Abstract
Introduction. Since 30 years, DBCG (Danish Breast Cancer Cooperative Group) has maintained a clinical database allowing the conduct of quality control studies, of randomised trials, examination of the epidemiology of breast cancer and of prognostic and predictive factors. Material and methods. The original database included patients with invasive breast cancer, but has later been expanded to patients with in situ breast cancer and hereditary breast and ovarian cancer families. Results. The multidisciplinary cooperative group has provided successive treatment guidelines and 70% of the 77284 registered patients have been enrolled and received treatment according to these guidelines. The standard treatments and the randomised trials included in the DBCG programmes are all briefly described. Among high-risk patients 48% have participated in randomised trials, and the results of these trials have largely been implemented in the next generation of treatment guidelines. Records within the clinical database of archival tumour tissue have established a basis for translational research and epidemiologic research has been enabled through linkage to other healthcare registries. Discussion. The joint conception of the multidisciplinary breast cancer group and a clinical database has provided improvements in the management of breast cancer patients and has enabled recruitment of patients onto randomised trials.
Clinical databases run by multidisciplinary cooperative groups are increasingly being used in healthcare to monitor performance of diagnostic and therapeutic measures with the aim to improve the quality of health care and eventually improve the prognosis. The nationwide breast cancer database was launched in 1977 Citation[1], and the conjunction with a multidisciplinary cooperative breast cancer group has provided an instrument for monitoring community breast cancer standards and for conducting a large series of randomised trials Citation[2]. These initiatives have significantly contributed to an improvement of the prognosis in breast cancer Citation[3] and have become a model for the construction of multidisciplinary groups within cancers.
Today the clinical database of the DBCG includes three parts. The central part concerns patients with invasive breast cancer. The other parts concern patients with in situ carcinomas and of hereditary breast cancer. Here we provide a status for the original database of invasive breast cancer and summarizes the other aspects of the registry.
Material and methods
The Danish Breast Cancer Cooperative Group (DBCG) was initiated by the Danish Surgical Society in 1976 and has since 1977 prepared guidelines for diagnostic and therapeutic procedures in primary invasive breast cancer later supplemented with guidelines for in situ carcinomas and hereditary breast cancer on a nationwide basis in Denmark Citation[2]. Individual patient data of demographic and histopathological variables, therapeutic interventions and follow-up has been reported to the DBCG registry by the use of standardized forms. All Danish units involved in the diagnosis and treatment of breast cancer have contributed to the clinical database. From the DBCG 89 programme patients gave their written informed consent to be registered in the DBCG database.
The reported histopatological data included histological type according to WHO, tumour size, examination of tumour margins, invasion into skin or deep resection line, malignancy grade, number of nodes examined, hereof tumour positive, vascular invasion, oestrogen (ER) and/or progesterone (PgR) status, HER2 status (from 2002) and TOP2A status (from 2007). Additional analysis and definitions are described in details elsewhere Citation[3], Citation[4]. The data of therapeutic interventions included type of surgery (mastectomy or breast conserving surgery), radiotherapy (target, dose, number of fractions), systemic therapy (type, doses, duration), haematological toxicities and other adverse events, and the results of the follow-up studies. A total number of 30000 patients are currently on treatment or in control. Annually approximately 1.5 mio variables are registered on 54 different forms.
Definition of risk groups
Throughout the DBCG programmes, DBCG 77, 82, 89, 99, 01, 04 and 07 patients were allocated, according to prognostic factors, to a low-risk group and a high-risk group.
The prognostic factors, defining the risk groups, evolved step by step as shown in . Allocation to the low-risk group required, in all programmes, node negativity and a tumour size of maximum 5 cm in programmes 77, 82 and 89, and 2 cm in the subsequent programmes. In addition, invasion to skin or chest wall/deep resection line excluded patients from the low-risk group in the first two programmes (77 and 82). Malignancy grade of ductal carcinomas was added as a selection criteria in pre-menopausal patients in DBCG 89 and in postmenopausal patients as well in the DBCG 99 programme, with grade I tumours (and non-ductal carcinomas) being allocated to the low-risk group and ductal carcinomas grade II–III to the high-risk group. Receptor status was used as selection criteria from DBCG 99 and onwards. Receptor positivity (ER and PgR both positive, or one positive, and the other unknown), and receptor unknown (both unknown) status led to allocation to the low-risk group. From the DBCG 01 programme age was added as selection criteria. Thus age 35 years or younger was considered a high-risk factor. In the subsequent DBCG 07 programme, positive HER2 status as well as abnormal TOP2A status has been added to the high-risk criteria.
Table I. Definition of low-risk groups in DBCG programmes.
From the DBCG 89 programme receptor status and, from the DBCG 04 programme in addition HER2 status have been used as predictive factors. In the DBCG 07 programme abnormal TOP2A status will be used to select patients to treatment with anthracyclines.
Definition of menopausal status
In the DBCG 77 and 82 programmes patients were defined postmenopausal if menostasia for more than 5 years, or prior hysterectomy or bilateral oophorectomy and 55 years or older, or menstruation during cyclic hormonal therapy and 55 years or older. All other were patients defined pre-menopausal.
In the DBCG 89 programme patients were defined postmenopausal if menostasia for 12 or more months, or prior bilateral oophorectomy or prior hysterectomy, or menstruation on cyclic hormonal therapy and 50 years or more. All other patients were defined pre-menopausal.
In the DBCG 99 programme patients were defined postmenopausal if menostasia for 12 or more months, or prior bilateral oophorectomy or prior hysterectomy, or menstruation on cyclic hormonal therapy and 55 years or more. In this programme perimenopausal status was introduced defined by menostasia for 2–12 months, or prior hysterectomy or menstruation on hormonal replacement therapy and age 50–54 years. All other were patients defined pre-menopausal.
In the DBCG 01, 04 and 07 programmes definitions were similar to those in the DBCG 99 programme with the exception that perimenopausal status was included in the pre-menopausal status.
Surgery
Mastectomy was the primary surgical procedure used in the vast majority of patients until the demonstration, in the DBCG TM trial, conducted as part of the DBCG 82 programme, of similar outcome with mastectomy and breast conserving surgery (BCS) plus irradiation against the residual breast in patients otherwise eligible for BCS Citation[5]. Since then an increasing proportion of patients had BCS as shown later in this paper. The primary surgical procedure was associated with axillary dissection at level I-II. The recommended number of examined lymph nodes increased with time. Initially it was recommended to remove five lymph nodes and it was demanded to examine at least one. From the 89 programme the requirement became strengthened and ended up demanding removal of at least 4, but preferably 10 lymph nodes, and this was further extended in 1994. Hereafter the minimum requirement was at least 10 removed lymph nodes. Since the introduction of the sentinel node technique from 2002 Citation[6] axillary dissection was limited to node positive cases and cases not eligible for this technique.
