Abstract
Background: Radiation therapy (RT) using a daily plan selection adaptive strategy can be applied to account for interfraction organ motion while limiting organ at risk dose. The aim of this study was to quantify the dosimetric consequences of daily plan selection compared with non-adaptive RT in cervical cancer.
Material and methods: Ten consecutive patients who received pelvic irradiation, planning CTs (full and empty bladder), weekly post-fraction CTs and pre-fraction CBCTs were included. Non-adaptive plans were generated based on the PTV defined using the full bladder planning CT. For the adaptive strategy, multiple PTVs were created based on both planning CTs by ITVs of the primary CTVs (i.e., GTV, cervix, corpus-uterus and upper part of the vagina) and corresponding library plans were generated. Daily CBCTs were rigidly aligned to the full bladder planning CT for plan selection. For daily plan recalculation, selected CTs based on initial similarity were deformably registered to CBCTs. Differences in daily target coverage (D98% > 95%) and in V0.5Gy, V1.5Gy, V2Gy, D50% and D2% for rectum, bladder and bowel were assessed.
Results: Non-adaptive RT showed inadequate primary CTV coverage in 17% of the daily fractions. Plan selection compensated for anatomical changes and improved primary CTV coverage significantly (p < 0.01) to 98%. Compared with non-adaptive RT, plan selection decreased the fraction dose to rectum and bowel indicated by significant (p < 0.01) improvements for daily V0.5Gy, V1.5Gy, V2Gy, D50% and D2%. However, daily plan selection significantly increased the bladder V1.5Gy, V2Gy, D50% and D2%.
Conclusions: In cervical cancer RT, a non-adaptive strategy led to inadequate target coverage for individual patients. Daily plan selection corrected for day-to-day anatomical variations and resulted in adequate target coverage in all fractions. The dose to bowel and rectum was decreased significantly when applying adaptive RT.
Introduction
Locally advanced cervical cancer patients are generally treated using external beam radiation therapy (EBRT) with concomitant chemotherapy, followed by brachytherapy [Citation1]. Radiation therapy (RT) with concurrent hyperthermia is the recommended treatment strategy for patients with a contraindication for chemotherapy [Citation2]. Intensity-modulated RT (IMRT) or volumetric modulated arc therapy (VMAT) allows highly conformal dose distributions and is most effective when combined with adequate online image guidance. However, large interfraction anatomical changes limit the efficacy of these advanced treatment techniques [Citation3]. Despite drinking instructions for cervical cancer patients, the bladder volume varies between treatment fractions and contributes to an increased risk of target underdosing [Citation3].
Adaptive RT (ART) has the potential to anticipate on anatomical changes during fractionated EBRT by adapting the radiation delivery during the treatment course based on pre-fraction imaging. Several adaptive strategies, both offline and online approaches, have been investigated [Citation4,Citation5]. The most widely reported approach for pelvic EBRT is the plan-library based plan-of-the-day strategy [Citation6–8]. Prior to treatment, a patient-specific plan library is defined by generating multiple treatment plans corresponding to different target volumes. Each treatment day the library plan best fitting the anatomy as observed on pre-fraction cone-beam CT (CBCT) imaging is selected in order to anticipate on interfraction anatomical changes.
Despite the use of large population-based margins added to the clinical target volume (CTV) to form the planning target volume (PTV), the interfraction anatomical changes in cervical cancer EBRT might still induce target underdosing. Furthermore, these large safety margins increase the dose to surrounding healthy tissues, which results in the enhancement of radiation-induced toxicity. Recently, several adaptive strategies in cervical cancer were investigated to anticipate on anatomical changes during the course of RT [Citation9,Citation10]. Next to the description of a clinically implemented adaptive strategy [Citation7], most studies reported on tools to support or automate adaptive workflows [Citation9,Citation11–13]. However, actual dosimetric improvements of ART compared with previously applied non-adaptive approaches in terms of target coverage and organ at risk (OAR) sparing are still unknown.
