Abstract
The invasive disease free survival, the overall survival, and the relative risk of death compared to the Danish population as well as the risk of recurrence and new malignancies is reported for low-risk breast cancer patients of the DBCG 89-A programme. The study includes a comparison between those patients who, according to the present criteria, would be defined low-risk and those who would be defined high-risk (the retrospective low→high-risk group) and a comparison of treatment by mastectomy and BCS combined with radiation therapy.
The DBCG 89-A programme scheduled 10 years of follow-up. Data was supplemented by record linkage to the Hospital Discharge Registry (date of event) and the Central Population Registry (date of death).
The study population consisted of 8 850 patients. With 12 years of follow-up 3 811 events (43%) were recorded: loco-regional recurrence 8%, distant recurrence 11%, contralateral cancer 6%, secondary cancer 8%, and deaths 11%. The DBCG registry had an incomplete reporting of events in these low-risk patients, due to premature discontinuation of control. The incidence of recurrences was higher for the retrospective low → high-risk group than for the low-risk group. The 10-year overall survival was 76%; lower in the retrospective low → high-risk group (71%) than in the low-risk group (83%). The 5-year survival following local recurrence was 68% after mastectomy and 81% after BCS. The risk of mortality was higher than in the general population for all subgroups of patients. The relative risk of mortality expressed in terms of the standardized mortality ratio was 10.4 for young patients (26–39 years) and 1.2 for old patients aged 70–74 years and 1.3 for patients in the retrospective low-risk group and 1.9 for patients in the low → high-risk group.
The loco-regional treatment given did not cure all patients, in particular young patients and those of the retrospective low → high-risk group.
In 1989 the Danish Breast Cancer Cooperative Group (DBCG) introduced new treatment programmes for early breast cancer. In the DBCG 89 protocol, which was active in the period 1989 to 2001, low-risk node negative patients were treated by mastectomy or breast conserving surgery (BCS) combined with radiation therapy (RT) of the residual breast. Systemic adjuvant treatment was not offered to these patients.
The DBCG 89 low-risk definition did not change substantially from the previous protocols and still included approximately 50% of the new-diagnosed patients with early breast cancer Citation[1]. On the other hand, the new definitions of the risk groups in the following protocols have decreased the low-risk group to 20% or less.
The intention with the present study was to document the overall results of the treatment strategy in the period 1989–2001 for the Danish low-risk group, and to investigate the recurrence pattern by analysis of the risk of local and distant recurrence as well as the occurrence of new contralateral breast cancer and new malignancies not related to breast cancer. It was also the aim to compare the outcome between those patients who, according to the present criteria, would be defined low risk and those who would be defined high risk. Furthermore, we wanted to describe the overall survival and the relative risk of death compared to the Danish population.
Material and methods
The DBCG 89-A programme included low-risk female breast cancer patients with the following characteristics: invasive breast carcinomas ≤5 cm, node negative, ductal grade I (for the premenopausal patients only, otherwise any grade), and age <75 years (age <70 years until March 21, 1991). Exclusion criteria were distant metastasis, bilateral breast cancer, inflammatory breast cancer, prior malignant disease apart from non-melanoma skin tumours or in situ cancer in cervix uteri, when observed up to 60 days after surgery. In the 89 programme, the requirement for the number of retrieved axillary lymph nodes was a minimum of 4, but preferably 10 lymph nodes. However, by 1994, DBCG addressed the surgical units and stated that at least 10 removed lymph nodes should make up the national standard.
The DBCG 89 programme was initiated in November 1989 and terminated April 2001. Both the initiation and the termination occurred gradually, as the individual counties joined the programme and finished inclusion of patients at different times. During this period, 9 655 patients fulfilled the inclusion criteria, but 805 patients had to be excluded, thus the study population consists of 8 850 patients ().
All Danish hospital departments engaged in treatment of breast cancer patients report prospectively to the DBCG database. The information collected includes data on pathology, surgery, radiotherapy, and clinical follow-up. For the present analysis, we have included information on age, tumour size, hormone receptor status, histological type, grade of malignancy, and number of lymph nodes removed. Histological type was defined according to the WHO histological classification. Tumour grade was assessed according to the method of Bloom and Richardson modified by Elston and Ellis Citation[2]. Hormone receptor status was determined by immunohistochemistry, and tumours were classified as receptor positive if 10% or more of the cells were stained positive for estrogen and/or progesterone receptors.