Radiotherapy
The target for radiotherapy following BCS includes the residual breast. In the DBCG 82 programme the median absorbed dose was 50 Gy/25 fractions over 5 weeks followed by 10–24 Gy/5–12 fractions over 1–2 weeks to the tumour bed. From the DBCG 89 programme the dose has been 48 Gy/24 fractions over 5 weeks followed by 10–16 Gy/5–8 fractions over 1 week. In all cases the treatment has been with high-voltage equipment. In addition, node positive patients received radiotherapy against the regional nodes, as described for post-mastectomy irradiation below.
Following mastectomy all high-risk patients received radiotherapy against the chest wall and regional lymph nodes (supraclavicular, infraclavicular and axillary) in the DBCG 77 programme. Most patients were treated with high-voltage equipment to a minimum dose of 41 Gy/22 fractions over 5 weeks or 36 Gy/12 fractions over 6 weeks. Approximately 15% of the patients were treated with low-voltage equipment with a dose of 36 Gy/20 fractions over 4 weeks.
In the DBCG 82 programme the target for the post-mastectomy irradiation included chest wall and regional nodes (supraclavicular, infraclavicular, axillary and parasternal). The recommended median absorbed dose was either 50 Gy/25 fractions over 5 weeks or 48 Gy/22 fractions over 5 weeks. During this period only 8% of the patients were treated with low-voltage equipment as described in the DBCG 77 programme above. The group of patients having irradiation was those high-risk patients randomised to arm 1 in the DBCG 82B and 82C trials ().
Table II. Adjuvant systemic therapy in high-risk patients in the DBCG programmes.
From the DBCG 89 programme all post-mastectomy irradiation was given using high voltage equipment against the chest wall and/or regional nodes, as in the 82 programme, with a recommended median absorbed dose of 48 Gy/24 fractions over 5 weeks. In the DBCG 89 programme all patients with invasion of the deep resection line had irradiation against the chest wall. According to the availability of data from the previous DBCG 82B and 82C trials, the recommendations for radiotherapy against the regional nodes changed. Before January 95 they included patients aged 45 years or younger with 4 or more positive axillary nodes, since these patients were the only subgroup which at that time showed a significant survival benefit of postmastectomy irradiation. After January 95 they included pre-menopausal patients with any number of positive axillary nodes or a tumour larger than 5cm. However the axilla was not included in the target if node positivity and 10 or more nodes had been removed or if node negativity and at least 4 nodes had been removed.
In the DBCG 99 and subsequent programmes recommendations for post operative irradiation included all pre-menopausal and postmenopausal women less than 70 years as described for the DBCG 89 programme since January 1995. In high risk patients above that age was adjuvant irradiation based on an individual decision.
As concerns the timing of chemotherapy and radiotherapy these two modalities were used simultaneously in the DBCG 77. In the DBCG 82 programme the chemotherapy was temporary interrupted, and in the DBCG 89 programme, treatment with metotrexate and fluorouracil were avoided during radiotherapy. From the DBCG 99 programme generally (if not otherwise indicated) radiotherapy was given after the completion of chemotherapy. This was however first implemented in 2001, and at the same time was the number of chemotherapy cycles (CEF or CMF) reduced from nine to seven.
Additional details about the radiotherapy are given elsewhere Citation[7–9].
Patient follow-up
The recommended follow-up included visits every 3–6 months during the first 5 years an hereafter annually for 5 years or until recurrence. The visits included physical examination and additional investigations as indicated. The patients went off study in case of an event including loco-regional or distant recurrence, contra-lateral breast cancer, other malignancy or inter-current death. All patients were followed for survival by linkage to the Danish Civil Registration System.
Systemic treatment, low-risk patients
Throughout the period patients allocated to the low-risk group received no systemic therapy but had local therapy only; surgery and, if BCS, radiation against the residual breast.
Systemic treatment, high-risk patients
In the DBCG 77 programme the recommended standard adjuvant therapy was post-mastectomy irradiation only (). The DBCG 77 programme included 2 randomised trials (DBCG 77B and 77C). All patients had post-mastectomy radiotherapy. Pre-menopausal patients (77B) were randomised to control or levamisole (2.5 mg/kg orally day 1 + 2, weekly times 48) or cyclophosfamide (130 mg/m2 orally days 1-14 every 4 weeks times 12) or CMF (cyclophosfamide 80 mg/m2 orally days 1-14 every 4 weeks times 12, metotrexate 30 mg iv. days 1 and 8 every 4 weeks times 12, fluorouracil 500 mg iv. days 1 and 8 every 4 weeks times 12). Postmenopausal patients (77C) were randomised to control or to levamisole (as in 77B) or tamoxifen, 30 mg per day, for 1 year. Both trials were activated October 1977 and closed December 1982. However, the levamisole arms were closed, and treatment discontinued, December 1979 due to considerable toxicity and early increased rate of recurrence Citation[10], and in DBCG 77B the radiotherapy only arm was closed January 1981 due to poorer outcome compared to the 2 chemotherapy arms. The DBCG 77B and 77C trials included 1144 and 1930 patients respectively. Data have been published and demonstrated superior outcome with chemotherapy and tamoxifen respectively Citation[11], Citation[12].
In the DBCG 82 programme the recommended adjuvant standard treatment was radiotherapy plus CMF (600, 40, 600 mg/m2 iv. day 1 every 4 weeks times 9) in pre-menopausal patients and tamoxifen, 30 mg daily for 1 year, in postmenopausal patients less than 70 years of age (). The DBCG 82 programme included 2 clinical trials, the DBCG 82B and DBCG 82C, who analysed the effect of avoiding radiotherapy in patients offered systemic therapy with CMF (pre-menopausal) or tamoxifen (postmenopausal). In addition these trials analysed the benefit of adding tamoxifen to CMF in pre-menopausal patients, and of adding CMF to tamoxifen in postmenopausal women less than 70 years of age. Doses and duration of chemotherapy and tamoxifen was similar to those used in the standard treatments. CMF and tamoxifen were administered simultaneously. The 2 trials were activated October 1982 and closed February 1990, however randomisation to the CMF + tamoxifen combination in DBCG 82B was terminated June 1986 due to higher early rate of mortality with the combination. Trial DBCG 82B included a total of 2028 patients and trial 82C 2030 patients. In both trials a benefit in terms of local and distant control was observed with the addition of radiotherapy Citation[13–17]. In trial DBCG 82B an updated analysis showed no significant benefit of adding tamoxifen to CMF Citation[18]. In the DBCG 82TM trial of mastectomy versus BCS, conducted as part of the DBCG 82 programme, high-risk patients were all allocated to receive the combined adjuvant therapy, i.e. radiotherapy plus CMF or radiotherapy plus tamoxifen in pre-menopausal and postmenopausal patients respectively Citation[5].
