TMA cores and full sections improved (κ = 0.6). For BCL2 a very good reproducibility was found between stainings of TMA cores and full sections (κ = 0.8) using a cutpoint of at least 10% invasive tumor staining with any intensity determining tumor positivity.
DBCG82 b&c
The subgroup of 1 000 patients was well-distributed between the two randomization arms for all classical clinical-pathological parameters including p53 and BCL2 ().
Table I. Distribution of different clinical-pathological parameters and biological parameters including p53 and BCL2, among 1 000 high-risk breast cancer patients randomized to +/− post-mastectomy radiotherapy.
Frequencies of p53 and BCL2
P53 expression was assessable in 928 TMA cores. Four hundred and eight tumors (44%) were recorded as p53 positive using the cutpoint of either 0% or at least 50% invasive tumor staining with a strong intensity. BCL2 expression was assessable in 933 TMA cores and 551 (59%) were recorded as BCL2 positive using the cutpoint of at least 10% invasive tumor staining with any intensity.
Prognostic value of p53 and BCL2
A significant association was found between p53 accumulation and other biological markers of poor prognosis such as grade 3 malignant tumors, hormonal receptor negative tumors, HER2 positive tumors and BCL2 negative tumors, but not with the classical clinical-pathological markers: more than 3 positive lymph nodes and large tumor size (). No significant association was found between p53 accumulation and increased overall mortality, DM or LRR probability in univariate or multivariate analyses in the total patient group with valuable p53 stainings, (univariate analyses: HR: 1.09 (0.93–1.27), HR: 1.05 (0.87–1.23) and HR: 1.21 (0.86–1.70), respectively).
Table II. Distribution of p53 and BCL2 between different clinical-pathological and biological parameters among high-risk breast cancer patients randomized to +/− postmastectomy radiotherapy.
Negative BCL2 expression was significantly associated with all other biological as well as clinical-pathological markers of poor prognosis, such as more than 3 positive lymph nodes, large tumor size, grade 3 malignant tumors, hormonal receptor negative tumors, HER2 positive tumors, and p53 positive tumors (). In addition, negative BCL2 expression was significantly associated with increased overall mortality, DM and LRR probability in both univariate and multivariate Cox regression analyses for the total patient population with valuable BCL2 stainings (multivariate analyses: HR: 1.26 (1.05–1.51), HR: 1.29 (1.08–1.55), and HR: 1.98 (1.41–2.77), respectively). In fact, BCL2 turned out a stronger prognostic marker of LRR than did positive lymph nodes (p < 0.001), it was only outperformed by randomization status.
A combined variable of positive p53 and negative BCL2 was significantly associated with increased overall mortality and LRR probability in univariate analyses but not with increased DM probability. Besides, the significance disappeared for OS in multivariate regression analyses and the combined variable was only significantly associated with increased LRR, however not as robustly as was BCL2 alone (HR 1.69 (1.16–2.47) (p = 0.006)).
P53 and BCL2 and response to postmastectomy radiotherapy
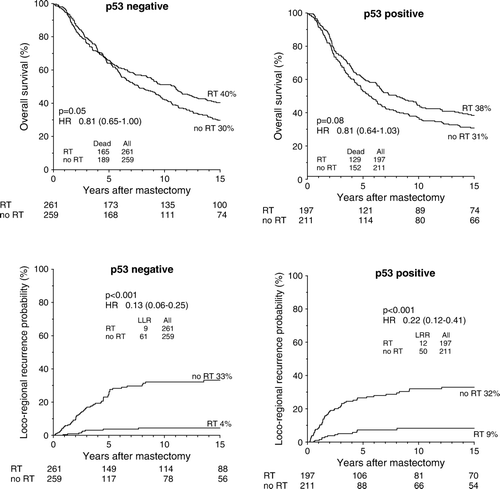
An improved OS probability after PMRT was seen both within the subgroup of p53 negative and the subgroup of p53 positive patients () as was reduced DM and LRR probabilities. Hazard ratios (HR)s and confidence intervals (CI)s did not differ significantly between the p53 positive and p53 negative subgroups for any of the three endpoints analyzed (p = 1.0), (p = 0.7), and (p = 0.3), respectively. Additionally, no significant interactions between p53 and randomization status were found in Cox multivariate regression analyses for any of the three endpoints analyzed. Changing the cutpoint determining tumor positivity did not improve the predictive value of positive p53 staining (data not presented).
