Abstract
DBCG (Danish Breast Cancer Cooperative Group) constitutes a multidisciplinary organization established in 1975 by the Danish Surgical Society. The purpose involves first and foremost a nation-wide standardization of breast cancer treatment based on novel therapeutic principles, collaboration between experts handling diagnostic work-up, surgery, radiotherapy, medical oncology, and basic research, and, further, complete registration of relevant clinical data in a national data base attached to DBCG. Data are processed by the Secretariat personnel composed of statisticians, data managers, and data secretaries making current analyses of outcome results feasible. DBCG is run by the Executive Committee consisting of expert members appointed by their respective society. From 1978 the DBCG project gained widely accession from participating units, and since then nearly all newly diagnosed breast cancer incident cases are reported and registered in the national data base. Today, the data base includes approximately 80 000 incidents of primary breast cancer. Annually, the Secretariat receives roughly 1.5 million parameters to be entered into the data base.
Over time DBCG has generated seven treatment programmes including in situ lesions and primary invasive breast cancer. Probands are subdivided into risk groups based on a given risk pattern and allocated to various treatment programmes accordingly. The scientific initiatives are conducted in the form of register- and cohort analysis or randomized trials in national or international protocolized settings. Yearly, about 4 000 new incident cases of primary invasive breast cancer and about 200 in situ lesions enter the national programmes. Further, about 600 women with hereditary disposition of breast cancer are registered and evaluated on a risk scale.
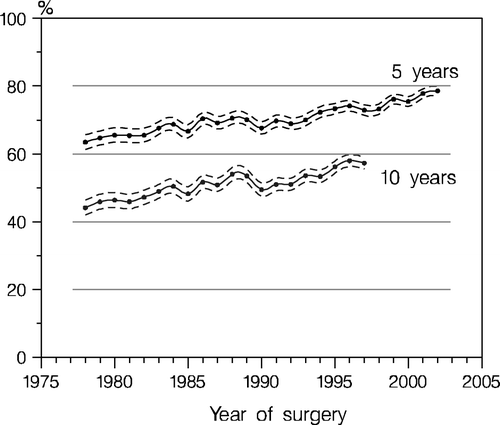
The main achievements resulted in a reduction of relative risk of death amounting up to 20% and increased 5-year overall survival ascending from 60% to roughly 80%.
This article is partly based on a Danish paper to be published in the Centenary Jubilee book of the Danish Surgical Society, 2008.
“DBCG constitutes an organization set up on the initiative of the Danish Surgical Society with the purpose of standardizing breast cancer treatment with reference to novel therapeutic principles and, further, executing a running registration and analysis of outcome results nation-wide”. This sentence prefaced the earliest paper on the history, organization, and treatment programmes of DBCG in the Danish Medical Journal in 1977 Citation[1]. Moreover, the paper emphasized the importance of a multidisciplinary approach to the handling of diagnostic work-up, treatment, and research within the field of breast cancer. The authors of the paper were Kaj Fischerman, at that time the Secretary General of the Danish Surgical Society and Chairman of DBCG, and Henning T. Mouridsen, medical oncologist and Secretary General of DBCG. The DBCG project appeared as a national innovator of novel treatment strategies including a national data base for registration and current analysis of treatment outcome and, remarkably, the first of its kind on a global scale. Thus, the objective of the DBCG project agreed with the aphorism from 1916 by the distinguished surgeon Dr. Charles H. Mayo, one of the founding brothers of the Mayo Clinic: “The keynote of progress in the 20th century is system and organization – in other words, “team-work””.
Background
The Danish Cancer Registry was founded in 1942 followed by the Norwegian, Finnish, and Icelandic Cancer Registries during the period 1952 to 1954 and the Swedish Cancer Registry in 1958. All registries include data which are based on a national scale Citation[2]. The initiatives made it feasible to analyse and compare mortality rates of cancer diseases. In Denmark, breast cancer mortality disclosed alarming results and no trend of improvements appeared during a time span of three to four decades Citation[2]. The reporting revealed that in Denmark breast cancer incidence as well as mortality reached the highest rates among the Nordic countries.