In the DBCG 89 programme the recommended standard adjuvant treatment appears from . The recommendation of tamoxifen 30 mg per day for 1 year in postmenopausal patients with receptor positive or unknown tumours was changed, from January 1997, to the duration of 5 years. CMF and CEF (cyclophosfamide, epirubicin, fluorouracil) were administered as 9 three-weekly cycles at 600, 40 and 600 mg/m2 and 600, 60 and 600 mg/m2 respectively. The DBCG 89 programme included 3 clinical trials, conducted in centres in Denmark and with contribution from centres in Sweden, The Netherlands and Iceland. DBCG 89B randomised pre-menopausal patients with node positive, receptor positive tumours to CMF (schedules as standard treatment) or to ovarian ablation by irradiation (15 Gy/5 fractions) or surgery. The trial was activated January 1990 and closed June 1998. A total of 762 patients were randomised, hereof 230 from Sweden and The Netherlands. Similar outcome was observed with the 2 regimes Citation[19]. DBCG 89C randomised postmenopausal patients less than 75 years with node positive, receptor positive or unknown tumours to tamoxifen 1 year versus 2 years versus tamoxifen 6 months followed by megestrol-acetate 160 mg daily for another 6 months. The trial was activated January 1990. When the pre-planned sample size was reached in December 1994 it was decided to continue randomization to tamoxifen 1 versus 2 year. Randomisation to the sequential treatment arm was not extended due to increased toxicity and no evidence of superiority compared to tamoxifen alone. One thousand six hundred and fifteen patients were randomised in arms 1–3 and further 703 patients in arms 1–2, and a recent analysis demonstrated similar outcome with the 3 regimes Citation[20]. DBCG 89D randomised pre-menopausal patients with receptor negative or unknown tumours and postmenopausal patients less than 70 years with receptor negative tumours and in addition pre-menopausal patients with ductal carcinomas malignancy grade II-III to 9 cycles of CMF or CEF (schedules as standard treatment) or to the same regimes plus pamidronate, 150 mg twice daily for 4 years. The CMF versus CEF comparison was activated December 1989 and closed June 1998. A total of 1224 patients, hereof 244 from Sweden and Iceland, were randomised and superiority of CEF in terms of recurrence rate and survival was observed Citation[21]. A subsequent translational analysis demonstrated that the superiority of CEF over CMF was limited to patients with abnormal TOP2ACitation[22]. The chemotherapy versus chemotherapy + pamidronate part of the study was activated July 1990 and closed January 1996 due to a lower than expected rate of bone recurrences and reports of severe gastrointestinal adverse events from trials in osteoporosis. This part of the trial randomised a total of 953 patients, hereof from Sweden and Iceland 205 patients, and a recent analysis has demonstrated no benefit, in terms of reduction in risk of bone metastases, with the addition of pamidronate, and gastrointestinal toxicity was not observed more frequently in the pamidronate group Citation[23].
The standard recommended adjuvant treatments in the DBCG 99 programme according to menopausal status and receptor status is shown in . The chemotherapy regimes included 9 cycles of CMF (600, 40, 600 mg/m2 iv. every 3 weeks) or CEF (600, 60, 600 mg/m2 iv. every 3 weeks) and the endocrine therapy included ovarian ablation or tamoxifen given for 5 years. As part of the DBCG 99 programme DBCG participated in 4 international trials as shown in . These trials are briefly summarised below.
The standard treatments in the DBCG 01 and 04 programmes according to menopausal status and receptor status are very similar (). Chemotherapy includes 7–9 cycles of CMF (600, 40, 600 mg/m2 iv. every 3 weeks) or CEF (600, 60, 600 mg/m2 iv. every 3 weeks) and the endocrine therapy includes tamoxifen given for 5 years. From June 2004 aromatase inhibitor were introduced for postmenopausal women who had completed 2–3 years of tamoxifen (sequential tamoxifen-aromatase inhibitor) or 4–5 years of tamoxifen (extended aromatase inhibitor). From January 2006 trastuzumab, three weekly for 1 year, was offered after chemotherapy to patients with HER2 positive tumours.
In the DBCG 07 programme standard treatment for patients younger than 60 years with receptor positive or unknown tumours was chemotherapy plus endocrine therapy. The chemotherapy is 3 three-weekly cycles of EC (600, 90 mg/m2) followed by 3 three-weekly cycles of docetaxel (100 mg/m2). The endocrine therapy is 5 years of tamoxifen in pre-menopausal and sequential tamoxifen-aromatase inhibitor in postmenopausal patients. Pre- and postmenopausal patients with receptor negative tumours and postmenopausal women 60 years or older with receptor positive or unknown tumours are offered chemotherapy and endocrine therapy respectively.
During the programmes 2001 to 2007 DBCG participated in international trials and conducted and planned another 2 national trials. These are shown in and are briefly summarized below.
International clinical trials
BIG 1-98.
The BIG 1-98 (Breast International Group) was a randomised double blind trial in postmenopausal patients with receptor positive tumours, comparing four endocrine therapy options: 1) monotherapy with tamoxifen, 20 mg daily, for 5 years; 2) monotherapy with letrozole, 2.5 mg daily, for 5 years; 3) sequential administration of tamoxifen for 2 years followed by letrozole for 2 years; 4) sequential letrozole for 2 years followed by tamoxifen for 3 years. Prior adjuvant chemotherapy was administered according to local/national guidelines. From March 1998 to May 2003 8010 patients entered the trial, hereof from Denmark 1396. The primary core-analysis with 25.8 months median follow-up demonstrated a significant reduction in the risk of event in patients treated with letrozole compared to tamoxifen Citation[24].
The IES-study
This Intergroup Exemestane Study recruited postmenopausal women previously diagnosed with a receptor positive or unknown breast cancer, who had received adequate local and adjuvant systemic therapy (including tamoxifen and chemotherapy if indicated). Women who remained free of disease on tamoxifen after 2–3 years were randomised to switch to exemestane 25 mg daily or to continue tamoxifen 20 or 30 mg (Denmark) per day for the remainder of 5 years endocrine treatment. Between 1998 and 2003 4704 patients were randomised, hereof from Denmark 136. After a median follow-up of 30.6 months a significant benefit in disease-free survival was reported in favour of exemestane Citation[25].
BIG 02-98
The BIG 02-98 (Breast International Group) trial recruited patients 70 years or younger with node positive breast cancer who were randomised to one of four treatments: 1) sequential control (4 cycles of doxorubicin 75 mg/m2 every 3 weeks, followed by 3 cycles of CMF); 2) concurrent control (4 cycles of doxorubicin 60 mg/m2 plus cyclophosfamide 600 mg/ m2 every 3 weeks followed by 3 cycles of CMF); 3) sequential docetaxel (3 cycles of doxorubicin 75 mg/m2 every 3 weeks followed by 3 cycles of docetaxel 100 mg/m2 every 3 weeks followed by 3 cycles of CMF); 4) concurrent docetaxel (4 cycles of doxorubicin 50 mg/m2+docetaxel 75 mg/m2 every 3 weeks, followed by 3 cycles of CMF). In all arms CMF was given every 4 weeks as oral cyclophosfamide 100 mg/m2 on days 1-14 and intravenous metotrexate 40 mg/m2+fluorouracil 600 mg/m2 on days 1 and 8. If oral cyclophosfamide could not be tolerated a switch to intravenous cyclophosfamide 600 mg/m2 on days 1 and 8 was allowed. Following chemotherapy patients got 5 years of endocrine therapy when indicated. From June 1998 to January 2001 2890 patients entered the study, hereof from Denmark 156. At 5 years, treatment with docetaxel resulted in an improvement of disease-free survival of borderline statistical significance compared with control treatment. However, disease-free survival in the sequential docetaxel arm was better than in concurrent docetaxel arm and in the sequential control arm Citation[26].