From 1999 (DBCG 99) patients with node negative tumours were allocated to the high-risk group if the tumour was >2 cm or ≤2 cm and receptor negative or ductal grade II–III. Retrospectively we could therefore isolate a group of patients, who had tumour size >2 cm or was receptor negative or ductal grade II–III. Patients with these tumour characteristics were allocated to the low-risk group in the DBCG 89 programme but would have been allocated to the high-risk group in the subsequent DBCG programmes, and thereby would have received adjuvant systemic therapy. In the following, this group is termed the retrospective low → high-risk group. The current high-risk criteria of patient age less than 35 years introduced in 2002 was not taken into consideration in the definition of the low→high-risk group.
The recommended follow-up programme consists of clinical examination twice a year for 5 years and once a year for additional 5 years to a total of 10 years of follow-up. Date, site of recurrence or new malignant disease, and end of follow-up without recurrence at 10 years are reported to the DBCG. Information on date of death is retrieved by record linkage to the Central Population Registry, which keeps electronically updated data on vital status and on emigration on the entire Danish population. Linkage with the registry is possible by using the civil person registration number, a unique personal identification number given to all Danes upon birth. Furthermore, the DBCG database information was by record linkage supplemented by information from the Hospital Discharge Register (HDR). This registry was established in 1977 and contains information on all hospitalisations registered on the individual level, with hospital and department number, dates of admission and discharge, and up to 20 diagnoses per hospitalisation and up to 6 operations per diagnosis. Diagnoses are coded according to a Danish five-digit version of International Classification of Diseases (lCD) 8th and 10th revision. Observations registered in HDR of malignant events not reported to the DBCG database, while the patient was included in the follow-up, were checked by reviewing the local medical records, and if the information was verified, the DBCG registration was updated accordingly. Follow-up data beyond 10 years were solely based on HDR registrations eventually supplied with review of records. Follow-up ended on April 1, 2007.
Events and end points
The end points were invasive disease free survival (IDFS) and overall survival (OS) Citation[3]. OS was defined as the time from the primary surgery to death, irrespective of cause. IDFS was defined as the time from primary surgery to one of the following events which ever occurred first. The events are: local recurrence (L), when occurring in the breast or scar, regional recurrence (R), when seen in the ipsilateral axilla or periclavicular region, distant recurrence (D), invasive contralateral breast cancer (CC), second primary non-breast invasive cancer (SC), or death attributable to any cause (DE). The pattern of malignant events was investigated. In case more than one event occurred simultaneously, recurrences were considered of higher priority than new cancers according to the sequence: D > R>L > CC > SC.
Statistics
Descriptive analysis included determination of the median time to event by type of event and according to retrospective risk group and type of surgery. Because only the first event was recorded, the time to event due to different types of events was analysed in a setting of competing risks. Cumulative incidence functions were determined by the cuminc-function of the cmprsk-package of R-software (version 2.6, R Development Core Team, 2007). Pair wise comparisons of the estimated curves according to the retrospective risk groups and surgery type were done by use of the test of Gray Citation[4]. Comparison of frequencies between groups was done by χ2 test.
The follow-up data were analysed for OS and IDFS calculated according to the Kaplan-Meier method, and the two types of surgery and the retrospective risk groups were compared by log rank tests in univariate models. The estimates of OS and IDFS were calculated for the total material and according to the retrospective risk groups and the type of surgery. In addition, overall survival from the time of the first malignant event was determined according to the type of event.
Standardized mortality ratios (SMR) were calculated as the number of observed deaths relative to the number of expected deaths, which were estimated by applying the appropriate number of person-years at risk to the female mortality figures of the general population specified by one-year age and calendar-time intervals. Time at risk was determined from the date of surgery + 60 days until death, immigration, or end of observation period (April 1, 2007). The time at risk was subdivided according to: age at surgery, calendar-time, time since surgery, type of surgery, and retrospective risk group. In addition, for the subgroup of the study populations that experienced a first malignant event, the subsequent time at risk was subdivided according to the type of event. The standardized mortality ratios were analysed using multivariate Poisson regression models. In the analysis of SMR following surgery, the observed variance was larger than the expected Poisson variance, and a correction for overdispersion was made by scaling the variance-covariance matrix by the deviance/df ratio. Parameter estimates was reported with 95% Wald confidence intervals. In addition, the observed and expected death rates per 100 000 person-years together with the crude SMR were reported. For the crude SMR, the confidence intervals were based on the assumption of Poisson variance. Models were fitted to data and maximum likelihood estimates of parameters were obtained by the proc genmod of SAS ver. 9.1 (SAS Institute, Cary, NC, USA).
Results
From November 1989 to April 2001 9 655 patients fulfilled the inclusion criteria, but 609 was excluded at the time of entry mainly due to previous malignancy or bilateral breast cancer, and further 18 patients were excluded during follow-up (). The data validation done afterwards by the linkage of DBCG 89-A records with HDR found another 178 patients who met the exclusion criteria, resulting in a study population of 8 850 patients.