Figure 1. Kaplan-Meier probability plots of overall survival and loco-regional recurrence in high-risk breast cancer patients as a function of randomization to postmastectomy radiotherapy within the subgroups of p53 negative and p53 positive patients.
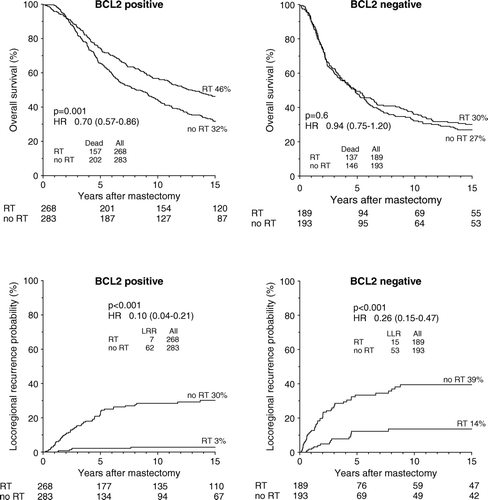
A highly significantly improved OS after PMRT was seen within the good prognostic subgroup of BCL2 positive patients, log rank test p = 0.001, HR 0.70, (0.57–0.86), whereas no significant survival improvement was seen after PMRT within the poor prognostic subgroup of BCL2 negative patients, log rank test p = 0.4, HR 0.94, (0.75–1.20), (). Similar tendency was found for DM probability, HR 0.66 (0.52–0.83) and HR 0.90 (0.69–1.16), respectively. Significant reductions in LRR probability after PMRT were recorded within both the BCL2 positive and BCL2 negative subgroups (). Hazard ratios (HR)s and confidence intervals (CI)s did not differ significantly between the BCL2 positive and BCL2 negative subgroups for any of the three endpoints analyzed, although they were all borderline-significant (p = 0.06), (p = 0.07), and (p = 0.06), respectively. Besides, no significant interactions between BCL2 and randomization status were found in Cox multivariate regression analyses for any of the three endpoints analyzed.
Figure 2. Kaplan-Meier probability plots of overall survival and loco-regional recurrence in high-risk breast cancer patients as a function of randomization to postmastectomy radiotherapy within the subgroups of BCL2 positive and BCL2 negative patients.
Combining p53 positive tumor status and BCL2 positive tumor status did not reveal a significantly smaller reduction in LRR probability after PMRT for that particular subgroup as compared with the remaining patients (p = 0.3). When the p53 positive subgroup was combined with the BCL2 negative subgroup a borderline-significantly smaller reduction in LRR probability after PMRT revealed as compared with the remaining patients (p = 0.06). No significant interactions were found between the BCL2 negative p53 positive subgroup and radiotherapy in multivariate regression analysis of LRR. A significant survival improvement after PMRT was found only for the good prognostic subgroup of BCL2 positive and/or p53 negative patients (HR 0.74 (0.62–0.89)) and not for the poor prognostic subgroup of BCL2 negative and p53 positive patients (HR 0.94 (062–1.28)). Hazard ratios and confidence intervals, however, were not significantly different (p = 0.2) and no significant interactions were found between the BCL2 negative p53 positive subgroup and radiotherapy in multivariate regression analysis of OS.