SBG 2001
This study, conducted within the frames of Scandinavian Breast Cancer Group (SBG) included high-risk patients, who received an initial cycle of CEF (600, 60 and 600 mg/m2=CEF level I). Haematological toxicity was carefully assessed following this initial cycle. Patients who experienced leucopoenia grade 4–5 continued treatment with CEF level I (450, 45, 600 mg/m2). If leucopoenia grade 3 patients continued treatment on level I. If leucopoenia grade 2 patients were randomized to further treatment with level I or II (600, 75, 900 mg/m2) and if grade 0–1 to further treatment with level I or II (600, 90, 1200 mg/m2). The patients received a total of 7 cycles three-weekly, and hereafter 5 years of endocrine therapy when indicated. From February 2001 to September 2003 a total of 1534 patients entered the trial, hereof from Denmark 564. The first analysis of the efficacy data is expected ultimo 2008.
HERA
The Herceptin Adjuvant BIG 0101 randomised trial compared 1 year against 2 years of trastuzumab given every 3 weeks with observation in patients with HER2 positive breast cancer who had completed loco-regional therapy and at least 4 cycles of neo-adjuvant or adjuvant chemotherapy. When indicated patients received 5 years of endocrine therapy following discontinuation of chemotherapy. From December 2001 to March 2005 5081 patients were enrolled in the study, hereof from Denmark 133. At the first planned interim analysis with a median follow-up of 1 year, a significantly reduced risk of event was observed in the trastuzumab group compared to the observation group Citation[27].
NICE
This is a neo-adjuvant study organized by DBCG and conducted in collaboration with centres in Sweden and Norway. Eligibility to the trial required the primary tumour to be at least 2 cm and ER receptor negative. Patients were randomised to receive EC (epirubicin at 90 mg/m2+cyclophosfamide at 600 mg/m2 for 4 cycles) plus placebo or EC plus gefitinib. The primary endpoint was complete pathological response. A translational research programme was pre-planned and frozen and paraffin embedded tumour tissue was obtained at baseline and if possible at definitive surgery. From October 2004 to January 2007 188 patients were randomised, hereof from Denmark 126. The first efficacy analysis is expected in 2008.
FACE
The Femara versus Anastrozole Clinical Evaluation trial compared 5 years of letrozole, 2.5 mg daily, with 5 years of anastrozole, 1 mg daily, in postmenopausal patients with receptor positive and node positive disease. Prior chemotherapy was allowed. From July 2005 to February 2008 a total of 4115 patients entered the trial, hereof from Denmark 269. Reports on early and late onset adverse events are expected 2008/2009. The first interim efficacy analysis is planned for 2009 and the final efficacy analysis for 2012.
TEACH
The Tykerb Evaluation After Chemotherapy trial compares 1 year of lapatinib against placebo in patients with HER2 positive breast cancer. Patients must have received prior adjuvant chemotherapy containing CMF, an anthracycline or a taxane and may continue to receive endocrine therapy. Prior therapy with a HER2 or EGFR inhibitor is not allowed. The first patient was randomised August 2006 and 2370 patients have since been enrolled, hereof 71 in Denmark. The recruitment goal is 3300 randomised patients and is expected to be reached in March 2008.
ALTTO
The international Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization study analyses the relative benefit, with lapatinib, trastuzumab, their sequence and combination. Patients with HER2 positive primary breast cancer are randomised to one of the following four options: 1) trastuzumab, iv. every 3 weeks for 1 year; 2) oral lapatinib daily for 1 year; 3) trastuzumab, iv. every 7 days for a total of 12 weeks followed by oral lapatinib to a total of 1 year; 4) treatment for 1 year of with lapatinib daily plus three weekly trastuzumab. Patients could be randomised following completion of prior adjuvant chemotherapy or concurrently with taxanes after any anthracycline based (neo-) adjuvant chemotherapy. Eight thousand patients are planned to be enrolled within 3.8 years. The study was activated May 2007 and the monthly accrual has increased steadily.
SOLE
The Study Of Letrozole Extension trial includes patients with hormone receptor positive tumours who remain free of disease following 4–6 years of endocrine therapy (tamoxifen and/or aromatase inhibitor). Prior treatment with cytotoxic agents or trastuzumab is allowed. Patients are randomised to another 5 years treatment with letrozole, administered continuously or intermittently (9 month treatment followed by 3 months break times 4, followed by 12 month treatment). The primary endpoint is disease-free survival. The trial was activated early 2008 and is planned to accrue 4800 patients in 3 years. DBCG plans participation during 2008.
National clinical trials
FEM 345D
This study conducted by DBCG included postmenopausal women with receptor positive, node positive or node negative high-risk tumours to 5 years of letrozole versus the sequence of 2 years of letrozole followed by 3 years of tamoxifen. The primary objective of this study was to closely monitor lipid- and bone metabolism (markers in blood, bone mineral density). From May 2005 to March 2006 267 patients entered the trial. The first data are expected to be available late 2008.
DBCG 07-READ
The DBCG 07-READ (Randomized trial of Epirubicin and cyclophosphamide followed by docetaxel Against Docetaxel and cyclophosphamide) is organized by DBCG. The trial includes patients with TOP2A normal tumours in patients otherwise eligible for chemotherapy according to the standard guidelines (). Patients are randomised to either 3 cycles of EC (epirubicin 90 mg/m2, cyclophosfamide 600 mg/m2) followed by 3 cycles of docetaxel (100 mg/m2 every 3 weeks) or to 6 cycles of the combination of CD (cyclophosfamide 600 mg/m2, docetaxel 75 mg/m2 every 3 weeks). Primary aim of the study is to test the hypothesis that a non-anthracycline combination is superior to an anthracycline combination in patients with TOP2A normal tumours. The study is planned to be activated April 2008 and to enrol 1932 patients in 3 years. DBCG invites international collaboration.
DBCG 07-REAL
The REAL (Randomised trial of Endocrine therapy Against Loco-regional therapy first) trial includes high-risk patients 60 years or older with hormone receptor positive tumours ≥2 cm. Patients are randomised to either surgery first or letrozole for 18 weeks before surgery. Following primary surgery patients will receive 5 years of adjuvant letrozole. Following preoperative letrozole patients with clinical benefit will continue with letrozole to a total of 5 years. Patients with progressive disease during preoperative letrozole will after surgery receive adjuvant chemotherapy with 3 cycles of EC (epirubicin 90 mg/m2, cyclophosfamide 600 mg/m2) followed by 3 cycles of docetaxel (100 mg/m2 every 3 weeks). The primary endpoint is invasive disease-free survival. Frozen and paraffin embedded tumour tissue is obtained at baseline and if possible at definitive surgery following preoperative letrozole. The study is planned to be activated June 2008 and to enrol 1500 patients in 5 years. DBCG invites international collaboration.