General characteristics of the study population are given in . The median age at surgery was 59 years (range 26–74), and 7 159 (81%) were postmenopausal. Relative to patients treated by BCS (n = 2 593) the patients treated by mastectomy (n = 6 257) were characterised by: higher age (median age 60 vs. 56 years), larger tumours, more lobular tumours, more receptor negatives, fewer grade I tumours (35 vs. 41%), and fewer removed lymph nodes. Consequently, a higher proportion of the patients treated by mastectomy were in the retrospective low→high-risk group (59%) than the BCS patients (44%). Overall 55% of the patients were in the retrospective low→high-risk group. These patients were older than those in the low-risk group: median age 60 vs. 57 years. The frequency of BCS decreased with increasing tumour size: 44% for tumour sizes ≤10 mm, 31% for tumour sizes 11–20 mm, and 15% for tumour sizes 21–50 mm. The frequency of patients treated by BCS increased over time from 25% in the first period 1989–1992 to 31% in the last period 1996–2001. The number of removed lymph nodes increased during the study period; ten or more nodes were harvested in 44, 62, and 83% in the periods 1989–1992, 1993–1995, and 1996–2001, respectively.
Table I. General characteristics of low-risk early breast cancer patients of DBCG 89-A cohort (n = 8850).
In total 3 811 events defining IDFS were recorded, 1 670 were recurrences, 1 166 were new cancers, and 975 were death as first event. A large proportion of the first events were recorded and validated following the linkage of DBCG 89-A records with HDR. To illustrate the effect of the record linkage a comparison at January 1, 2004 was performed. The record linkage resulted in the finding of 1280 previously unrecorded recurrences and second primary cancers (). A majority of the reclassifications of first events were done for patients subject to premature discontinuation of control (n = 929) and happened within the intended 10 years follow-up period (n = 859). Reclassifications before the original time of censoring or first event constituted only 380 events equivalent to 4.3% of the study population. The findings of previously unrecorded recurrences or second primary cancers differed proportionally from the distribution among first events originally recorded in the DBCG data base (p < 0.0001): more second primary cancers (37 vs. 9%), more contralateral cancers (20 vs. 13%), and fewer local recurrences (9 vs. 26%) were found, while the proportions of distant recurrences (36 vs. 34%) and regional recurrences (9 vs. 7%) were similar.
Table II. Reclassification of first events in the DBCG 89-A study population and findings of previously unrecorded recurrences and second primary cancers following record linkage to the Hospital Discharge Register.
The record linkage to HDR up to January 1, 2004 did increase the median potential follow-up before this date by two years from 6.8 years (95% CI: 6.7–6.9) to 8.8 years (95% CI: 8.7–8.9), while the five-year estimates of IDFS decreased from 79.8% (95% CI: 78.9–80.7%) to 77.6% (95% CI: 76.7–78.4%). At the end of the study period (April 1, 2007) the median potential follow-up was 12.0 years (95% CI: 11.9–12.1) and the total risk-time was 90 470 person-years. Of these 78 670 person-years was before first malignant event and 11 800 person-years was after first malignant event ().
Table III. Risk time for DBCG 89-A cohort by age at surgery and event status.
The 10-year estimated OS was 76.3% (95% CI: 75.3–77.2) for the whole group. The OS of the retrospective low → high-risk group was lower than that of the low-risk group (p < 0.0001, ); the 10-year estimates were 70.6% (95% CI: 69.3–71.9) and 83.1% (95% CI: 81.9–84.3), respectively. Likewise, IDFS of the retrospective low → high-risk group was lower than that of the low-risk group (p < 0.0001) with 10-year estimates of 56.7% (95% CI: 55.2–58.1) and 67.9% (95% CI: 66.4–69.3), respectively. The OS was lower after mastectomy than after BCS (p < 0.0001, ); the 10-year estimates were 73.3% (95% CI: 72.2–74.4%) and 83.5% (95% CI: 82.0–84.9%), respectively. Also, IDFS was lower after mastectomy than after BCS (p < 0.0001) with 10-year estimates of 65.1% (95% CI: 63.9–66.3%) and 72.3% (95% CI: 70.5–74.1%), respectively.
Figure 2. IDFS (left side panels) and OS (right side panels) total and according to retrospective risk group (upper panels) and type of operation (lower panels) in the DBCG 89-A cohort.