Discussion
Neither positive p53 status nor positive BCL2 status were associated with a significantly smaller reduction in LRR probability after PMRT. The probability of locating a single marker associated with radioresistance in a clinical study randomizing patients to receive or not receive radiotherapy, however, may also be minimal as a single marker will constitute only a tiny piece of a large picture consisting of many markers probably influencing response to radiotherapy in each direction and maybe each other as well. Not surprisingly, considering the Kaplan-Meier probability plots, combining positive p53 and positive BCL2 status did not reveal an even smaller reduction in LRR probability. Based on these findings our first hypothesis was rejected. It may though seem from Kaplan-Meier probability plots that a slightly smaller LRR reduction after PMRT was found among p53 positive patients as compared with p53 negative patients. If true, that could be in agreement with findings from a large study by Zellars et al.Citation[35], in which accumulation of p53 protein was examined in 1 530 mastectomy-treated breast cancer patients, of whom 259 were additionally treated with radiotherapy. Among the 1 271 patients not receiving radiotherapy the proportion of patients having a LRR was 9.1% among the p53 negative and 16.5% among the p53 positive. In the radiotherapy group similar proportions were found: 9.3% among the p53 negative and 21.5% among the p53 positive patients. The association was statistically significant in multivariate analyses. Nevertheless, a study by Silvestrini et al. suggested that p53 accumulating tumors had a preferential benefit on LRR probability from radiotherapy Citation[36]. The same study, however, suggested a preferential benefit on LRR probability from radiotherapy for tumors with negative BCL2 status, which was also in disagreement with the findings presented in this study. Furthermore, in disagreement with our results, a clinical study including 124 laryngeal cancers found an association between positive BCL2 and radio-resistance Citation[37]. One point of criticism is our use of Kaplan-Meier probability plots to examine LRR probability. This statistical methodology is not appropriate for endpoints subjected to competing risk, such as LRR. Kaplan Meier probability plots may underestimate the true rates of LRR by assuming that patients with competing risk (DM, contra lateral breast cancer, death) have a LRR risk equivalent to that of the overall population in the study, although these patients probably have more aggressive disease and thereby a higher risk of LRR. Furthermore, although being the largest study so far having addressed this issue in breast cancer patients randomized to receive not receive radiotherapy, the subgroup analyses are not appropriately powered for the statistical analyses, and the results need to be confirmed in a larger scaled study.
The second hypothesis was verified. Negative BCL2 expression was significantly associated with reduced OS and no obviously improved survival probability after PMRT was seen for this subgroup of patients, whereas p53 accumulation was not significantly associated with reduced survival and an improved survival probability after PMRT was located both among p53 positive and p53 negative patients. The fact that we did not locate a significant association between p53 accumulation and reduced survival or increased LRR probability is not entirely surprising as contradictory results have been reported so far. Nevertheless, many large IHC studies, examining several hundreds of patients, have reported an independent prognostic value of p53 accumulation in Cox multivariate regression analyses Citation[9], Citation[10], Citation[12], Citation[13]. And in two recent studies of respectively 438 and 1 076 node negative and positive patients, in which IHC markers were examined with clustering analyses, p53 accumulation was significantly associated with survival in univariate analyses (multivariate analyses were not presented) Citation[38] and moreover turned out very important for the formation of prognostic clusters Citation[38], Citation[39]. The prognostic significance of p53 for LRR is suggestive as well, but also for this endpoint conflicting data exist Citation[40]. It cannot, however, be ruled out that the lacking prognostic and predictive value of p53 expression may owe to the IHC method applied. Immunohistochemically detected p53 accumulation certainly encompass some methodological concerns and p53 protein accumulation measured by immunohistochemistry has been shown to correlate with TP53 mutations detected by sequencing in less than 75% of breast carcinomas Citation[41], Citation[42] and even worse in a study be our group, in which TP53 mutations were found in only 40% of the tumors with strong p53 staining of at least 50% of invasive tumor Citation[34]. This finding may among others owe to normal non-mutated p53 protein accumulating in some cells as a result of either a response to DNA damage, binding to other cellular components, or the failure of feedback loops or degradation pathways to appropriately down-regulate protein levels. Notably, we also found that 40% of tumors with practically no p53 staining had null-mutations, which was the reason that we chose to use the cutpoint determining p53 positivity of either 0% or more than 49% strong staining for p53. After publications by Sjögren et al. Citation[43], Norberg et al. Citation[41], and Geisler et al. Citation[42] it has been commonly accepted that p53 gene mutations obtained by sequencing provide superior prognostic information to p53 accumulation determined by IHC. Other methodological aspects explaining part of the lacking prognostic relevance found for p53 in this study may relate to the use of TMAs. A poor agreement was found for p53 stainings of TMA cores and full sections using a cutpoint of at least 10% invasive tumor staining but was improved a little (although not to a fully acceptable level) using the cutpoint of either 0% or at least 50% invasive tumor staining with a strong intensity. Other studies, however, have reported a better agreement, although with minor regional variations Citation[44], Citation[45]. Nevertheless, in conclusion, examining p53 status with IHC may explain the lacking prognostic value of p53 in this study.