To investigate the dosimetric consequences of ART and determine the area of improvement, differences in dose delivery between an adaptive and non-adaptive strategy need to be assessed. Therefore, the purpose of this study was to quantify the potential dosimetric advantages of a daily adaptive plan selection treatment strategy compared with a non-adaptive treatment approach in cervical cancer RT.
Material and methods
Patients and imaging
In this retrospective study, ten consecutive cervical cancer patients who received pelvic irradiation and additional CT imaging were included. All patients, treated between January 2014 and August 2015, gave written informed consent after local medical ethical approval for additional CT imaging initially acquired for a study on adaptive proton therapy [Citation14]. Besides two planning CTs (i.e., full and empty bladder), all patients received pre-fraction CBCT imaging (Synergy platform, Elekta AB, Stockholm, Sweden) and weekly CT imaging in treatment position directly after irradiation. Weekly post-fraction CT imaging resulted in a unique set of on average 7 CTs per patient with varying anatomy. Regarding the pre-fraction imaging, five out of the 230 CBCTs were acquired with a limited field of view or limited number of projections resulting in poor image quality and were excluded from analysis. For tumor localization on CBCT, fiducial markers (2–4 per patient, Visicoil, 0.35 mm diameter, IBA Dosimetry GmbH, Schwarzenbruck, Germany) were implanted in the cervix during the pre-treatment examination under anesthesia.
Six patients were treated in the recommended prone position using a belly board device and for four patients the supine position was applied since the prone position was not possible due to comfort and stability issues. Aiming at irradiations with a full bladder, patients were instructed to empty their bladder, to drink 0.5 liter of water, and to refrain from voiding 1.5 h prior to each treatment fraction. As a result of the clinical introduction of ART at our department in April 2015, two out of the ten included patients were actually treated according to the adaptive strategy. For these two patients, the non-adaptive strategy was simulated. The other patients were clinically treated according to the non-adaptive strategy while the adaptive strategy was simulated for this analysis ().
Table 1. Patient characteristics.
Target and OAR definition
According to clinical guidelines [Citation15], the gross tumor volume (GTV), corpus-uterus, cervix, upper part of the vagina and lymph nodes were delineated on all CTs (i.e., planning CTs and weekly repeat CTs) by an experienced radiation oncologist. Also, rectum, bladder and bowel cavity, as a surrogate for small bowel, were delineated according to RTOG guidelines. The bladder wall was created using a 3 mm inwards expansion of the delineated bladder [Citation16].
The library of target structures for the adaptive strategy was created based on the planning CTs acquired with an empty and a full bladder. After bony registration of both planning CTs, corresponding primary CTVs (pCTVs) which encompassed the GTV, cervix, corpus-uterus and upper part of the vagina were registered using a structure-based deformable image registration (DIR) algorithm [Citation17]. The patient-specific full-range primary internal target volume (pITV) was divided in pITV subranges by scaling the deformation vectors (Supplementary Figure A1). According to our clinically implemented adaptive strategy, the full-range pITV was divided into one (pITV0-100), two (pITV0-50, pITV50-100) or three (pITV0-33, pITV33-67, pITV67-100) subranges when the top of corpus-uterus displacement was below 10 mm, between 10 mm and 20 mm or above 20 mm, respectively. For each pITV subrange, a primary PTV was generated by enlarging the part of the pITV including the corpus-uterus with an 8 mm isotropic margin and the part of the pITV including the cervix and vagina with a margin of 8 mm, 8 mm and 13 mm in left–right, superior–inferior and anterior–posterior direction, respectively. The extended margin in anterior–posterior direction was derived clinically and introduced to anticipate on possible large inter- and intrafraction rectum filling. The lymph nodes were enlarged with an 8 mm isotropic margin and PTVs were created by combining primary PTVs with the expanded lymph nodes.
In our non-adaptive strategy, target definition was based on only the full bladder planning CT using associated delineations. The pCTV, encompassing the GTV, cervix, corpus-uterus and upper part of the vagina was expanded with an isotropic margin of 10 mm. Also, the lymph nodes were expanded with an 8 mm isotropic margin and combined with the expanded pCTV to form the PTV.