New radiotherapy trials
In addition to the studies described above is a series of new trials under way which explore in the possibilities of reducing the extend and time involved in postoperative radiotherapy. This includes both a reduction of the irradiated volume after breast conserving surgery, so called partial breast irradiation, and at study of the feasibility of using hypofraction i.e. fewer but larger doses of irradiation per fraction. Both trials will be designed as randomized phase II studies using radiation induced morbidity as the primary endpoint. Each trial will include 600 patients (300 in each arm) and is planned to start in 2008.
Patients with ductal or lobular carcinoma in situ
From 1982 registration of in situ carcinoma was added to the DBCG programme as an observational study which included patients with histological verified lobular (LCIS) or ductal (DCIS) carcinoma in situ treated by BCS. From the 1989 programme and onwards patients treated by mastectomy was also included. The follow-up programme in the 82-IS and 89-IS protocols consisted of annual clinical examination and mammography every second year for ten years. In 2004 the in situ protocol was changed and differentiated recommendations for treatment were launched based on the van Nuys histological classification and distribution of the lesion. Since, the standard treatment is BCS followed by radiotherapy, although this is omitted in small lesions up to 20 mm with van Nuys group I histology and with 10 mm free margins. In case of diffuse or very large lesions mastectomy with or without immediate reconstruction is recommended. From 2006 it is recommended to perform sentinel lymph node biopsy in case of larger lesions especially in conjunction with mastectomy.
There are 3228 patients registered in the database with a primary lesion of carcinoma in situ (1982–2006). Of these 35% are treated by mastectomy, 38% by BCS and radiotherapy and 27% biopsy only. Four hundred and three patients (13.2%) have developed invasive carcinoma and 73 of these have died (2.2% of the whole cohort). A more detailed presentation of the results from the in situ protocols is presented elsewhere in this issue Citation[28].
Breast and ovarian cancer families
A steering committee was established by the DBCG in 1997 and produced evidence-based guidelines for genetic and clinical counselling of breast and ovarian cancer families. In 1999 these guidelines were implemented nationwide in Denmark and a family registry, the Hereditary Breast and Ovarian Cancer Registry (HBOC) was launched as an extension to the clinical database of the DBCG. The committee consists of a medical geneticist from each of the centres involved in genetic counselling of breast and ovarian cancer families in Denmark, and representatives from the specialities of breast surgery, gynaecology, reconstructive surgery, radiology, molecular biology and oncology.
The families are identified through genetic counselling at one of the centres and each family is assigned a unique family identifier by the DBCG. Data is collected and whenever possible family history of cancer is verified through the DBCG registry, pathology reports, and hospital charts. The pedigree is reviewed and the family is assigned to the low, moderate or high-risk group by the DBCG criteria. Testing for mutations in BRCA1 and BRCA2 is offered to patients in the moderate and high-risk groups. Family data and individual patient data regarding risk reductive surgery, gynaecologic and breast screening have been reported to the DBCG by the use of standardized forms.
A total of 3121 breast and ovarian cancer families were entered into the HBOC part of the DBCG registry from 1999 through December 2006. Thomassen and colleagues present the results of mutation screening in BRCA1 and BRCA2 elsewhere in this issue Citation[29].
Data-management
Data entry
The primary key of the database is the personal identification number (CPR). This is a unique 10-digit number, which encodes date of birth and sex of all individuals in Denmark.
The data entry module has been upgraded continuously since the beginning of the registry. Until 1990, paper data forms were send to the DBCG office, recoded by the data-secretaries, punched on cards, and computer processed once every month using a batch programme written in the statistical modelling language SAS. Online systems have been in use since 1990, available only for secretaries at the DBCG office. In the computerisation of data forms double entry is used to minimize errors. A web-based data entry has been introduced and allows direct data entry and queries at the individual clinic. The use of web-based data entry interface is expanding rapidly and will gradually be made available for all clinical end-users.
Data validation
At entry, the on-line screens contain a validation of individual variables. A date must be a valid date, number variables must be within relevant range, and codes must be valid. There is a test for missing values, so that missing, zero and blank is not confused. From the internet interface, end-users can produce valid codes only.
The database receives updates and corrections on a daily basis, by means of a batch programme. The batch programme produces an error list regarding discrepancies in the double entry, a cross-validation report for data validations dependent on several variables, and remainder lists covering for instance missing flow sheets.
A reminder is send immediately if any important prognostic parameter is missing, in order to determine the recommended treatment of the patient. At some time intervals the database is controlled for missing follow-up, e.g. if the periods since last follow-up is more than expected then first and second reminders are sent. If the data are still missing the patients are regarded lost to follow-up.
Quality control
Completeness has been validated by linkage to The Danish Cancer Registry and to the National Pathology Registry (NPR).
In connection with analyses of the DBCG 82 and DBCG 89 studies special validation has been conducted. It includes new questionnaires and inspection of original patient records, as well as linkage to the National Hospital Discharge Registry (NHDR).
Computer systems
The computing setup at DBCG consists of a number of components: A logical data model, a physical database, a data entry interface, service programmes and analysis programmes.
Logical data model: This is a description of the concepts and events recorded in the database and their interrelations. The logical model of the DBCG database has changed very little during its history.
Physical database: This is the data model as implemented on an actual computer platform. Since 1995, DBCG has used a relational Oracle database running on a UNIX platform hosted by UNI-C (Danish Computing Centre for Education and Research). The programming language in the database is SQL. For analysis purposes, there is a mirror of the Oracle database in SAS files. The SAS database includes historically data.
Data entry interface: The data entry consists of screen definitions and programming logic. The logic takes care of screen dialogue part of the data validation.
Service programmes: These are programmes run at regular intervals with the purpose of validation. Of critical importance to the maintenance of the database is the batch programme running on a daily basis. This programme transfers user records from a temporary to a permanent state.
Analysis programmes: These are programmes written by DBCG statisticians for analysis. Statistical analysis is primarily performed by the statistical software tool SAS.
Results
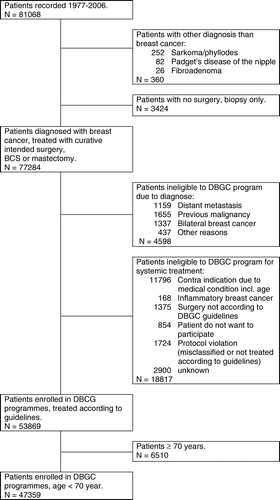
From 1977 through 2006 records from 81068 patients with malignant breast tumours was entered into DBCG's clinical database, and 77284 of these patients were diagnosed with an invasive carcinoma following BCS or mastectomy (). A part of the patients (N = 23415) were ineligible to be enrolled in the DBCG treatment programmes, and 53869 (70%) eligible patients were enrolled. Co-morbidity and age were the main causes for non-eligibility, while only 854 (1%) did not consent to registration and 3099 (4%) were either not allocated correctly or not treated according to DBCG's guidelines (). The upper age limit for patients to be eligible to systemic treatment has differed from no limit in DBCG 77 and DBCG 01-04 programmes, 69 years in DBCG 82 programme and 74 years in DBCG 89 and 99 programmes (), therefore the subgroup of patients enrolled and being younger than 70 years has been considered to illustrate the patient characteristics over time (n = 47359).