Up to about 1970, approximately 25% of women with breast cancer had died after one and a half years from time of diagnosis, and 50% within four and a half years Citation[3]. Further, cohort survival varied geographically showing better survival in the Copenhagen area compared with the remainder of Denmark. Particularly, among the younger age groups the geographic differences in survival were most distinct. The findings seem to express geographic variations in diagnostic work-up and treatment of the disease. Naturally, the phenomenon is difficult to explain prior to the introduction of the DBCG data base due to lack of valid knowledge as concerns the handling of breast cancer among institutions nation-wide. By that time local therapy (surgery plus radiotherapy) was still the only option in the treatment of primary breast cancer, but data had just been published indicating benefit with a new approach: systemic therapy added to the primary local therapy Citation[4], Citation[5]. That is how the situation appeared in the mid 1970s at the time, when the Danish Surgical Society took the lead and introduced the DBCG project as a nation-wide endeavour.
Establishment of DBCG and further advance
The beginning took place at the annual meeting of the Danish Surgical Society in 1975 where the launching of the DBCG project appeared on the agenda and obtained approval. A steering committee consisting of representatives from appropriate professional specialties (surgery, pathology, radiotherapy, medical oncology) was set up. The main objectives focused on ensuring of coordination, standardization, and quality control and development of the professional handling of breast cancer in Denmark on a nation-wide scale. The Secretary General of the Danish Surgical Society at that time, Kaj Fischerman, held the position as chairman of the steering committee, ex-officio. The steering committee became the harbinger of the future Executive Committee of DBCG. Unfortunately, no inaugural documents of DBCG can be traced from the archives of the Danish Surgical Society, since records are not filed until after 1981.
The enterprise launching DBCG proved quite demanding from the very start. During the first two years the steering committee held ten meetings. In October 1978, the DBCG project gained widely accession from the departments responsible for diagnosis and treatment of breast cancer and about 75% of new breast cancer cases were registered in the DBCG data base. A half year later in May 1979, the figure reached 90%, i.e. approximately 150 new breast cancer cases per month. Since then, nearly all patients with newly diagnosed breast cancer are reported and registered in the DBCG data base – at present approximately 300 incident cases a month.
At a meeting of the steering committee, September 18, 1979, the chairman, Kaj Fischerman, declared that the Danish Surgical Society now considered its contribution as mediator as concluded. Henceforth, DBCG should continue as an independent organization run by an Executive Committee with representatives appointed by the appropriate scientific societies and with surgical representation on equal terms with the remainder professions. Kaj Fischerman, Rigshospitalet, became President of the Executive Committee and held the position up to 1989. The succeeding President, Mogens Blichert-Toft, surgeon-in-chief of the Breast & Surgical Endocrine Clinic, Rigshospitalet, undertook his presidency until 2002 followed by the present President, Peer Christiansen, head of the Breast & Surgical Endocrine Unit, Aarhus University Hospital. Henning T. Mouridsen, medical oncologist, Finsen Institute, Rigshospitalet, became Secretary General, a position he still holds.
Infrastructure and organization of DBCG
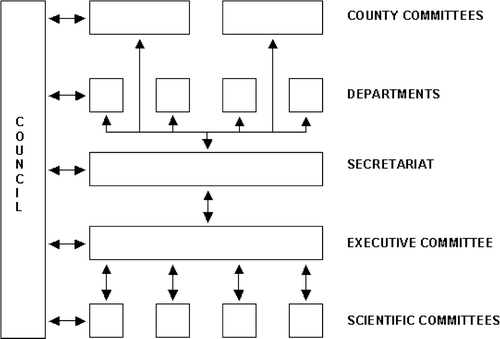
From the beginning the Executive Committee fully realized the immense challenges connected with a project like DBCG. It was entirely decisive that the project seemed realistic, that the initial support from the departments responsible for diagnosis and treatment of primary breast cancer could be maintained, and that they were invited to take an active part in the progress of the project, and were kept fully informed about factual status of ongoing or closed trials, and that the nation-wide activities became strengthened locally by the formation of local county committees. Finally, binding collaboration between the clinical disciplines and basic research was strongly addressed. The issues of intent did not appear merely as declarations free from obligations. Contrary, every item became implemented as binding commitments in the future cooperation within DBCG ().
The following bodies made up the infrastructure and organization of DBCG and a more detailed description of the various elements are presented in the statutes of DBCG Citation[6].
Council
The Council represents the supreme governing body of the organization consisting of the multidisciplinary representatives from participating departments, all members of local county committees, members of scientific committees, and members of the Executive Committee. A national meeting takes place at least once a year led by the President and the Secretary General of DBCG.