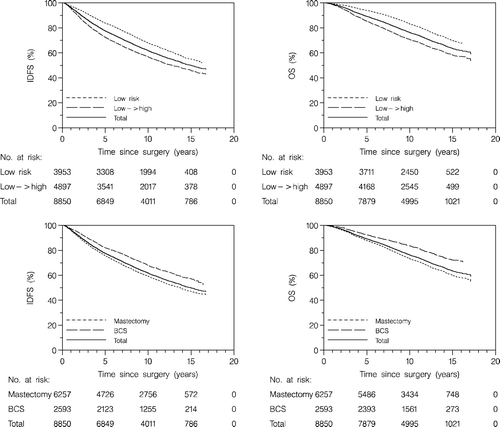
Figure 3. Cumulative incidence functions for first events by retrospective risk group and by type of surgery. L: local recurrence, R: regional recurrence, D: distant recurrence, CC: contralateral breast cancer, SC: second primary cancer, and DE: death as first event.
The type and frequencies of recurrences and secondary cancers are further presented in . Recurrence, contralateral breast cancer, or second primary cancer was observed in 2 836 patients (32.1%), death as first event in 975 patients (11.0%), and no events were seen in 5 039 patients (56.9%). Among patients treated by mastectomy more events were observed than in the group with BCS, 46.0 vs. 35.9%, respectively (p < 0.0001). Patients in the retrospective low→high-risk group had more events and shorter median time of IDFS compared to the low-risk group. Of the 8 850 patients 972 had distant recurrence; metastases were located in lungs (N = 209, 2.3%), liver (N = 118, 1.3%), central nervous system (N = 86, 1.0%), and bone (N = 377, 4.3%). Three hundred and four patients had bone metastasis alone (3.4%). The median time to first events increased from regional recurrence (2.9 years) over local recurrence (3.5 years), distant recurrence (3.8 years), contralateral cancer (5.0 years), and secondary cancer (6.3 years), to death as first event (6.6 years). For local recurrence, the median time was shorter after mastectomy (2.7 years) than after lumpectomy (5.6 years). Second new primary cancers observed during the follow-up period are listed in ; the pattern did not differ between surgery by mastectomy and BCS (p = 0.47).
Table IV. First event following surgery (recurrence, new malignant disease, death, or censoring), frequency and median time to event by type of surgery and retrospective risk group.
Table V. Location of second primary cancer except contralateral breast cancer by type of surgery†.
The time to first events are illustrated by cumulative incidence curves taking the competing risks into consideration (). For patients in the retrospective low→high-risk group the cumulative incidence curve estimates differed for local recurrence (p = 0.005), regional recurrence (p = 0.0006), distant recurrence (p < 0.0001), and death as first event (p = 0.003). In all cases the cumulative incidence estimates after ten years follow-up were higher for the retrospective low→high-risk group than for the low-risk group: local recurrence 6.2% (95% CI: 5.5–6.9%) versus 4.8% (95% CI: 4.1–5.5%), regional recurrence 2.8% (95% CI: 2.3–3.2%) versus 1.6% (95% CI: 1.3–2.1%), distant recurrence 13.5% (95% CI: 12.6–14.5%) versus 6.7% (95% CI: 6.0–7.6%), and death as first event 9.7% (95% CI: 8.8–10.5%) versus 7.7% (95% CI: 6.9–8.6%). The cumulative incidence curves for contralateral breast cancer (p = 0.34) or other second cancers (p = 0.46) did not differ among retrospective risk groups.
Among patients treated by mastectomy or BCS, the cumulative incidence curve estimates differed for distant recurrence (p < 0.0001) and for death as first event (p < 0.0001). For distant recurrence the cumulative incidence estimates after ten years follow-up were higher for mastectomy 11.4% (95% CI: 10.7–12.3%) than for BCS 8.0% (95% CI: 7.0–9.2%). Also for death as first event the ten-year estimates were higher for mastectomy 10.1% (95% CI: 9.3–10.9%) than for BCS 5.6% (95% CI: 4.8–6.6%). For contralateral cancer, the cumulative incidence tended to be higher after mastectomy than after BCS (p = 0.058), while no differences where found for secondary cancers (p = 0.93) and regional recurrences (p = 0.83). For local recurrence, the estimated cumulative incidence curves for mastectomy and BCS crossed each other after 9 years. When the first 5 years of follow-up was analysed, the curves differed significantly (p = 0.0006). The five-year estimate was higher for mastectomy 4.2% (95% CI: 3.7–4.7%) than for BCS 2.7% (95% CI: 2.1–3.4%).