The proportion of patients enrolled increased steadily from 65% in the 77 programme to 81% in the 04 programme (). In the 77, 82 and 89 programmes half of the enrolled patients were allocated to the low-risk group, and this fraction was reduced to approximately 20% in following programmes in parallel with modifications of the selection criteria. Among 32300 high-risk patients 15605 (48%) participated in a randomised trial. Patients enrolled in the 77 and 82 programmes were all randomised prior to information about the trials. The fraction of high-risk patients enrolled in a randomised trial have decreased from 58% in the DBCG 89 programme to 20% in the DBCG 01 programme with a further decrease in the DBCG 04 programme.
Table III. Number of patients diagnosed with invasive breast cancer in the DBCG programmes, 1977–2006.
The surgical procedure was almost exclusively mastectomy in the 1977 programme. Seven percent of the patients in the 1982 programme received a BCS and this fraction steadily increased to 49% in the 2004 programme (). contains all patients diagnosed with invasive breast cancer while is restricted to patients enrolled in the DBCG programmes with an upper age limit of less than 70 years. The 77 and 82 programmes enrolled a higher proportion of patients 44 years and younger compared to later programmes (). Tumour size decreased steadily from programme to programme, while the proportion of patients with lymph node positive disease is less distinctive. The difference observed in number of lymph nodes visualized is in part reflecting the introduction of sentinel node technique.
Table IV. All patients diagnosed with invasive breast cancer in the DBCG database 1977–2006.
Table V. Patients < 70 years with invasive breast cancer enrolled in the DBCG programmes, 1977–2006.
More details about the changes according to time as concerns surgery, age, tumour size, number of examined and positive nodes, malignancy grade, and receptor status are presented elsewhere in this issue Citation[3].
Quality control
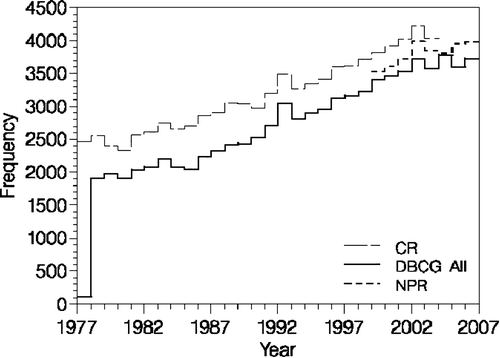
A past comparison Citation[30] of cases reported during the period 1979–1994 to DBCG and to The Danish Cancer Registry, which is considered to have a close to complete reporting of all cases, demonstrated a missing reporting to DBCG of approximately 20% of cases in the beginning of the period decreasing to approximately 10% of the cases in 1994. In addition, the vast majority of missing cases were elderly women over 70 years. Validation by linkage to the National Pathology Registry (NPR) was done in 2004 covering 1999–2004. In the group aged 18–69 years, approximately 3% of the cases were missing in the DBCG, 8% for patients diagnosed at 70–79 years of age, and 18% for the group of 80 years or older. Also, the NPR was not complete to the DBCG database. A new comparison in 2006 showed the same tendency ().
Figure 2. Number of patients according to time, registered to DBCG, the Danish Cancer Registry (CR) and the National Pathology Registry (NPR).
Data quality and completeness was investigated in patients diagnosed between 1983 and 1989 in one county (n = 1765) comparing DBCG recordings with a complete population-based database holding detailed information on patient characteristics, treatment and outcome Citation[31]. The DBGC hold high-quality data for the patients registered (83%), however, a substantial subset of patients (17%) were not recorded to DBCG, and for those the stage distribution was poorer and the life expectation was lower. When considering only patients < 70 years of age, there was no significant difference in overall survival between the population-based cohort and the patients notified in DBCG.
Follow-up data have been validated in different settings. For 4455 patients included in DBCG 82B and DBCG 82C, off-study data were validated by reviewing the patient's records Citation[32]. Both type of recurrence and time to event were due to inconsistencies. Incorrect data were observed in 16% of the cases. The amount of inconsistencies did not significantly influence the disease-free survival, with Kaplan-Meier estimates at 5 years at 49.9% and 49.4%, respectively.
In the DBCG 89D randomised trial comparing CMF with CEF, all 980 patient records have been reviewed. The results from the retrospective data enquiry using specific forms were compared with the original data collected prospectively. Information on adjuvant treatment was concordant. Type of first event (loco-regional recurrence, distant recurrence, new malignancy, death) matched in 87% of the cases, 7% had an event not recorded in the regular follow-up, 1% of the events from regular follow-up were not found in the review and in 5% of the cases, the type of first event were different. Comparing disease-free survival estimated from the two data sources showed no difference (p = 0.81), with estimates at 5 years at 60.0% and 59.2%, respectively, following the regular follow-up and the review [DBCG, non-published report].
A similar retrospective validation was performed for patients enrolled (both randomised and non-randomised) in the DBCG 89B programme, including 1575 patients. Again, high agreement was found in data concerning adjuvant treatment, and the results on type of first event and time to first event were in accordance with the above, finding 91% with exact match, 7% and 1%, respectively, missing in one of the cases, and 2% with non-matching type of events [DBCG, non-published report].
The retrospective data validation in the DBCG 89C trial with 2322 randomised patients was done differently, as queries were sent only for patients with missing follow-up and for patients still on study with no events recorded (13% of the patients). Thus, this type of analysis does not validate the total study population as concerns frequency and site of metastases. The recorded time of follow-up was extended for 150 patients (6%) resulting in updating of observations during treatment for 54 patients (2%), 78 additional observations of first event (3%), and prolonged period without event for 18 patients (1%), [DBCG, non-published report].
DBCG 89A programme including 9028 low-risk patients was validated retrospectively by record linkage to the National Hospital Discharge Registry (NHDR), which contains information on all hospitalizations registered on the individual level, with dates of admission and discharge, one or more diagnoses per hospitalization and up to 6 operations per diagnosis. Observations registered in NHDR of malignant events were compared to the DBCG register, and if the event was recorded in NHDR only, the patient journal was reviewed. The data validation resulted in the finding in 1280 previously unrecorded recurrences and second primary cancers, however, the majority (N = 929) related to patients with premature discontinuation of control (censuring). Reclassification before censuring constituted 4.3% of the study population. The validation resulted in a significant decrease in disease-free survival from 80 to 78% Citation[33]. However, it should be emphasized that using the NHDR as reference also confers problems due to missing validation studies of the quality of this database.