Treatment planning
In the adaptive strategy, plans based on the defined PTVs were created to form the plan library and for the non-adaptive strategy, a single treatment plan was generated based on the corresponding PTV. Treatment plans using a dual-arc VMAT technique (356° per arc, 10 MV, 20° collimator angle) were created (Oncentra, Elekta AB, Stockholm, Sweden) with a prescribed PTV dose of 46 Gy (23 × 2 Gy). All plans were optimized on a uniform 3 mm dose grid with the beam isocenter set to the PTV center of mass using the full bladder planning CT. Plan optimizations were performed using the clinically used set of planning objectives in order to minimize OAR dose while maintaining ICRU-based PTV coverage (D98% > 95%, D2% < 107%).
Daily dose calculation
Since CBCT Hounsfield units (HU) are inaccurate, CBCT images are not directly suitable for dose calculation. To enable daily dose distribution calculation, CT HU were mapped to CBCT images by registering selected CTs to CBCT images deformably (VelocityAI, version 3.1.0, Varian Medical Systems, Inc., Palo Alto, CA) [Citation18]. For each pre-fraction CBCT image, one of the planning CTs or weekly repeat CTs best representing the daily pelvic anatomy (i.e., the CT with the highest initial anatomical similarity) was selected in order to maximize DIR accuracy. After rigid alignment based on bony anatomy, selected CTs with accurate HU were deformed to represent CBCT images. The performances of CT-to-CBCT deformable registration in the pelvic area using the VelocityAI software were validated previously and accurate DIR results were reported [Citation19,Citation20]. Additionally, an experienced observer visually assessed the DIR results to verify plausible HU modification for CBCT-based dose calculation.
Daily plan selection was simulated according to the clinical adaptive protocol. The deformed CTs representing daily anatomy were rigidly aligned with the full bladder planning CT based on bony anatomy. Next, patient-specific PTVs were projected on the daily image and the PTV encompassing the target with the implanted fiducial markers inside the PTV was selected.
The library plan corresponding to the selected PTV and the non-adaptive plan were recalculated to obtain daily adaptive and non-adaptive dose distributions, respectively.
Data analysis
Potential dosimetric advantages of ART were determined by comparing fraction dose distributions obtained using the adaptive and non-adaptive strategy. For evaluation purposes, original structures were deformed after DIR in order to match the deformed CT. Although plausible delineation deformation was obtained due to the high initial anatomical similarity between CBCTs and selected CTs combined with the reported high DIR accuracy, possible deformation inaccuracies are present in both strategies and will not affect the outcome when comparing both strategies. Supplementary Figure A2 shows a typical example of the DIR procedure including deformed delineations. Dose-volume histograms (DVHs) of daily dose distributions were calculated for the pCTV, lymph nodes, CTV, bladder, bladder wall, bowel cavity and rectum. For the target structures, the fraction dose to 98%, 50% and 2% of the volume (D98%, D50%, D2%) were calculated and differences between ICRU-based coverage (D98%>95%) were tested pairwise for significance (McNemar chi-square test). Besides the median (D50%) and near-maximum (D2%) fraction dose, the V0.5Gy, V1.5Gy and V2Gy for OARs were extracted from daily DVHs and tested pairwise for significance using a non-parametric statistical test (Wilcoxon signed-rank test).
Results
Large (>20 mm) pre-treatment displacements of the corpus-uterus top were observed in seven out of the ten patients, resulting in plan libraries consisting of three plans. For two patients, the plan library consisted of two plans and a one-plan library was generated for one patient. Compared with the average PTV volume for the non-adaptive strategy of 1601 cm3, the average volume of all generated PTVs was decreased to 1487 cm3 in the adaptive strategy.