Regarding the prognostic value of BCL2 contradictory results have been published, as well. Some studies did not reveal an independent prognostic significance of BCL2 Citation[26–28], whereas many studies have shown a significant association between BCL2 positivity and favorable survival in multivariate analyses Citation[15–20]. In conclusion, many of the studies reported were small and examined only few markers in parallel. But recently, a large Canadian TMA study examining expression of 13 biomarkers in 930 patients (30% node negative), was published Citation[46]. It showed that BCL2 expression in addition to ER and the Nottingham Prognostic Index (NPI) was significantly associated with survival in Cox multivariate regression analysis. The findings were validated in a larger series of 1 930 (64% node negative) patients. The prognostic effect was smaller than for the series of 930 patients, and with a maximal prognostic effect in the first 5 years after diagnosis. It was argued that maybe BCL2 was a better prognostic parameter in high-risk patients, which is in agreement with the findings presented in this study. It was a bit surprisingly, though that negative BCL2 turned out that strongly associated with increased LRR probability in multivariate regression analyses in this study. To our knowledge BCL2 has not been associated with LRR probability in other studies, only with recurrence probability or disease-free survival Citation[18], Citation[47]. The fact, that positive BCL2 are associated with favorable outcome, is in conflict with the known anti-apoptotic effect of BCL2, which has been explained by a stronger growth inhibitory effect of BCL2 Citation[48], as well as a role of BCL2 in prolonging the cell cycle Citation[49–51]. Examining BCL2 expression with IHC does not seem to be associated with particular methodological concerns. BCL2 status determined by IHC seems to be a commonly accepted method and others have also reported a high agreement between stainings of TMA cores and full sections Citation[45].
The results presented in this study may lend support to the spectrum hypothesis, suggesting that the breast cancer disease covers a large spectrum of diseases. This spectrum extends from tumors destined to remain localized, to tumors with the potential to metastasize but without micrometastases at diagnosis, and to tumors that always present with at least micrometastases at diagnosis. Tumors in the aggressive end of the spectrum may be characterized by different biological markers associated with reduced survival (e.g., BCL2) and may if these biological characteristics are sufficiently severe result in micrometastases that are unresponsive to the systemic therapy applied. As a consequence this patient group will not experience an improved survival after locoregional radiotherapy. Supporting this hypothesis as well, we recently presented results from the DBCG82 b&c study showing that the poor prognostic subgroups of hormonal receptor negative and HER2 positive patients also did not experience any survival improvement after PMRT Citation[52].
In conclusion, we did not verify our first hypothesis but found significant reductions in LRR probability after PMRT among all subgroups of p53 positive, p53 negative, BCL2 positive, and BCL2 negative patients. The second hypothesis, however, was verified. Negative BCL2 turned out a significant prognostic marker of reduced survival and no survival improvement after PMRT was found for this subgroup of patients, whereas p53 accumulation was not significantly associated with reduced survival and survival improvements after PMRT were found both for the p53 positive and the p53 negative patients.
Acknowledgements
This study was supported by grants from the Danish Cancer Society, the University of Aarhus, the Danish Medical Research Council, ML Jørgensen and Gunnar Hansen's foundation, and the Novo Nordisk Foundation. The funding sources had no role in the writing of the manuscript. In addition, we would like to thank technical assistants Mogens M. Johannesen, Birthe Hermansen and Tine Bovtrup for excellent technical assistance, and MD Trine Tramm for consultancy assistance during immunohistochemical scoring. The study was conducted on behalf of the DBCG (Danish Breast Cancer Cooperative Group).