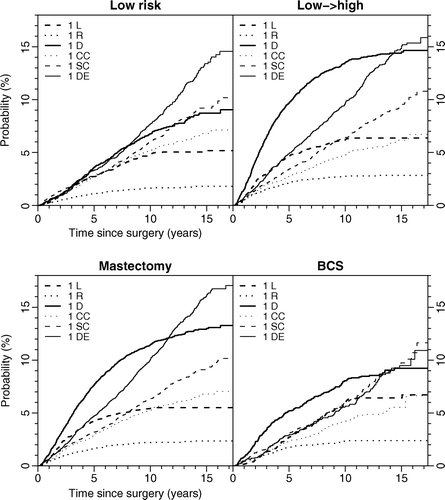
The survival following recurrence or second primary cancer was strongly affected by the type of event (). Distant recurrence had the worst prognosis with a five-year survival estimate of 23.0% (95% CI: 20.1–25.8%). Following second primary cancer, the five-year survival estimate was 45.5% (95% CI: 41.3–49.6%), after regional recurrence 50.6% (95% CI: 43.4–57.8%), and after contralateral breast cancer 80.4% (95% CI: 76.5–84.4%). The prognosis after local recurrence was depending on type of surgery; after mastectomy the estimate was 67.9% (95% CI: 62.8–72.9%) and after BCS 80.6% (95% CI: 73.7–87.6%).
Figure 4. Survival following recurrence or second primary cancer (p < 0.0001). L Mast: local recurrence after mastectomy, L BCS: local recurrence after BSC, R: regional recurrence, D: distant recurrence, CC: contralateral breast cancer, and SC: second primary cancer.
The analysis of SMR following surgery showed that type of surgery, age at surgery, follow-up period, and retrospective risk group all had significant effects, while the effect of period of surgery was not significant (). The age at surgery had a marked effect on SMR. Thus, compared with patients aged 50–59 years (reference level), younger patients had a significantly higher relative risk of dying: 5.31 (95% CI: 3.79–7.43) and 1.59 (95% CI: 1.33–1.90) for patients in the age groups 26–39 years and 40–49 years, respectively. On the other hand, lower RR's were observed in older patients with RR's of 0.67 (95% CI: 0.60–0.75) and 0.52 (95% CI: 0.45–0.59) in 60–69-year-old and 70–74-year-old patients, respectively. The observed and expected death rates showed that the excess mortality rate was largest for the young patients aged 26–39 years (1 642 per 100 000 person-years) and lowest for patients aged 40–49 years and 70–74 years (approximately 880 per 100 000 person-years).
Table VI. Effect of age at surgery, period of surgery, time since surgery, type of surgery, and retrospective risk group on standardized mortality ratio (SMR) in the DBCG 89-A cohort. The crude SMR is the ratio between the observed and expected death rates. Relative risk (RR) estimates* in a multivariate model for patients in the reference category (age at surgery 50-59, period of surgery 1996-2001, surgery by mastectomy, and retrospective low-risk group) relative to the mortality risk in the general population.
Patients in the retrospective low → high-risk group had an increased relative risk of 1.50 (95% CI: 1.37–1.65) compared with the retrospective low-risk group (reference level) and likewise a much larger excess mortality: 1 650 versus 481 per 100 000 person-years. The retrospective risk group further had significant interactions with age at surgery and follow-up period, respectively (not shown). Patients in the low-risk group aged 60–74 years had a lower relative risk than patients in the low→high-risk group, while there was no difference for patients aged 26–59 years. Thus, the estimates of relative risk for the youngest patients aged 25–39 years were high also for the retrospective low-risk group. Ten years after surgery the relative risk did not differ between the retrospective low-risk group and the low→high group.
The relative risk of patients treated by BCS was 0.76 (95% CI: 0.68–0.85) and lower than for patients operated by mastectomy (reference level). Although the type of surgery did not have a significant interaction with the age at surgery in the regression analysis (p = 0.10), the estimates of crude SMR indicated an interaction. For patients aged 40–69 years the estimates of crude SMR showed a better prognosis after BCS than after mastectomy while the opposite was the case for the youngest patients aged 26–39 years, for which the estimates of crude SMR were 13.4 (95% CI: 9.0–20.0) after BCS and 8.6 (95% CI: 5.7–12.8) after mastectomy.
The results of the analysis of SMR following first malignant event are shown in . The type of event in combination with the type of surgery and retrospective risk group had marked effects on the estimates of relative risk. If the overall effects of first malignant events are ranked then the relative risk decreased in the order: D > SC > R>L > CC. For distant recurrence, local recurrence, and contralateral breast cancer the relative risk of patients in the retrospective low→high-risk group was larger than for the low-risk group: distant recurrence 10.1 (95% CI: 7.6–13.4) versus 5.8 (95% CI: 4.3–7.8), local recurrence 2.6 (95% CI: 1.9–3.6) versus 1.6 (95% CI: 1.1–2.4), and contralateral breast cancer 1.5 (95% CI: 1.1–2.1) versus 1.0 (reference). For local recurrence the relative risk after BCS of 0.51 (95% CI: 0.35–0.76) was lower than the value of 1.00 after mastectomy (reference level).