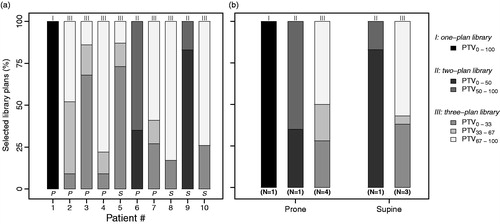
shows the selected adaptive plans per patient. For most of the patients, in the majority of fractions the selected adaptive plan corresponded to the target position related to a full bladder (PTV67-100, PTV50-100). However, despite drinking instructions, the preferred irradiation with a full bladder was not achieved for all fractions and library plans were selected corresponding to target positions related to low or intermediate bladder volumes. For patients with a three-plan library, the selection frequency of the adaptive plan with the target position related to a full bladder (PTV67-100) was on average 50% and 57% for the prone and supine treatment position, respectively ().
Figure 1. (a) Frequency of the selected library plans during the course of treatment for each patient. The number on top of each bar represents the number of available library plans and the character in the figure (P; S) represents the used treatment position (prone; supine). (b) Average percentage of selected library plans during the course of treatment for both the prone and supine treatment position. The number on top of each bar represents the number of available library plans.
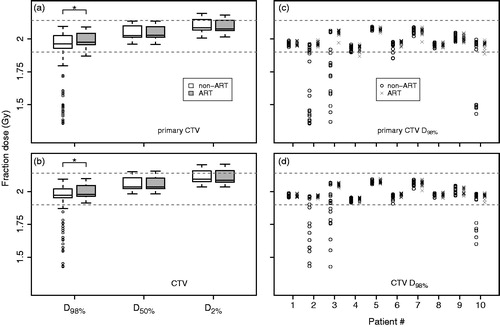
shows a typical example of fraction DVHs for the target structures of one patient and overall results are presented in . Although 24 (11%) and 38 (17%) fractions in the non-adaptive approach showed inadequate coverage (D98% < 95%) for, respectively, the CTV and pCTV, daily plan selection resulted in adequate target coverage in 225 (100%) and 220 (98%) fractions for the CTV and pCTV, respectively (). Compared with non-ART, ART significantly (p < 0.01) improved daily coverage (D98% > 95%) for both the pCTV and CTV. The overall inadequate target coverage is largely caused by three patients and thereby illustrates the potential benefit of the adaptive strategy for individual patients ().
Figure 2. For patient 3, DVHs of recalculated fraction dose distributions are shown for target volumes (CTV, pCTV) and OARs (bladder, bladder wall, rectum, bowel cavity) based on the non-adaptive (non-ART; solid lines (red)) and adaptive (ART; dotted lines (blue)) treatment strategy. The intersection of the 2 thin solid lines (black) in the DVHs for target structures indicates V95% = 98%.
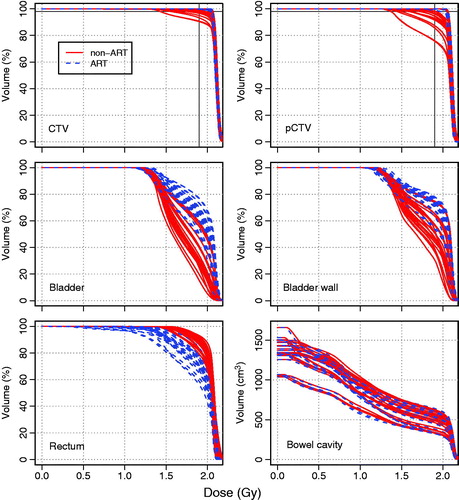
Figure 3. Overall and patient-specific results of the recalculated fraction dose distributions based on the primary CTV (upper) and the CTV (lower), with the dotted gray horizontal lines indicating 95% and 107% of the prescribed fraction dose. (a,b) The boxplots of daily dose parameters over all analyzed fractions of all included patients are shown for both the non-adaptive (non-ART) and the adaptive (ART) strategy. Boxes represent upper and lower quartiles (IQR), the band inside the box the median value and the whiskers the highest (lowest) value within 1.5 IQR of the upper (lower) quartile. Horizontal lines including an asterisk indicate statistical significant difference (p < 0.01). (c,d) Patient-specific coverage of the primary CTV and CTV (D98%) for all analyzed fractions are shown for both the non-ART and ART strategy.