References
- McMillan, TJ, Steel, GG. DNA damage and cell killing. In: Basic clinical radiobiology. GG Steel, London: Hodder Arnold; 2002. p 71–83.
- McMillan, TJ, Begg, AC. Genetic control of the cellular response to ionizing radiation. In: Basic clinical radiobiology. GG Steel, London: Hodder Arnold; 2002. p 84–93.
- Lowe SW, Bodis S, McClatchey A, Remington L, Ruley HE, Fisher DE, et al. p53 status and the efficacy of cancer therapy in vivo. Science 1994; 266: 807–10
- Delia D, Mizutani S, Lamorte G, Goi K, Iwata S, Pierotti MA. p53 activity and chemotherapy. Nat Med 1996; 2: 724–5
- Xia F, Wang X, Wang YH, Tsang NM, Yandell DW, Kelsey KT, et al. Altered p53 status correlates with differences in sensitivity to radiation-induced mutation and apoptosis in two closely related human lymphoblast lines. Cancer Res 1995; 55: 12–5
- Askmalm MS, Carstensen J, Nordenskjöld B, Olsson B, Rutqvist LE, Skoog L, et al. Mutation and accumulation of p53 related to results of adjuvant therapy of postmenopausal breast cancer patients. Acta Oncol 2004; 43: 235–44
- Schwartz JL, Jordan R, Slovic J, Moruzzi AM, Kimmel RR, Liber HL. Induction and loss of a TP53-dependent radioadaptive response in the human lymphoblastoid cell model TK6 and its abrogation by BCL2 over-expression. Int J Radiat Biol 2007; 83: 153–9
- Guo WF, Lin RX, Huang J, Zhou Z, Yang J, Guo GZ, et al. Identification of differentially expressed genes contributing to radioresistance in lung cancer cells using microarray analysis. Radiat Res 2005; 164: 27–35
- Silvestrini R, Daidone MG, Benini E, Faranda A, Tomasic G, Boracchi P, et al. Validation of p53 accumulation as a predictor of distant metastasis at 10 years of follow-up in 1400 node-negative breast cancers. Clin Cancer Res 1996; 2: 2007–13
- Allred DC, Clark GM, Elledge R, Fuqua SA, Brown RW, Chamness GC, et al. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst 1993; 85: 200–6
- Silvestrini R, Benini E, Daidone MG, Veneroni S, Boracchi P, Cappelletti V, et al. p53 as an independent prognostic marker in lymph node-negative breast cancer patients. J Natl Cancer Inst 1993; 85: 965–70
- Thor AD, Moore DH, II, Edgerton SM, Kawasaki ES, Reihsaus E, Lynch HT, et al. Accumulation of p53 tumor suppressor gene protein: An independent marker of prognosis in breast cancers. J Natl Cancer Inst 1992; 84: 845–55
- MacGrogan G, Bonichon F, de M, I, Trojani M, Durand M, Avril A, et al. Prognostic value of p53 in breast invasive ductal carcinoma: An immunohistochemical study on 942 cases. Breast Cancer Res Treat 1995; 36: 71–81
- Daidone MG, Luisi A, Martelli G, Benini E, Veneroni S, Tomasic G, et al. Biomarkers and outcome after tamoxifen treatment in node-positive breast cancers from elderly women. Br J Cancer 2000; 82: 270–7
- Kymionis GD, Dimitrakakis CE, Konstadoulakis MM, Arzimanoglou I, Leandros E, Chalkiadakis G, et al. Can expression of apoptosis genes, bcl-2 and bax, predict survival and responsiveness to chemotherapy in node-negative breast cancer patients?. J Surg Res 2001; 99: 161–8
- Neri A, Marrelli D, Roviello F, De Marco G, Mariani F, De Stefano A, et al. Bcl-2 expression correlates with lymphovascular invasion and long-term prognosis in breast cancer. Breast Cancer Res Treat 2006; 99: 77–83
- Kroger N, Milde-Langosch K, Riethdorf S, Schmoor C, Schumacher M, Zander AR, et al. Prognostic and predictive effects of immunohistochemical factors in high-risk primary breast cancer patients. Clin Cancer Res 2006; 12: 159–68