Other research activities
Aside from therapeutic development the scientific committees of the DBCG have been focusing on three main research themes.
Lifestyle factors have been at the centre of DBCG's scientific interest, both as a cause of breast cancer and as an indicator of prognosis following the diagnosis of breast cancer. Continuous efforts have been aimed at unravelling the relative importance of risk factors such as dietary, smoking, alcohol, socio-economic status, and hormonal and reproductive factors. The prognostic impact of body mass index and pregnancy following breast cancer are among the landmark research activities of the DBCG. Other epidemiological research aspects include new primary malignancies and late toxicities (e.g. bone fractures and heart diseases).
Molecular pathology and breast cancer genetics has constantly been an area of research. DBCG very early acquired a leading position regarding the clinical usefulness of ER and PgR receptors, and has made a large contribution to the standardization and quality control of steroid receptors. The effort of the DBCG to individualize treatment of breast cancer has more recently been extended to chemotherapy, and in particular development of TOP2A as a biomarker for treatment with anthracyclines. The prognostic properties of more than 70 biomarkers have been assessed.
Imaging research focusing at earlier diagnosis and improved staging of breast cancer is closely connected to therapeutic development and has also attained major awareness. A recent example being the introduction of sentinel node biopsy while earlier studies clarified that imaging not should be used as a screening tool for distant recurrences.
We only provided a brief account of the research activities within the DBCG, and must emphasize the achievements only have been possible through the collaboration with other research groups and institutions primarily in Denmark. This short overview is not comprehensive and considerable more information about the research activities of the DBCG can be obtained from the website of the DBCG [www.dbcg.dk].
Discussion
A very high acceptance has been accomplished from breast cancer patients as well as their physicians of registration in DBCG's nationwide clinical database. In a 30 years period only 1% of patients has not consented to have data about diagnostic procedures, treatment and follow-up entered onto the registry. Correspondingly, more than 95% of patients and their physicians seem to have adhered to the DBCG's clinical practice guidelines regarding surgery and medical therapies. These figures are however subject to some uncertainty resulting from data missing from a minority of patients with operable breast cancer ranging from less than 5% of patients younger than 70 years to less than 10% for patients diagnosed at 70–79 years of age and less than 20% for patients, 80 years or older.
Advanced age at diagnosis of breast cancer was the single most common reason for non-entry into the registry. Although the physicians throughout the period were encouraged to register all new patients, co-morbidities and, until 2000, the upper age limits to offer control or treatment according to well defined guidelines may explain that more than 10% of patients 70 years or older are not registered in the database. The latter is also supported by recent finding of a close to complete registration when compared with figures from the National Pathology Registry. Reports have claimed that elderly patients are less likely to receive the treatment recommended by the guidelines Citation[34–36], but the incomplete registration of elderly patients in the past have hampered our ability to substantiate whether this also is the case within a Scandinavian health care system. Elderly patients are typically underrepresented in clinical trials but are probably as likely as younger patients to participate if given the opportunity Citation[37]. From the beginning of this century age has no longer been considered an exclusion criteria concerning enrolment in DBCG programmes, and the DBCG has recognized a need to develop clinical trials that will facilitate participation of elderly patients.
Recruitment of breast cancer patients to clinical trials has persistently been a major goal of the DBCG. A total of 15605 patients corresponding to 48% of all Danish high-risk breast cancer patients diagnosed since 1977 have participated in a randomised trial. This achievement is to our knowledge unsurpassed, but has not been obtained without difficulties.
The professional practice standard of informed consent has continuously been improved since 1977, and the long-lasting collaboration with ethical committees concerning several generations of randomised trials has stimulated this process. The patient's rights to be informed about any randomised trial conducted within Denmark has emerged as a fundamental principle of veracity and this has major implications for the comprehensiveness of the information patients receive today. The standards for obtaining informed consent has evolved from oral information without specific information concerning randomization to shared decision-making following detailed information, orally and in writing.
The decrease in the proportion of randomised patients in the DBCG 04 programme is considered to be temporary and generated by gab in-between trials. The activation of two major trials, READ and REAL, and DBCG's participation in the SOLE trial will during 2008 increase patient's opportunity to participate in a clinical trial. Indeed, following some reluctance among patients to participate in randomised trials in the late 90's as a result of past public discussion about the format of the informed consent patients interest to learn about and participate in clinical trials seems to be increasing.
The establishment of the multidisciplinary breast cancer group with its associated database has provided the opportunity to improve the quality of the diagnostic and therapeutic aspects of breast cancer and to run trials, national or in international collaboration. The diagnostic and therapeutic guidelines set up by DBCG according to national and international scientific evidence has insured early introduction, on a nationwide basis, of new treatment strategies and has significantly contributed to the steady improvement of the prognosis in breast cancer through the past 30 years Citation[3]. The availability of the clinical data in the database combined with the availability of tumour tissue (paraffin embedded from essentially all, fresh frozen tissue from approximately 25%) and the Danish legislation as concerns examination of the tumour tissue has provided the ideal conditions for translational research. Large studies are ongoing with the aim to tailor the therapeutic interventions. Also linkage between the DBCG database and other registries offers the ideal platform to run epidemiological studies. The combined efforts of DBCG have also significantly contributed to an improved knowledge of breast cancer in both the primary and the secondary health care and improved awareness about the disease in the public.
References
- Andersen KW, Mouridsen HT. Danish Breast Cancer Cooperative Group (DBCG). A description of the register of the nation-wide programme for primary breast cancer. Acta Oncol 1988; 27: 627–47
- Blichert-Toft M, Christiansen P, Mouridsen HT. Danish Breast Cancer Cooperative Group – DBCG: History, organisation, and status of scientific achievements at 30-year anniversary. Acta Oncol 2008; 47: 497–505
- Mouridsen HT, Bjerre KD, Christiansen P, Jensen M-B, Møller S. Improvement of prognosis in breast cancer in Denmark –2006, based on the nationwide reporting to the DBCG registry. Acta Oncol ;47 1977; 2008: 525–36
- Kier HV, Nielsen BB, Bjerre KD, Lænkholm AV. Classical pathological variables recorded in the Danish Breast Cancer Cooperative Group's register –2006. Acta Oncol ;47 1978; 2008: 778–83
- Blichert-Toft M, Nielsen M, Düring M, Møller S, Rank F, Overgaard M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol 2008; 47: 672–81
- Christiansen P, Friis E, Balslev E, Jensen D, Møller S. Sentinel node biopsy in breast cancer: Five years experience from Denmark. Acta Oncol 2008; 47: 561–68
- Juul Christensen J, Overgaard M. Postoperative radiotherapy in DBCG during 30 years. Development of techniques, evaluation of indications and clinical radiobiological perspectives. Acta Oncol 2008; 47: 639–53
- Nielsen HM, Overgaard J, Grau C, Christensen JJ, Overgaard M. Audit of the radiotherapy in the DBCG 82 b&c trials--a validation study of the 1,538 patients randomised to postmastectomy radiotherapy. Radiother Oncol 2005; 76: 285–92
- Thomsen MS, Berg M, Nielsen HM, Pedersen AN, Overgaard M, Ewertz M, et al. Post-mastectomy radiotherapy in Denmark: From 2D to 3D treastment planning guidelines of The Danish Breast Cancer Cooperative Group. Acta Oncol 2008; 47: 654–61
- Brincker H, Mouridsen HT, Andersen KW, Andersen J, Castberg T, Fisherman K, et al. Increased breast-cancer recurrence rate after adjuvant therapy with lavamisole. Lancet 1980; 2: 824–7
- Dombernowsky P, Brincker H, Hansen M, Mouridsen HT, Overgaard M, Panduro J, et al. Adjuvant therapy of premenopausal and menopausal high-risk breast cancer patients. Present status of the Danish Breast Cancer Cooperative Group Trials 77-B and 82-B. Acta Oncol 1988; 27: 691–7
- Mouridsen HT, Rose C, Overgaard M, Dombernowsky P, Panduro J, Thorpe S, et al. Adjuvant treatment of postmenopausal patients with high-risk primary breast cancer. Results from the Danish adjuvant trials. DBCG 77 C and DBCG 82 C. Acta Oncol 1988; 27: 699–705
- Overgaard, M, Hansen, PS, Overgaard, J, Rose, C, Andersson, M, Bach, F, for the Danish Breast Cancer Cooperative Group 82b Trial, et al. Postoperative radiotherapy in high-risk pre-menopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med 1997;337:945–55.