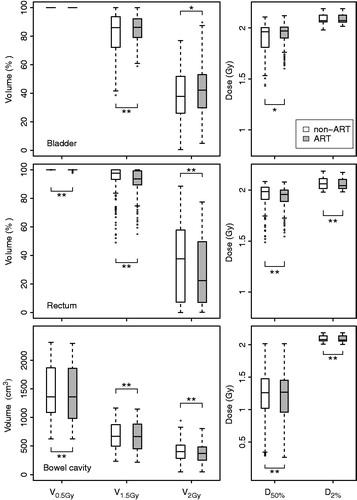
As an example, for one patient also fraction DVHs for OARs are shown (). Supplementary Figures B1–B10 show fraction DVHs of target volumes and OARs for each patient. Compared with non-adaptive RT, daily plan selection reduced the dose to rectum and bowel cavity indicated by significant improvements (p < 0.01) of all DVH parameters of interest (). However, the D50%, V1.5Gy and V2Gy for bladder were increased significantly (p < 0.05) when applying the adaptive strategy instead of the non-adaptive approach. Supplementary Table A1 presents the mean DVH parameter values for the OARs and the absolute and relative differences between non-adaptive and adaptive RT.
Figure 4. For the non-adaptive (non-ART) and adaptive (ART) strategy, boxplots of fraction DVH parameters over all analyzed fractions of all patients are shown for bladder (upper), rectum (middle) and bowel cavity (lower). For the meaning of box, whiskers and dots: see Figure 3. Horizontal lines including asterisks indicate statistical significant difference (*p < 0.05; **p < 0.01).
Discussion
In this first realistic dosimetric analysis on ART in cervical cancer, we investigated the potential advantages of a daily plan selection strategy compared with a non-adaptive approach in cervical cancer RT. A plan-library based plan-of-the-day strategy was used to adapt for day-to-day anatomical variations and dose distributions according to the adaptive as well as the conventional non-adaptive treatments were calculated using pre-fraction CBCT imaging. Compared with non-adaptive RT, a daily plan selection adaptive strategy allowed us to anticipate on anatomical changes and consequently improved fraction-based target coverage significantly. Additionally, daily plan selection reduced the dose to rectum and bowel significantly; however, the clinical relevance of the limited dose differences has to be investigated prospectively.
Previously conducted research on cervical cancer ART focused either on the quantification of inter- and intrafraction anatomical changes [Citation11], the optimization of various adaptive strategies [Citation9,Citation10,Citation21], tools to guide or automate adaptive workflows [Citation12,Citation13] or the clinical implementation [Citation7]. However, the actual benefit of ART in terms of delivered dose while taking into account day-to-day anatomical variations is essential to further improve current adaptive strategies. Our recalculated daily adaptive and non-adaptive dose distributions based on pre-fraction imaging resulted in a representative comparison in terms of differences in target coverage and OAR sparing. Therefore, this study is an important next step to optimize the presented adaptive strategy in cervical cancer RT.
The simulated adaptive strategy resulted in a significant improvement on daily target coverage (D98% > 95%) while the daily dose to rectum and bowel decreased significantly. However, the anticipation on anatomical changes by plan selection resulted in an increased dose to the adjacent bladder, indicated by the V2Gy, V1.5Gy and D50% bladder parameters. Since adaptive target volumes were created based on bladder volume variations, bladder sparing in ART was expected to be less [Citation22]. Besides considering CTV-to-PTV margin reductions to avoid the increase of bladder dose during ART, target volume definitions could be optimized based on magnetic resonance imaging (MRI) by excluding the healthy part of the corpus-uterus from the target volume [Citation23,Citation24].
