Abstract
Background: Women who have been treated for cervical cancer have persistent changes in their sexual function, which result in considerable distress. The aim of this study was to investigate the morphology of the vaginal epithelium in cervical cancer survivors treated with radiotherapy and its correlation to serum levels of sex steroid hormones and sexual function.
Material and methods: We included 34 patients treated for cervical cancer with radiotherapy and 37 healthy age-matched control women scheduled for benign gynecological surgery. After inspection and grading of vaginal atrophy, vaginal biopsies were taken. Epithelial structures were analyzed by measuring epithelial thickness as well as the number, height and width of the dermal papillae and the dermal papillae distance. Sex steroid hormone levels were analyzed and a questionnaire designed to assess sexual function was filled out.
Results: In the cervical cancer survivors treated with radiotherapy, the vaginal epithelium volume was reduced compared to control women. Longer distance between the dermal papillae (p < .001) and a shorter distance from basal layer to epithelial surface (p < .05) were measured. Mucosal atrophy was observed in 91% of the survivors. There was no difference in serum estradiol between cancer survivors and control women, implying that the cancer survivors were sufficiently substituted. The epithelial thickness correlated to serum levels of estradiol. The cervical cancer survivors reported more physical sexual symptoms. The highest relative risk (RR) was found for insufficient vaginal lubrication (RR 12.6), vaginal inelasticity (RR 6.5), reduced genital swelling when sexually aroused (RR 5.9), and for reduction of vaginal length during intercourse (RR 3.9).
Conclusion: We found that cervical cancer treatment including radiotherapy is associated with vaginal epithelial atrophy and sexual dysfunction. To hamper the atrophic process affecting the sexual function, an early start of local estrogen after therapy might be of importance.
Introduction
Sexual dysfunction is one of the most distressful symptoms among cervical cancer survivors. Cancer treatment including radiotherapy results in a high degree of vaginal morbidity and persistent sexual dysfunction [Citation1]. The vaginal symptoms reported after cervical cancer treatment, with sore membranes, reduced lubrication and genital swelling severely affect the women’s sexual health [Citation2–4]. Logically, the morphological structure of the vaginal epithelium plays an important role in maintaining the sexual function after therapy, but has so far received little attention, especially concerning long-term effects after treatment.
The majority of women contracting cervical cancer are young or middle-aged, and survival rates are continuously increasing [Citation5]. A growing number of cervical cancer survivors are therefore at risk of experiencing long-term side effects that may compromise their health. The side effects may be induced by surgery, irradiation or cytotoxic chemotherapy. Morphological changes in the bladder and intestines have been reported among cervical cancer survivors after pelvic radiation [Citation6]. In a previous study, we have shown that radiation causes fibrosis in the vaginal connective tissue, which may result in inelasticity and pain during intercourse [Citation7]. We do not know, however, to what extent the radiotherapy affects the epithelium either as a result of the cessation of the estrogen production from the ovaries or by causing direct tissue damage. Little is known about the underlying normal-tissue reactions concerning radiation-induced decreased lubrication. A normal lubrication requires functional vessels as well as normal mucosal structures. Ionizing radiation may hamper these requirements.
The aim of the study was to investigate the morphology of the vaginal epithelium in cervical cancer survivors treated with radiotherapy and its correlation to serum levels of sex steroid hormones and sexual function.
Material and methods
We identified 66 cervical cancer survivors treated with radiotherapy at Radiumhemmet, Karolinska University Hospital in Stockholm, between 1 January 2004 and 31 December 2007. The inclusion criteria were, apart from a diagnosis of cervical cancer, age less than 51 years (mean age for menopause for Swedish women) and time from treatment was between two and five years. All women were treated with radiotherapy, either primary or in combination with surgery and/or chemotherapy. Exclusion criteria were recurrence of cervical cancer and significant co-morbidity. As control women, we included patients undergoing benign gynecological surgery at Danderyd Hospital, Stockholm. The control women had no history of cancer and were premenopausal, age less than 51 years. General exclusion criteria were current pregnancy, history of cancer and systemic diseases. A clinical examination was made to exclude vulvovaginal infection and/or inflammatory lesions, which also were exclusion criteria. The study was approved by the Regional Research Ethics Committee (EPN Stockholm, Dnr 2003-753) and the women gave their written informed consent.