Table VII. Effect of first malignant events by type of event, type of surgery, and retrospective risk group on standardized mortality ratio (SMR) in the DBCG 89-A cohort. The crude SMR is the ratio between the observed and expected death rates. Relative risk (RR) estimates in a multivariate model (only significant interactions included) adjusted for the effects of age at surgery and period of surgery.
Discussion
This study shows breast cancer recurrence in approximately 19% of patients in the DBCG 89-A programme within 12 years of follow-up. More than half of these were distant metastases of witch three-quarter of patients died within 5 years. Regional recurrence was followed by death in 50% within 5 years. Local recurrence after mastectomy lead to death of one third, while a local relapse after BCS had a better prognosis with a mortality rate of only 20%. Furthermore, it was found that up to 6% of these patients had new contralateral breast cancer, and just fewer than 8% of patients had new non-breast cancers. Throughout the follow-up period, this cohort of low-risk patients, as defined in the early 1990's, had a substantial higher mortality than the general population.
The DBCG 89-A programme was a purely observational study. Therefore, the comparisons presented in this paper between mastectomy and BCS are confounded with different prognostic factors and should be looked at as merely explorative, including pursuing previously defined hypothesis, as well as generating new hypothesis.
A weakness of the follow-up registration in this study is the large proportion of patients lost to follow-up early due to premature discontinuation. Almost one quarter of the events in the present study took place after discontinuation and was therefore not originally recorded in the database. A similarly insufficient reporting has not been observed in high risk patients Citation[1] and may be explained by the lack of intervention after primary treatment and the expectation of low recurrence rate, which led some centres to stop follow-up of the low-risk patients early on, despite the DBCG guidelines. Another explanation for the missing data is that patients suffering from new non-breast malignancies to some extent are lost to follow-up because diagnosis and treatment take place in departments not engaged in treatment of breast cancer. Although the difference was small, the record linkage to the HDR and subsequent data-validation resulted in decreased estimates of invasive disease free survival. Thus a part of the censorings in the original DBCG register were not independent of the risk of new malignancies, but should be regarded as “informative censorings” most likely related to the loss of second primary non-breast cancers from follow-up.
The present DBCG 89-A protocol reflects the results of a local treatment strategy in low-risk patients and thus the outcome is completely dependent on the quality of the surgery and, for the BCS treated patients, the radiotherapy. The data was compared with the results from the 2005 overview from EBCTCG Citation[5]. In this meta-analysis of a large number of randomised studies, the loco-regional recurrence rates at 5 or 10 years were 6.7 and 10.0%, respectively, for the node negative patients treated by BCS plus radiotherapy. In the mastectomy group the corresponding figures were 6.3 and 8.0%, respectively. The observed rates in the present series (L + R) are very close to these figures, and all values are below the mean values from the meta-analysis. Thus, it seems fair to conclude that the loco-regional treatment in the present series was comparable to what is considered “the gold standard” for that time.
Follow-up of the low-risk group of breast cancer patients according to DBCG in the period 1977-1990 (DBCG 77-A and DBCG 82-A) has previously been published by Axelsson et al. Citation[6]. Mean follow-up was 9 and 3 years, respectively. Overall survival and recurrence free survival curves were identical for the two protocols. All patients were treated by mastectomy, and the overall survival was 70% and the recurrence free survival 55% after 10 years. There was a slight difference in the risk criteria between DBCG 77-82 and the present DBCG 89 programme. Thus, from 1989 and onward premenopausal patients with grade II and III tumours were classified as high-risk and offered adjuvant therapy. Furthermore, the axillary surgery became more sufficient in the 89-programme where two thirds of the patients had 10 or more lymph nodes removed, as opposed to less than 20% in the DBCG 77-A and 82-A series. Most likely these changes lead to a better prognosis for the whole low-risk group and may partly explain why the present group of low-risk patients has a better overall survival and disease free survival. We were not able to show a significant change in results during the study period, as the improved axillary surgery could suggest, but there were a trend in the SMR estimates which will be further explored later.
The analysis based on the retrospective risk groups reflects the effect to be expected of the change in strategy performed in 1999. The data clearly show that marked improvement in the outcome for the low-risk group of patients according to the 1999 risk criteria could be anticipated. Moreover, the addition of age below 35 as a high-risk definition from 2002 has lead to further diminishment of the low-risk group and to supplementary improvement in prognosis for these patients.