There are limitations to this study. Firstly, our dosimetric analysis was performed based on a relatively small patient population of ten patients because prospectively collected data from a previous study was used [Citation14]. All included patients received additional CT imaging during the treatment course, resulting in a unique set of on average 7 CTs per patient with varying anatomy. Unfortunately, patients who received para-aortic lymph nodes irradiation were unsuited for the presented analysis. Due to the limited field of view of CBCT imaging, volumes of interest were not completely visualized on pre-fraction CBCTs. The majority of patients (i.e., eight patients) were treated using the non-adaptive approach. For these patients, we simulated adaptive treatments according to the clinically implemented daily plan selection strategy. For the two patients actually treated using the adaptive strategy, non-adaptive treatments were simulated to fairly quantify dosimetric differences compared with the adaptive strategy. Although we illustrated the benefit of ART for individual patients, based on this data it is difficult to estimate the number of patients that will actually benefit from ART. Therefore, a study including a larger number of patients is required to provide definitive information on target coverage improvements, maximum achievable OAR sparing and the percentage of patients that will benefit from ART.
Secondly, possible consequences of intrafraction anatomical changes are not represented by our recalculated dose distributions since we used pre-fraction CBCT images for both plan selection and dose recalculation. Intrafraction organ motion during cervical cancer irradiation can be considerable (e.g., passing rectal gas) and may affect dose delivery [Citation11]. However, the reported intrafraction motion is based on pre- and post-fraction CBCT imaging with a relatively large interval time of 20.8 minutes [Citation11]. Our clinical experience indicated that all operations between pre-fraction CBCT imaging and the end of dose delivery, including patient positioning, library plan selection and VMAT dose delivery, is determined to take up to at most 7 min. Consequently, the intrafraction variation present in our patient population is assumed to be smaller compared with the reported intrafraction displacements. In addition, the use of ITVs in our adaptive strategy also compensates for possible intrafraction target motion induced by intrafraction bladder filling. Hence, dosimetric uncertainties induced by intrafraction anatomical changes in cervical cancer RT are assumed to be limited.
Thirdly, daily dose distributions were calculated based on deformed CTs after CT-to-CBCT DIR for HU modification using the implemented algorithm in VelocityAI [Citation18]. The algorithm performance for CT-to-CBCT registration in the pelvic area was previously evaluated in terms of registration errors and small errors across the whole pelvic area (mean: 1.9 mm) were reported [Citation19,Citation20]. However, only the reported registration error for bladder was relatively large (mean: 4.6 mm) due to the initial large bladder volume differences in their study [Citation19]. In our study, registration errors were further minimized by selecting one of the planning CTs or weekly repeat CTs best representing the daily pelvic anatomy (i.e., the CT with the highest initial anatomical similarity). Next to the reported DIR accuracy and the high initial similarity between CBCTs and selected CTs, deformed CTs were visually inspected by an experienced observer to ensure correct HU modification for reliable dose calculation. Moreover, Onozato et al. [Citation20] evaluated the accuracy of dose calculation for pelvic anatomy after CT-to-CBCT DIR and reported average dose uncertainties of 1.2%. Furthermore, deformed CTs including possible deformation inaccuracies are used in both strategies and will not affect the outcome when comparing both strategies. The effect of residual deformation errors on our results was therefore expected to be negligible.
Besides HU modification, DIR between pre-fraction CBCTs and selected CTs with a high initial anatomical similarity was also used to deform original delineations in order to match deformed CTs. Next to the earlier mentioned justification (i.e., reported DIR accuracy, high initial similarity between images, negligible dose uncertainties induced by HU modification), deformed delineations were also visually inspected by an experienced observer to ensure plausible delineation deformation. Although the deformed delineations could include small deformation errors, these delineations were used for both the non-adaptive and the adaptive strategy. Consequently, possible small deviations are included in an identical way in both strategies and will not affect our outcome when comparing both strategies. Given the previously evaluated DIR accuracy [Citation19], the reported negligible dose uncertainties induced by HU modification [Citation20], the high initial similarity between pre-fraction CBCTs and selected CTs, delineation deformation validation by an experienced observer and the use of identical deformed delineations for both strategies, we consider our presented dose differences realistic and definitely representative for advantages of cervical cancer ART.