- Sirvent JJ, Aguilar MC, Olona M, Pelegri A, Blazquez S, Gutierrez C. Prognostic value of apoptosis in breast cancer (pT1-pT2). A TUNEL, p53, bcl-2, bag-1 and Bax immunohistochemical study. Histol Histopathol 2004; 19: 759–70
- Sjostrom J, Blomqvist C, von Boguslawski K, Bengtsson NO, Mjaaland I, Malmstrom P, et al. The predictive value of bcl-2, bax, bcl-xL, bag-1, fas, and fasL for chemotherapy response in advanced breast cancer. Clin Cancer Res 2002; 8: 811–6
- Swellam M, Ismail M, Eissa S, Hamdy M, Mokhtar N. Emerging role of p53, bcl-2 and telomerase activity in Egyptian breast cancer patients. IUBMB Life 2004; 56: 483–90
- Isola J, Visakorpi T, Holli K, Kallioniemi OP. Association of overexpression of tumor suppressor protein p53 with rapid cell proliferation and poor prognosis in node-negative breast cancer patients. J Natl Cancer Inst 1992; 84: 1109–14
- Ostrowski JL, Sawan A, Henry L, Wright C, Henry JA, Hennessy C, et al. p53 expression in human breast cancer related to survival and prognostic factors: An immunohistochemical study. J Pathol 1991; 164: 75–81
- Jacquemier J, Moles JP, Penault-Llorca F, Adelaide J, Torrente M, Viens P, et al. p53 immunohistochemical analysis in breast cancer with four monoclonal antibodies: Comparison of staining and PCR-SSCP results. Br J Cancer 1994; 69: 846–52
- Sjostrom J, Blomqvist C, Heikkila P, Boguslawski KV, Raisanen-Sokolowski A, Bengtsson NO, et al. Predictive value of p53, mdm-2, p21, and mib-1 for chemotherapy response in advanced breast cancer. Clin Cancer Res 2000; 6: 3103–10
- Alsner, J, Olsen, KE, Jensen, V, Yilmaz, M, Knoop, A, Overgaard, J. TP53 mutation and overexpression of the HER2 receptor, but not p53 expression, are strong indicators of poor prognosis in both node-negative and node-positive early breast cancer. Proc Am Ass Canc Res 2002;43.
- Linjawi A, Kontogiannea M, Halwani F, Edwardes M, Meterissian S. Prognostic significance of p53, bcl-2, and Bax expression in early breast cancer. J Am Coll Surg 2004; 198: 83–90
- Choi DH, Kim S, Rimm DL, Carter D, Haffty BG. Immunohistochemical biomarkers in patients with early-onset breast carcinoma by tissue microarray. Cancer J 2005; 11: 404–11
- Daidone MG, Luisi A, Veneroni S, Benini E, Silvestrini R. Clinical studies of Bcl-2 and treatment benefit in breast cancer patients. Endocr Relat Cancer 1999; 6: 61–8
- Kyndi, M, Sorensen, FB, Knudsen, H, Overgaard, M, Nielsen, HM, Andersen, J, et al. Tissue microarrays compared with whole sections and biochemical analyses. A subgroup analysis of DBCG82 b&c. Acta Oncol 2008;47:591–99.
- Offersen BV, Sorensen FB, Yilmaz M, Knoop A, Overgaard J. Chalkley estimates of angiogenesis in early breast cancer--relevance to prognosis. Acta Oncol 2002; 41: 695–703
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997; 337: 949–55
- Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999; 353: 1641–8
- Nielsen HM, Overgaard M, Grau C, Jensen AR, Overgaard J. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: Long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol 2006; 24: 2268–75
- Alsner, J, Jensen, V, Kyndi, M, Offersen, BV, Vu, P, Borresen-Dale, AL, et al. A comparison between p53 accumulation determined by immunohistochemistry and TP53 mutations as prognostic variables in tumours from breast cancer patients. Acta Oncol 2008;47:600–7.