The mortality among low-risk breast cancer patients is an important indicator of local treatment quality including treatment side-effects and the quality of post-malignancy clinical surveillance. In this investigation we extended the overall survival curves with the estimation of SMR, and the absolute excess mortality derived from observed and expected death rates, which gave valuable information of the relationship between age and prognosis. Thus, a special focus was brought on the young age group. It has previously been shown that young breast cancer patients have a worse prognosis than more elderly patients regardless of the type of surgery Citation[7–10], but the data from the previous DBCG study by Kroman et al. Citation[7] also indicated that in the low-risk group, not having chemotherapy, the outcome was worse among the very young patients treated by BCS compared with patients having a mastectomy. The present data clearly indicates that the disease has a more aggressive course in the young age group. This in spite of the fact that the young patients in the present series are restricted to grade I histology, and the observations can, therefore, not be exclusively explained by tumour biology, as it has been proposed Citation[11]. The present investigation gives no clear-cut answer to the remaining question whether mastectomy would be a better treatment to young patients with breast cancer, but as better survival in the young patients in the mastectomy group was documented by the crude standard mortality ratios for the young patients, this study gives further evidence for a restrictive attitude towards BCS in the young age group.
The difference in outcome following mastectomy and BCS reflects that the two groups are not comparable, but at the same time, the study indicated different local recurrence patterns, which is in line with other results also published in this issue Citation[12]. Based on a histopathology review, more true recurrences were found in the mastectomy group, whereas new primary tumours were more pronounced in the BCS group, and the recurrences appeared later in the BCS group. This is in accordance with the present findings.
Along with the observed difference in cumulative incidence, the present study also showed a worse prognosis after local recurrence in the mastectomy group compared with patients having local recurrence following BCS. This is in agreement with most other observations Citation[13], but seems to contradict the results from the combined EORTC-DBCG study Citation[14], where similar survival rates were observed after salvage treatment of recurrence in patients randomised to mastectomy or BCS. The difference in outcome is probably related to differences in the materials, though. In the EORTC-DBCG study both local and regional recurrences were included whereas the present study distinguished between these events. Furthermore, the number of observations in the EORTC-DBCG study was fairly low (N = 135) compared to the number of events in the present study. A factor not taken into consideration here is the treatment of patients with local failure. It has been most common to perform salvage mastectomy in case of local recurrence after BCS, and the treatment of recurrent lesions in the chest wall after mastectomy has usually been local resection and RT, but during the period there were no formal recommendations in the guidelines, and no registration of the treatment given. It is therefore not possible to explore this topic further with the present data, but it is obvious that the present findings point to further studies based on review of patient charts etc.
The relative risk of invasive cancer in the contralateral breast following a first primary breast cancer is increased, and overall the risk was estimated to be 2.8 in a previous Danish study Citation[15]. The risk is most pronounced in young patients Citation[16] and in patients with a family history Citation[17], and there is a tendency towards a higher risk among patients with lobular carcinoma or tumours with more severe prognostic factors Citation[18]. It has also been shown that radiotherapy increases the risk Citation[5]. This effect was not evident in the present series, where contralateral breast cancer ratios were 5% both following mastectomy and BCS. There were significant differences between the groups though (BCS + RT and mastectomy without RT): BCS patients were younger but on the other hand they had more favourable tumour characteristics. None the less, at least within a 12-year time frame, the radiotherapy seemed not to lead to an increased risk of new contralateral breast cancers.
It is well-known that the risk of a second primary cancer excluding contralateral cancer is increased by approximately 25% Citation[19] in breast cancer patients. Some of this increase is related to treatment side-effects and includes radiation induced cancer, especially lung cancer. In the study by Andersson et al. Citation[20] also published in this issue, the overall incidence of lung cancer was not increased in early breast cancer patients, but the sub-group who had radiotherapy had a 33% higher risk compared to not treated patients. This strongly indicates that lung cancer is an adverse effect of radiotherapy. The present study showed no predominance of lung cancer among the BCS treated group. This could indicate that the radiotherapy given after BCS is less likely to induce carcinogenesis, but it is probably too early to draw any conclusions, as the radiation induced lung cancers seem to appear with several years latency, and was found most pronounced 20 years or more after treatment Citation[20].