Executive Committee
The Executive Committee coordinates and promotes decisions made by the Council and the various committees. Further, the Executive Committee will conduct affairs concerning the practical implementation of DBCG projects, coordinate the tasks of scientific committees, and serve as advisor to the local county committees and participating departments as well as to public health authorities. Further, the Executive Committee will take the initiative preparing evidence-based guidelines for diagnostics and treatment in agreement with existing international guidelines.
The Executive Committee consists of 3 surgeons, of whom one is a plastic surgeon, 2 histopathologists, 4 oncologists, 1 clinical physiologist, 1 diagnostic radiologist, 1 clinical geneticist, and 1 tumour biologist representing basic science, all appointed by their respective scientific society. In addition, the Executive Committee includes the Secretary General of DBCG and the managing director (head statistician) of the Secretariat. Among its members the Executive Committee elects a President for a term of two years and shall be eligible for re-election in the same post.
Local county committees
In their respective counties the local committees are responsible for drawing up the guidelines regarding patient referrals, diagnostic work-up, courses of treatment, and clinical follow-up assessment in accordance with DBCG recommendations. Members of the local committee are appointed by their respective collegiate health board. By January 1, 2007 a new Local Government Act has been implemented abolishing the smaller counties for the benefit of larger regions. Accordingly, the DBCG statutes await an amendment with reference to the item.
Scientific committees
The purpose of the scientific committees is to ensure evidence-based treatment programmes and to initiate concurrent scientific projects. A scientific committee may be formed on the initiative of the Executive Committee and will in such circumstances preferably include representatives from the Committee, or by enterprise from persons with a special expertise of importance for DBCG activities. The scientific committees may be formed as standing committees or ad hoc committees. They will draw up their own bylaws, constitute a chairman and, if necessary, a secretary.
DBCG Secretariat
The Secretariat, housed at Rigshospitalet, conducts the centralized data registration, current data processing, and statistical analysis and shall ensure a link between the Secretariat and participating departments and committees. By January 1, 2006, the permanent staff includes a managing director (head statistician), 2 statisticians besides the managing director, 5 data secretaries, and a physician employed half time. In addition, one data manager and one statistician are employed by external funding.
Once a year the Secretariat publishes the DBCG News Journal giving an update of ongoing and closed trials. Further, the Journal carries the oral communications given at the annual Council meeting for general information.
Participating departments
The participating departments include surgical departments responsible for primary diagnostics in association with imaging departments, surgical interventions, and follow-up of low risk patients who do not receive adjuvant treatment. Moreover, the professional groups involve departments of pathology and oncological departments carrying out the oncological therapies and subsequent follow-up according to DBCG recommendations. In addition, there are the departments taking care of a variety of other activities, i.e., diagnostic radiology, clinical physiology/nuclear medicine, clinical biochemistry, and genetics. Finally, scientific groups working within the field of basic research, translational research, and epidemiology associated with breast cancer are also attached to the DBCG infrastructure and organization. More details about the organization and DBCG guidelines are described elsewhere Citation[6].
Quantitative items
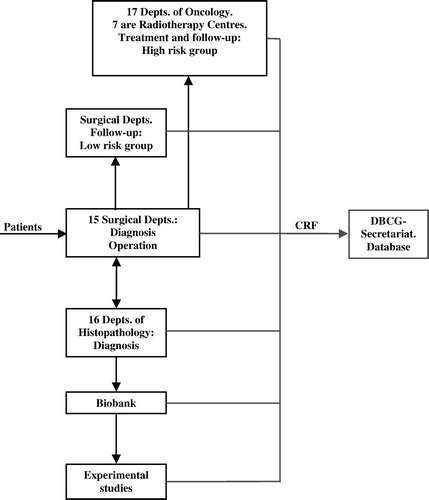
Since 1977 DBCG has generated seven treatment programmes, i.e. DBCG-77, DBCG-82, DBCG-89, DBCG-99, DBCG-01, DBCG-04, and DBCG-07. The initiatives are conducted in the form of register- and cohort analysis or randomized trials, national or in international collaboration Citation[7]. The treatment and control programmes encompass operable primary invasive breast cancer, in situ lesions, and (since 1999) hereditary breast and ovarian cancer. The main scientific achievements have aimed at development of new surgical procedures, improvement of radiation quality, novel chemotherapy- and endocrine strategies, introduction of antibody specific agents such as trastuzumab, and evaluation of predictive and prognostic factors. Data entering the DBCG database are submitted on standardized case report forms Citation[6] including a collective follow-up of 10 years (). Results are currently published in national and international peer-reviewed medical journals, so far approximately 250 papers Citation[6]. A brief review of the results of national and international trials is presented elsewhere in this issue Citation[7]. Further, DBCG data have contributed to the metaanalyses conducted by the Early Breast Cancer Trialists’ Collaborative Group Citation[8], Citation[9] and to the scientific content of several theses for the doctorate and Ph. D. degrees. In addition, DBCG conducts an extensive biobank storing leftover tissue as well as plasma/serum samples obtained preoperatively.