The presented adaptive strategy is designed to anticipate on day-to-day anatomical variations using pre-treatment predicted deformations. However, anatomical changes not represented by the library plans (e.g., changing bowel or rectum volume) can limit the efficiency of our adaptive strategy. To overcome this problem, a motion-robust backup plan with very generous margins can be added to the plan library. Heijkoop et al. [Citation7] selected such a motion-robust backup plan in 17.5% of all fractions to cover unpredicted variations, resulting in limited OAR sparing while adequate target coverage was ensured.
In this study, the potential dosimetric benefit of a daily plan selection adaptive approach was retrospectively investigated by comparing adaptive and non-adaptive fraction dose distributions. The definitive dosimetric gain of ART will be determined when comparing accumulated dose distributions; however, reliable dose accumulation requires accurate voxel-to-voxel correspondence. Despite the reported DIR accuracy for anatomical borders, the limited soft-tissue contrast in CBCT imaging prevents accurate correspondences within structures. Specific structure-based algorithms are developed to overcome this limitation [Citation17]; however, the accuracy of such algorithms for dose accumulation is unknown and first need to be derived in an independent study. Also, a prospective evaluation of ART based on a large number of patient is required to determine the actual benefit of ART in terms of tumor control and toxicity.
In future, our adaptive procedure will be optimized to reduce clinical workload and minimize OAR dose. Also, the use of online plan adaptations based on daily MRI will be implemented [Citation25]. First of all, MRI guidance can be introduced to avoid plan selection difficulties due to limited CBCT image quality or to implement additional boost techniques based on tumor response. Moreover, the clinical introduction of MRI-guided RT allows online plan adaptations and could be the next step in online ART in cervical cancer [Citation25].
In conclusion, an adaptive strategy using daily plan selection allows to correct for day-to-day anatomical variations in cervical cancer RT. Compared with the conventional non-adaptive strategy, significant improvements in target coverage were found when applying the adaptive strategy. Additionally, a significant reduction in dose to bowel and rectum was observed with a yet unclear clinical relevance.
IONC_1287949_Supplemental_material.pdf
Download PDF (2.8 MB)Acknowledgements
The authors would like to thank Dr M. Hoogeman (Erasmus MC, Rotterdam, the Netherlands) for making the Erasmus RTStudio, an application of the Erasmus MatterhornRT Software Development Platform, available.
Disclosure statement
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the manuscript.
References
- Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of Radiation Therapy Oncology Group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–880.
- Franckena M, Stalpers LJA, Koper PCM, et al. Long-term improvement in treatment outcome after radiotherapy and hyperthermia in locoregionally advanced cervix cancer: an update of the Dutch Deep Hyperthermia Trial. Int J Radiat Oncol Biol Phys. 2008;70:1176–1182.
- Jadon R, Pembroke CA, Hanna CL, et al. A systematic review of organ motion and image-guided strategies in external beam radiotherapy for cervical cancer. Clin Onco. 2014;26:185–196.
- Stewart J, Lim K, Kelly V, et al. Automated weekly replanning for intensity-modulated radiotherapy of cervix cancer. Int J Radiat Oncol Biol Phys. 2010;78:350–358.
- Vestergaard A, Søndergaard J, Petersen JB, et al. A comparison of three different adaptive strategies in image-guided radiotherapy of bladder cancer. Acta Oncol. 2010;49:1069–1076.
- Lutkenhaus LJ, Visser J, de Jong R, et al. Evaluation of delivered dose for a clinical daily adaptive plan selection strategy for bladder cancer radiotherapy. Radiother Oncol. 2015;116:51–56.
- Heijkoop ST, Langerak TR, Quint S, et al. Clinical implementation of an online adaptive plan-of-the-day protocol for nonrigid motion management in locally advanced cervical cancer IMRT. Int J Radiat Oncol Biol Phys. 2014;90:673–679.