- Overgaard M, Jensen M-B, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999; 353: 1641–48
- Højris I, Overgaard M, Christensen JJ, Overgaard J. Morbidity and mortality of ischaemic heart disease in high-risk breast-cancer patients after adjuvant postmastectomy systemic treatment with or without radiotherapy: Analysis of DBCG 82b and 82c randomised trials. Lancet 1999; 354: 1425–30
- Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol 2007; 82: 247–53
- Nielsen HM, Overgaard M, Grau C, Jensen AR, Overgaard J. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: Long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol 2006; 24: 2268–75
- Andersson M, Kamby C, Jensen MB, Mouridsen H, Ejlertsen B, Dombernowsky P, et al. Tamoxifen in high-risk pre-menopausal women with primary breast cancer receiving adjuvant chemotherapy. Report from the Danish Breast Cancer Co-Operative Group DBCG 82B trial. Eur J Cancer 1999; 35: 1659–66
- Ejlertsen B, Mouridsen HT, Jensen MB, Bengtsson NO, Bergh J, Cold S, et al. Similar efficacy for ovarian ablation compared with cyclophosphamide, methotrexate, and fluorouracil: From a randomized comparison of premenopausal patients with node-positive, hormone receptor-positive breast cancer. J Clin Oncol 2006; 24: 4956–62
- Andersen J, Kamby C, Ejlertsen B, Cold S, Ewertz M, Jacobsen EH, et al. Similar efficacy of tamoxifen for one year versus two years versus 6 month of tamoxifen followed by 6 month of megestrol acetate: Results from a randomized comparison in postmenopausal high-risk patients with hormone receptor positive or unknown breast cancer (DBCG trial 89C). Acta Oncol 2008; 47: 718–24
- Ejlertsen B, Mouridsen HT, Jensen M-B, Andersen J, Jensen BB, Cold S, et al. Improved outcome from substituting methotrexate with epirubicin: Results from a randomised comparison of CMF versus CEF in patients with primary breast cancer. Eur J Cancer 2007; 43: 877–84
- Knoop AS, Knudsen H, Balslev E, Rasmussen BB, Overgaard J, Nielsen KV, et al. Retrospective analysis of Topoiso-merase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, methotrexate and fluorouracil or cyclophosphamide, epirubicin and fluorouracil: Danish Breast Cancer Cooperative Group. J Clin Oncol 2005; 23: 7483–90
- Kristensen B, Ejlertsen B, Mouridsen HT, Jensen M-B, Andersen J, Bjerregaard B, et al. Bisphosphonate treatment in primary breast cancer: Results from a randomised comparison of oral pamidronate versus no pamidronate in patients with primary breast cancer. Acta Oncol 2008; 47: 740–46
- Thürlimann B, Keshaviah A, Coates AS, Mouridsen HT, Mauriac L, Forbes JF, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 2005; 350: 2747–57
- Coombes RC, Hall E, Gibson LJ, Paridaens R., Jassem J, Delozier T, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 2004; 350: 1081–92
- Francis P, Crown J, Di Leo A, Buyse M, Balil A, Andersson M, et al. Adjuvant chemotherapy with sequential or concurrent anthracycline and docetaxel: Breast International Group 02-98 randomized trial. J Natl Cancer Inst 2008; 100: 121–33
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Traztuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005; 353: 1659–72
- Lænkholm A-V, Jensen M-B, Kroman N, Rank F. Breast cancer in situ. From pre-malignant lesion of uncertain significance to well-defined non-invasive malignant lesion. The Danish Breast Cancer Cooperative Group –2007 revisited. Acta Oncol ;47 1977; 2008: 765–71
- Thomassen T, Hansen TO, Borg A, Lianee HT, Wikman F, Pedersen IS, et al. BRCA1 and BRCA2 mutations in Danish families with hereditary breast and/or ovarian cancer. Acta Oncol 2008; 47: 772–77
- Rostgaard K, Holst H, Mouridsen HT, Lynge E. Do clinical databases render population-based cancer registers obsolete. The example of breast cancer in Denmark. Cancer Causes Control 2000; 11: 669–74
- Jensen AJ, Storm HH, Møller S, Overgaard J. Validity and Representativity in the Danish Breast Cancer Cooperative Group. Acta Oncol 2003; 42: 179–85
- Hansen PS, Andersen E, Andersen KW, Mouridsen HT. quality control of end results in a danish adjuvant breast cancer multi-center study. Acta Oncol 1997; 36: 711–4
- Christensen, P , Al-Suliman , Bjerre, KD , Møller, S . Low-risk breast cancer – Follow-up from the DBCG 89-A program. Acta Oncol 2008;47:691–703.
- August DA, Thomas R, Sondak VK. Age-related differences in breast cancer treatment. Ann Surg Oncol 1994; 1: 45–52
- Ballard-Barbash R, Potosky AL, Harlan LC, Nayfield SG, Kessler LG. Factors associated with surgical and radiation therapy for early stage breast cancer in older women. J Natl Cancer Inst 1996; 88: 716–26
- Tyldesley S, Zhang-Salomons J, Groome PA, Zhou S, Schulze K, Paszat LF, et al. Association between age and the utilization of radiotherapy in Ontario. Int J Radiat Oncol Biol Phys 2000; 47: 469–80
- Kemeny M, Peterson BL, Kornblith AB, Muss HB, Wheeler J, Levine E, et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol 2003; 21: 2268–75