Already from the very beginning DBCG established a data base for continuous registration of clinical parameters. The participating departments representing surgery, pathology, and oncology as well as scientific committees and biobanks report on diagnostics, histopathological findings, treatment, follow-up events, and research using standardized case report forms (). Annually, the data base registers about 4 000 new incident cases of primary invasive breast cancer, about 200 incidents of in situ lesion, and 600 women with hereditary disposition of breast cancer.
By January1, 2006 the data base includes approximately 80 000 cases of primary breast cancer, hereof about 29 000 patients participating in ongoing trials or follow-up. Annually, DBCG Secretariat receives roughly 1.5 million parameters to be entered into the data base.
Financing
Financing offered quite an intricate course over time. Initially, DBCG received support from external funds. By July 1, 1982, the Secretariat became part of the health services based on an agreement between the National Health Service, the Ministry of the Interior, and the Ministry of Education. From then on, Rigshospitalet, Copenhagen, assumed the financial responsibility. Expenditures to run the clinical database and the associated organization were reimbursed from the various hospital municipalities paying a certain amount of money for each patient registered for the first time in the DBCG database – according to a government circular of December 22, 1980. The arrangement continued until the end of 1986 at which time the agreement was re-negotiated with the hospital counties. By mutual consent the funding lasted until January 1, 2007. As a consequence of the new Local Government Act by January 1, 2007 the existing financial arrangement ceased. Placed on the same footing as the other Danish multidisciplinary cancer groups, DBCG will now have to obtain financial support from a common governmental pool founded for invigoration of infrastructure of clinical cancer research. Since its establishment DBCG has received significant external funding to cover scientific research activities.
Impact on diagnostic work-up
Diagnostic work-up marks an essential step on the path towards an exact diagnosis prior to definitive surgery. Consequently, one of the main objectives emphasized by DBCG consists in enhancing diagnostic efficacy without unnecessary excisional biopsies removing benign lesions – not otherwise in need of surgery. Moreover, sorting out the diagnosis of malignant breast lesions prior to definitive surgery facilitates women's opportunity for taking part in decision-making concerning choice of surgical procedure. Besides, breast conserving surgery seems more easily accomplished in breasts not otherwise harmed by preceding cuttings.
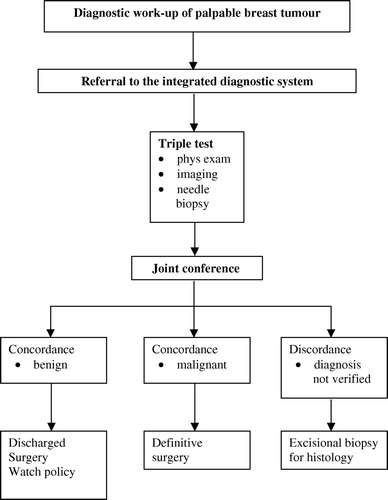
During the existence of DBCG the diagnostic procedures underwent remarkable innovations and refinements. One major advance consisted in setting up a teamwork affiliated to specialty units in which the expert surgeon together with the expert diagnostic radiologist and pathologist collaborate within the frames of the so-called integrated diagnostic system Citation[10–12]. The teamwork made it feasible to introduce the so-called triple assessment composed of physical examination, appropriate imaging procedures combined with needle biopsies carried out by respective experts and as a same-day intervention Citation[10], (). Introduction of triple assessment significantly reduces the number of excisional biopsies in benign as well as in malignant findings Citation[13].
Based on such a progress, DBCG in 2004 advanced the following recommendations on a national scale Citation[6]:
The local county committees are urged drawing up guidelines in writing for referred women for diagnostic work-up on the basis of a national strategy.
Women with breast symptoms or findings suspicious of malignancy are referred to further diagnostic work-up within the integrated diagnostic system.