- Meijer GJ, van der Toorn P-P, Bal M, et al. High precision bladder cancer irradiation by integrating a library planning procedure of 6 prospectively generated SIB IMRT plans with image guidance using lipiodol markers. Radiother Oncol. 2012;105:174–179.
- Bondar L, Hoogeman M, Mens JW, et al. Toward an individualized target motion management for IMRT of cervical cancer based on model-predicted cervix-uterus shape and position. Radiother Oncol. 2011;99:240–245.
- Ahmad R, Bondar L, Voet P, et al. A margin-of-the-day online adaptive intensity-modulated radiotherapy strategy for cervical cancer provides superior treatment accuracy compared to clinically recommended margins: a dosimetric evaluation. Acta Oncol. 2013;52:1430–1436.
- Heijkoop ST, Langerak TR, Quint S, et al. Quantification of intra-fraction changes during radiotherapy of cervical cancer assessed with pre- and post-fraction Cone Beam CT scans. Radiother Oncol. 2015;117:536–541.
- van de Schoot AJAJ, Schooneveldt G, Wognum S, et al. Generic method for automatic bladder segmentation on cone beam CT using a patient-specific bladder shape model. Med Phys. 2014;41:031707.
- Bondar ML, Hoogeman M, Schillemans W, et al. Intra-patient semi-automated segmentation of the cervix-uterus in CT-images for adaptive radiotherapy of cervical cancer. Phys Med Biol. 2013;58:5317–5332.
- van de Schoot AJAJ, de Boer P, Crama KF, et al. Dosimetric advantages of proton therapy compared with photon therapy using an adaptive strategy in cervical cancer. Acta Oncol. 2016;55:892–899.
- Lim K, Small W, Portelance L, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int J Radiat Oncol Biol Phys. 2011;79:348–355.
- Wright P, Muren LP, Høyer M, et al. Evaluation of adaptive radiotherapy of bladder cancer by image-based tumour control probability modelling. Acta Oncol. 2010;49:1045–1051.
- Bondar L, Hoogeman MS, Va´squez Osorio EM, et al. A symmetric nonrigid registration method to handle large organ deformations in cervical cancer patients. Med Phys. 2010;37:3760–3772.
- Veiga C, McClelland J, Moinuddin S, et al. Toward adaptive radiotherapy for head and neck patients: Feasibility study on using CT-to-CBCT deformable registration for "dose of the day" calculations. Med Phys. 2014;41:031703.
- Stanley N, Glide-Hurst C, Kim J, et al. Using patient-specific phantoms to evaluate deformable image registration algorithms for adaptive radiation therapy. J Appl Clin Med Phys. 2013;14:4363.
- Onozato Y, Kadoya N, Fujita Y, et al. Evaluation of on-board kV cone beam computed tomography based dose calculation with deformable image registration using Hounsfield unit modifications. Int J Radiat Oncol Biol Phys. 2014;89:416–423.
- Oh S, Stewart J, Moseley J, et al. Hybrid adaptive radiotherapy with on-line MRI in cervix cancer IMRT. Radiother Oncol. 2014;110:323–328.
- Bondar ML, Hoogeman MS, Mens JW, et al. Individualized nonadaptive and online-adaptive intensity-modulated radiotherapy treatment strategies for cervical cancer patients based on pretreatment acquired variable bladder filling computed tomography scans. Int J Radiat Oncol Biol Phys. 2012;83:1617–1623.
- van de Schoot AJAJ, de Boer P, Buist MR, et al. Quantification of delineation errors of the gross tumor volume on magnetic resonance imaging in uterine cervical cancer using pathology data and deformation correction. Acta Oncol. 2014;54:224–231.
- de Boer P, Adam JA, Buist MR, et al. Role of MRI in detecting involvement of the uterine internal os in uterine cervical cancer: systematic review of diagnostic test accuracy. Eur J Radiol. 2013;82:e422–e428.
- Kontaxis C, Bol GH, Lagendijk JJW, et al. A new methodology for inter- and intrafraction plan adaptation for the MR-linac. Phys Med Biol. 2015;60:7485–7497.