As described, the recurrence and death rates were not negligible, and the analysis of the standardized mortality ratios clearly shows that the group of patients selected by the risk criteria valid for the period still had a higher mortality than the background population, thus all the patients were not cured by the loco-regional treatment given. Since then, the criteria have changed, and according to the present criteria, as expressed in the St. Gallen 2007 meeting highlights Citation[21], about half of the patients in the present cohort would today be offered adjuvant systemic treatment. Based on the present findings, this is expected to lead to better results. A detailed analysis taking individual risk factors in consideration is beyond the scope of this paper, but it will be further explored in a planned following investigation of the present cohort of patients. At this point, we conclude that the low-risk criteria valid in the 1990's were not able to discriminate between patients who needed further systemic treatment and those who did not.
Acknowledgements
We thank Henning Mouridsen and Bent Ejlertsen for valuable comments on earlier drafts of the present paper and Jørgen Olsen for discussions of the SMR-analysis.
References
- Møller S, Jensen MB, Ejlertsen B, Bjerre KD, Larsen M, Hansen HB et al. The clinical database of the Danish Breast Cancer Cooperative Group (DBCG); its 30-years experience and future promise. Acta Oncol 2008; 47:506–24.
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991; 19: 403–10
- Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 2007; 25: 2127–32
- Gray RA. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 2008; 16: 1141–54
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366: 2087–106
- Axelsson CK, Andersen JA, Andersen KW, Blichert-Toft M, Dombernowsky P, Hansen M, et al. Prognosis after surgical treatment of breast cancer. 3. The low-risk group of the DBCG (Danish Breast Cancer Cooperative Group)]. Ugeskr Laeger 1991; 153: 2276–9
- Kroman N, Holtveg H, Wohlfahrt J, Jensen MB, Mouridsen HT, Blichert-Toft M, et al. Effect of breast-conserving therapy versus radical mastectomy on prognosis for young women with breast carcinoma. Cancer 2004; 100: 688–93
- de la Rochefordiere A, Asselain B, Campana F, Scholl SM, Fenton J, Vilcoq JR, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet 1993; 341: 1039–43
- Aebi S, Gelber S, Castiglione-Gertsch M, Gelber RD, Collins J, Thurlimann B, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer?. Lancet 2000; 355: 1869–74
- Bollet MA, Sigal-Zafrani B, Mazeau V, Savignoni A, de la RA, Vincent-Salomon A, et al. Age remains the first prognostic factor for loco-regional breast cancer recurrence in young (<40 years) women treated with breast conserving surgery first. Radiother Oncol 2007; 82: 272–80
- Zhou P, Gautam S, Recht A. Factors affecting outcome for young women with early stage invasive breast cancer treated with breast-conserving therapy. Breast Cancer Res Treat 2007; 101: 51–7
- Blichert-Toft M, Nielsen M, Düring M, Møller S, Rank F, Overgaard M et al. Long term results of breast conserving surgery vs. mastectomy for early stage invaqsive breast cancer: 20-year follow-up of the Danish randomized DBCG-82 TM protocol. Acta Oncol 2008; 47:672–81.
- Overgaard M, Christiansen P. The role of local management in locally recurrent and metastatic breast cancer. EJC supplements 2003; 1: 265–73
- van Tienhoven G, Voogd AC, Peterse JL, Nielsen M, Andersen KW, Mignolet F, et al. Prognosis after treatment for loco-regional recurrence after mastectomy or breast conserving therapy in two randomised trials (EORTC 10801 and DBCG-82TM). EORTC Breast Cancer Cooperative Group and the Danish Breast Cancer Cooperative Group. Eur J Cancer 1999; 35: 32–8
- Storm HH, Jensen OM. Risk of contralateral breast cancer in Denmark 1943–80. Br J Cancer 1986; 54: 483–92
- Broet P, de la RA, Scholl SM, Fourquet A, Mosseri V, Durand JC, et al. Contralateral breast cancer: annual incidence and risk parameters. J Clin Oncol 1995; 13: 1578–83
- Vaittinen P, Hemminki K. Risk factors and age-incidence relationships for contralateral breast cancer. Int J Cancer 2000; 88: 998–1002
- Li CI, Malone KE, Porter PL, Daling JR. Epidemiologic and molecular risk factors for contralateral breast cancer among young women. Br J Cancer 2003; 89: 513–8
- Mellemkjaer L, Friis S, Olsen JH, Scelo G, Hemminki K, Tracey E, et al. Risk of second cancer among women with breast cancer. Int J Cancer 2006; 118: 2285–92
- Andersson M, Jensen MB, Engholm G, Storm HH. Risk of second primary cancer among patients with early operable breast cancer registered or randomised in Danish Breast Cancer cooperative Group (DBCG) protocols of the 77, 82 and 89 programmes during 1977–2001. Acta Oncol 2008; 47:755–64.
- Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 2007; 18: 1133–44