The integrated diagnostic system in accordance with recommendations by the National Health Service Citation[11], Citation[12] should become the leading principle in diagnostic work-up.
Triple assessment should become the mainstay as the preferred diagnostic tool.
The accountability imposed on each expert member of the diagnostic team should be specified unequivocally in the guidelines covering the complete course.
The accepted international outcome measure saying that in women with breast cancer at least 70%, preferably 90% requires a verified diagnosis preoperatively Citation[13]. A quality demand also endorsed by DBCG.
Impact on breast surgery
Surgical treatment presents a first step in the majority of patients with newly diagnosed breast cancer. Apparently, in roughly 95% of incident cases in Denmark the disease are confined loco-regionally by clinical examination at the time of diagnosis Citation[7].
Prior to DBCG, breast cancer treatment appeared quite straightforward. The initial operation, when feasible, included a simple mastectomy without axillary dissection and followed by radiotherapy targeted against the chest wall and neighbouring lymph node basins.
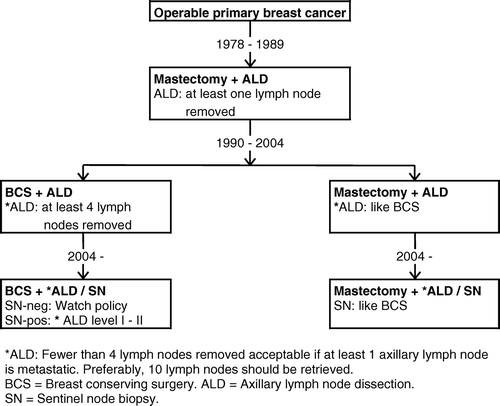
From the very beginning, DBCG tightened up the surgical requirements underlining the intent to cure principle from a loco-regional point of view. In particular, two items were emphasized. Firstly, the mastectomy should be carried out fulfilling the demands of a modified radical mastectomy including axillary dissection of level I–II Citation[14]. The requirement for the number of retrieved axillary lymph nodes became strengthened and ended up demanding removal of at least 4, but preferably 10 lymph nodes by year 1990 (). However, by 1994, DBCG addressed the surgical units and stated that at least 10 removed lymph nodes should make up the national standard. Secondly, the surgical specimen should be removed in such a way that it allowed the pathologist to measure relevant prognostic and predictive markers of importance for allocation to risk groups and choice of adjuvant strategies.
Until 1990, the recommended surgical method comprised mastectomy. Breast conserving surgery was introduced during the 1990s following satisfactory results of a randomized trial during the 1980s comparing mastectomy with breast conservation Citation[15]. The sentinel node technique for axillary staging became standard procedure by year 2004 provided the surgical unit had passed an adjudicating panel for quality assessment Citation[16].
At the start of DBCG, 77 surgical departments carried out breast surgery and participated in the project. Along with increasing requirements involving diagnostic work-up () and surgical expertise () the number of surgical departments doing breast operations fell. The smaller ones were closed down and the breast operations became centralized gathered in larger units. Provisionally, the number of recognized surgical breast units today comprises 15 departments handling at least 150 incident cases a year (). By 2002, breast surgery in Denmark is classified as a subspecialty embracing specified requirements for surgical skills, expertise, training, and education quite in accordance with international rules Citation[17–19].
Impact on non-surgical oncology
When DBCG was established 30 years ago the standard treatment was surgery followed by radiotherapy according to the McWhirter method. Since then the criteria for offering radiotherapy in primary breast cancer has varied over time as described in detail elsewhere Citation[7]. The present guidelines recommend irradiation against the residual breast following breast conserving surgery and against the chest wall and regional nodes following mastectomy in patients with node positive disease. The latter recommendation is based upon the outcome from the large DBCG 82B and 82C trials Citation[20], Citation[21] demonstrating the inferior outcome when avoiding to add radiotherapy to systemic therapy. The adjuvant systemic therapy has, over the past 30 years changed dramatically from no systemic therapy in all cases to systemic therapy in a group of patients at a certain risk of recurrence and death, a group which according to time has changed from including approximately 50 to 80% of the patients Citation[7]. The endocrine therapy has developed from tamoxifen 1 year to tamoxifen 5 years and recently to the inclusion of aromataseinhibitors and chemotherapy has developed from CMF (cyclofosfamide, methotrexate, fluorouracil) to the inclusion of anthracyclines and recently the taxanes Citation[4]. In addition we have seen since 2006 biological therapy has been included in the standard treatment programmes Citation[7]. DBCG has contributed actively to this development through the conduct of national trials and participation in international trials Citation[7].