- Zellars RC, Hilsenbeck SG, Clark GM, Allred DC, Herman TS, Chamness GC, et al. Prognostic value of p53 for local failure in mastectomy-treated breast cancer patients. J Clin Oncol 2000; 18: 1906–13
- Silvestrini R, Veneroni S, Benini E, Daidone MG, Luisi A, Leutner M, et al. Expression of p53, glutathione S-transferase-pi, and Bcl-2 proteins and benefit from adjuvant radiotherapy in breast cancer. J Natl Cancer Inst 1997; 89: 639–45
- Nix P, Cawkwell L, Patmore H, Greenman J, Stafford N. Bcl-2 expression predicts radiotherapy failure in laryngeal cancer. Br J Cancer 2005; 92: 2185–9
- Makretsov NA, Huntsman DG, Nielsen TO, Yorida E, Peacock M, Cheang MC, et al. Hierarchical clustering analysis of tissue microarray immunostaining data identifies prognostically significant groups of breast carcinoma. Clin Cancer Res 2004; 10: 6143–51
- Abd El-Rehim DM, Ball G, Pinder SE, Rakha E, Paish C, Robertson JF, et al. High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J Cancer 2005; 116: 340–50
- Haffty BG. Molecular and genetic markers in the local-regional management of breast cancer. Semin Radiat Oncol 2002; 12: 329–40
- Norberg T, Lennerstrand J, Inganas M, Bergh J. Comparison between p53 protein measurements using the luminometric immunoassay and immunohistochemistry with detection of p53 gene mutations using cDNA sequencing in human breast tumors. Int J Cancer 1998; 79: 376–83
- Geisler S, Lonning PE, Aas T, Johnsen H, Fluge O, Haugen DF, et al. Influence of TP53 gene alterations and c-erbB-2 expression on the response to treatment with doxorubicin in locally advanced breast cancer. Cancer Res 2001; 61: 2505–12
- Sjogren S, Inganas M, Norberg T, Lindgren A, Nordgren H, Holmberg L, et al. The p53 gene in breast cancer: prognostic value of complementary DNA sequencing versus immunohistochemistry. J Natl Cancer Inst 1996; 88: 173–82
- Torhorst J, Bucher C, Kononen J, Haas P, Zuber M, Kochli OR, et al. Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol 2001; 159: 2249–56
- Chhieng DC, Frost AR, Niwas S, Weiss H, Grizzle WE, Beeken S. Intratumor heterogeneity of biomarker expression in breast carcinomas. Biotech Histochem 2004; 79: 25–36
- Callagy GM, Pharoah PD, Pinder SE, Hsu FD, Nielsen TO, Ragaz J, et al. Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin Cancer Res 2006; 12: 2468–75
- Lee KH, Im SA, Oh DY, Lee SH, Chie EK, Han W, et al. Prognostic significance of bcl-2 expression in stage III breast cancer patients who had received doxorubicin and cyclophosphamide followed by paclitaxel as adjuvant chemotherapy. BMC Cancer 2007; 7: 63
- Pietenpol JA, Papadopoulos N, Markowitz S, Willson JK, Kinzler KW, Vogelstein B. Paradoxical inhibition of solid tumor cell growth by bcl2. Cancer Res 1994; 54: 3714–7
- Lipponen P, Pietilainen T, Kosma VM, Aaltomaa S, Eskelinen M, Syrjanen K. Apoptosis suppressing protein bcl-2 is expressed in well-differentiated breast carcinomas with favourable prognosis. J Pathol 1995; 177: 49–55
- O'Reilly LA, Huang DC, Strasser A. The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO J 1996; 15: 6979–90
- Knowlton K, Mancini M, Creason S, Morales C, Hockenbery D, Anderson BO. Bcl-2 slows in vitro breast cancer growth despite its antiapoptotic effect. J Surg Res 1998; 76: 22–6
- Kyndi, M, Sorensen, FB, Knudsen, H, Overgaard, M, Nielsen, HM, Overgaard, J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: The Danish Breast Cancer Cooperative Group. J Clin Oncol 2008;26: 1419–26.