Scientific achievements
Over the years, the most substantial gains achieved by DBCG cover a wide field of quality marks including diagnostic work-up, therapeutic attitudes and strategies, education of the health professionals, and research in the management of primary breast cancer Citation[6], Citation[7]. As concerns therapeutic landmarks, the efficacy of a variety of surgical interventions and radiotherapy is based on the outcome of randomized trials and cohort studies Citation[7]. Furthermore, DBCG initiated numerous experimental studies evaluating various combinations of chemotherapy, endocrine treatment, and biological modalities on a national and international scale Citation[7]. Besides, DBCG participates on a large scale in collaborative studies conducted by international cooperative groups Citation[7]. The issue of these efforts led to improved evidence-based treatment regimens nation-wide and, further, tightened up the attention towards breast cancer management as a whole.
Another experimental field involved analysis of prognostic and predictive factors. Especially, during recent years DBCG has been instrumental in identification of novel molecular and biological predictive markers carried out in collaboration with international groups Citation[7]. One example of such efforts affects the influence of abnormal expression of TOP2A as predictive marker for therapeutic effect of anthracycline containing regimens Citation[22]. DBCG carries the ideal prospects of performing that sort of translational research due to the nation-wide staging, clinical information kept in the national data base, and biobank access to paraffin-embedded tissue samples from roughly all patients as well as fresh frozen tissue samples from approximately 25% of incident cases with breast cancer Citation[7].
Over the years, improved surgical radicality and reliable axillary staging increased significantly survival and reduced the risk of loco-regional recurrence, especially among premenopausal high risk women Citation[23]. Further, in meta-analyses, novel policies of radiotherapy and, in particular, medical treatment demonstrate significant improvement of overall prognosis in breast cancer Citation[8], Citation[9].
Since the establishment of DBCG the prognosis in primary breast cancer has steadily improved with a 5 year survival increasing from approximately 60% to close to 80% (). The significant improvement of the prognosis seems primarily to be associated with more effective therapeutic interventions and to earlier diagnosis as described in details elsewhere in this issue Citation[24].
Concluding remarks
Highlights of DBCG achievements over 30 years are enumerated as follows:
Quality assurance of diagnostic work-up and treatment regimens. Team work has been accomplished, and all patients nation-wide are managed by equal standards as concerns diagnostics and treatment policies independent of geographic residence.
Protocolized research activities are endorsed nation-wide as well as on an international basis resulting in larger and more rapid patient recruitment.
Novel evidence-based treatment modalities are implemented immediately nation-wide and evaluated in a non-selected patient population.
DBCG activities led to an improved prognosis with 5-year survival ascending from 60% to roughly 80%.
The DBCG clinical data base allows for identification of specific indicators fit for national quality assurance projects and monitoring of quality outcome measures.
DBCG promotes translational research by coordination of clinical research with laboratory and biobank research.
DBCG has been instrumental in improving patient care and education of specialized health professionals as well as general practitioners.
Reaching the goals of DBCG has not been without obstacles. The course, however, entailed profound learning. The timely words by Daniel Andersen, a distinguished Danish surgeon and medical ethicist, unveil the dilemma quite instructively:” It takes time learning to integrate clinical practice and clinical science with due respect to the necessity of either issue or simultaneously coming to terms with their problematic reconciliation” Citation[25].
References
- Fischerman K, Mouridsen HT. Danish Breast Cancer Cooperative Group – DBCG. Ugeskr Læger 1977; 139: 2493–4
- Engeland A, Haldorsen T, Dickman PW, Hakulinen T, Möller TR, Storm HH, et al. Relative survival of cancer patients. A comparison between Denmark and the other Nordic countries. Acta Oncol 1998; 37: 49–59
- Andreasen AH, Mouridsen HT, Andersen KW, Lynge E, Mouridsen M, Olesen KP. Prognoseforbedring for brystkræft. Ugeskr Læger 1994; 156: 6512–7
- Fisher B, Carbone P, Economu SG, Frelick R, Glass A, Lemer H, et al. L-phenylalanine mustard (L-PAM) in the management of primary breast cancer. N Engl J Med 1975; 292: 117–22
- Bonadonna G, Brusamolino E, Valagussa P, Rossi A, Brugnatelli L, Brambilla C, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 1976; 294: 405–10
- Danish Breast Cancer Cooperative Group. www.dbcg.dk.
- Møller, S, Jensen, M-B, Ejlertsen, B, Bjerre, KD, Larsen, M, Hansen Hanne, B, et al. The clinical database and the treatment guidelines programmes of the Danish Breast Cancer Cooperative Group (DBCG); its 30-years experience and future promise. Acta Oncol 2008;47:506–24.
- EBCTCG, Early Breast Cancer Trialists’ Collaborative Group.Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and on 15-year survival: An overview of the randomised trials. Lancet 2005;366:2087–106.
- EBCTCG, Early Breast Cancer Trialists’ Collaborative Group.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomized trials. Lancet 2005;365:1687–717.
- Hermansen C, Poulsen SH, Jensen J, Langfeldt B, Steenskov V, Frederiksen P, et al. Diagnostic reliability of combined physical examination, mammography, and fine needle puncture (“Triple test”) in breast tumours. Cancer 1987; 60: 1866–71
- Sundhedsstyrelsens udvalg vedr.“Brystkræft: Tidlig opsporing og undersøgelse”. Sundhedsstyrelsen. 1994.
- Sundhedsstyrelsens notat vedr.”Vejledning om diagnostisk udredning af patienter med symptomer på eller hvor der er rejst mistanke om brystkræft”. ( 2. rev. udg.). Sundhedsstyrelsen, 25 oktober, 1999.
- Perry NM of behalf of the EUSOMA Working Party.Quality assurance in the diagnosis of breast disease. Eur J Cancer 2001;37:159–72.
- Cady B. Total mastectomy and partial axillary dissection. Surg Clin North Am 1973; 53: 313–8
- Blichert-Toft M, Rose C, Andersen JA, Overgaard M, Axelsson C, Andersen KW, et al. Danish randomized trial comparing breast conservation with mastectomy: Six years of life-table analysis. J Natl Cancer Inst Monogr 1992; 11: 19–25
- Christiansen, P, Friis, E, Balslev, E, Jensen, D, Møller, S. Sentinel node biopsy in breast cancer: Five years experience from Denmark. Acta Oncol 2008;47:561–68.
- Blamey, R, Cataliotti L on behalf of the EUSOMA revision committee.The requirements of a specialist breast unit. Chapter 9. In: NM Perry, M Broeders, D de Wolf, et al. European Guidelines for quality assurance in breast cancer screening and diagnosis. 4th ed. Brussels; European Commission: 2006. p 343–354.
- Blichert-Toft M, Kroman N. The specialist breast unit and breast surgery as a specialty. Scand J Surgery 2002; 91: 227–31
- Cataliotti L, Wolf de C, Holland R, Marrotti L, Perry N, Redmond K, et al. EUSOMA guidelines on the standards for the training of specialised health professionals dealing with breast cancer. Eur J Cancer 2007; 43: 660–75
- Overgaard, M, Hansen, PS, Overgaard, J, Rose, C, Andersson, M, Bach, F, for the Danish Breast Cancer Cooperative Group 82b Trial, et al.Postoperative radiotherapy in high-risk pre-menopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med 1997;337:945–55.
- Overgaard M, Jensen M-B, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999; 353: 1641–8
- Knoop AS, Knudsen H, Balslev E, Rasmussen BB, Overgaard J, Nielsen KV, et al. Retrospective analysis of topoisomerase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to Cyclophosphamide, Methotrexate, and Fluorouracil or Cyclophosphamide, Epirubicin, and Fluorouracil: Danish Breast Cancer Cooperative Group. J Clin Oncol 2005; 23: 7483–90
- Grabau DAa, Jensen M-B, Blichert-Toft M, Andersen JA, Dyreborg U, Carstensen B, et al. The importance of surgery and accurate axillary staging for survival in breast cancer. Eur J Surg Oncol 1998; 24: 499–507
- Mouridsen, HT, Bjerre, KD, Christiansen, P, Jensen, M-B, Møller, S. Improvement of prognosis in breast cancer in Denmark 1977–2006, based on the nationwide reporting to the DBCG registry. Acta Oncol 2008;47:525–36.
- Andersen D. Læge og klinisk forsker (editorial). Ugeskr Læger 1991; 153: 2269