The cervical cancer patients were treated according to regional guidelines at our institution at the time of the study. For stage IA2-IB1 and small IIA tumor, the standard treatment was radical hysterectomy with lymphadenectomy (Piver type II) with or without preoperative intracavitary brachytherapy (BT) pulse dose rate (PDR) insertions (38 Gy in two fractions). Patients with lymph node metastases or close surgical margins were typically treated with post-operative conformal chemoradiotherapy. This treatment consisted of 45 Gy (1.8 Gy/fraction) external beam radiotherapy (EBRT) to a standard pelvic field and two vaginal high dose rate (HDR) insertions BT, 5 Gy/fraction if not preoperative BT was given and five concomitant courses of cisplatin. In stage IB2 and more advanced disease, the standard treatment was definitive conformal chemoradiotherapy (45 Gy (1.8 Gy/fraction) EBRT to a pelvic field, an integrated external boost to the tumor to 50 Gy (2.0 Gy/fraction), three fractions of BT with 8 Gy, PDR BT, to point A, and six concomitant courses of cisplatin). In the case of co-morbidity or low risk of disseminated spread (no lymph node metastases, no lymphovascular space invasion), chemotherapy was excluded according to regional guidelines.
Clinical assessment
Thirty-four patients agreed to take part in the study and were planned for a research visit. One gynecological oncologist (AH) did the gynecological examinations. During the inspection, the vaginal atrophy and the existence and quantification of telangiectasia were assessed. The vaginal atrophy was graded, modified from Common Terminology Criteria for Adverse Events v3.0 (CTCAE) [Citation8], as absent (normal mucosa), mild (slightly pale mucosa, reduced folding), moderate (moderately pale, reduced folding) or severe (pronounced pallor, absent folding). Vaginal length was measured in cm by a vaginal measuring cylinder. The women in the control group were examined according to the protocol by one gynecologist (NBS). Before the study commenced, the two examiners together checked the procedures to make sure that the biopsy sampling, the clinical examination and the use of the vaginal measuring cylinder were carried out in the same way in the cancer survivors and the control women.
Sampling and tissue preparation
Vaginal biopsies were taken after a five-minute application of lidocaine/prilocaine cream (EMLA®, AstraZeneca, Sweden). Two biopsies were taken from each woman, with a 3 mm forceps at the three and nine o’clock position, 3–4 cm from the vaginal introitus. The biopsies were placed in neutral buffered 4% formaldehyde solution for 6–12 h. Dehydration, fixation and paraffin embedding were performed according to a standard protocol. Sections were cut perpendicularly to the surface, with a nominal thickness of 5 µm. When preparing and sectioning the biopsies effort was made to obtain vertical sections with full-thickness epithelium. From each tissue block nine sections in three series were obtained. Between each series the four subsequent sections were discarded. The nine sections from each tissue block were mounted on slides and stained. The first section from the first, second and third series were stained with hematoxylin and eosin, and the other sections were used for immunohistochemical analyses. All biopsies and sections were handed and analyzed in a blinded manner throughout all analyses (HJ).
Blood samples were taken and serum estradiol, progesterone, testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and sex hormone-binding globulin (SHBG) were determined by direct chemiluminescent immunometric assay (Immulite; Siemens, Munich, Germany).
Epithelial measurement
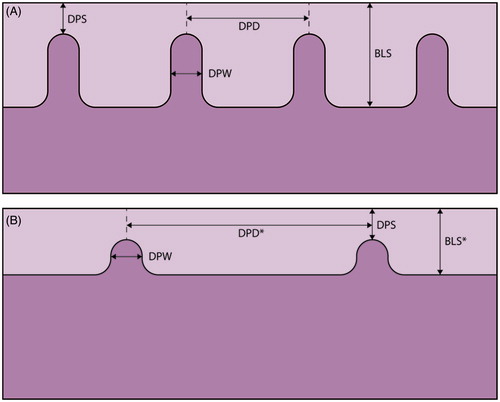
Squamous epithelium consists of various layers of keratinocytes and dermal papilla depending on body location. A computer-assisted method was used to analyze the epithelial structures. The epithelial thickness as well as the number, height and width of the dermal papillae and the dermal papillae distance were measured in µm (). The specimens were placed in the microscope (Axioplan 2, Carl Zeiss) under a 5 × objective and an image of the specimen was captured in 8-bit RGB-mode into the computer (Sun SparcStation 20, Sun Microsystems Computer Corporation) equipped with Micro-GOP 2000s (Context Vision) image analysis software. By manually setting the threshold on the basal layer and thereafter removing larger subepithelial objects, the epithelial profile is outlined and measurement operations begin with the above-described parameters [Citation9]. Every specimen was measured from the left side to the right with five equally spaced intervals of 411 µm each.
Figure 1. A schematic illustration of the vaginal wall in healthy women (A), and cervical cancer survivors with thin epithelia and sparse dermal papillae (B). Parameters measured in each specimen; DPS: distance from dermal papilla top to epithelial surface; DPD: interdermal papilla distance; DPW: dermal papilla width; BLS: distance from basal layer to epithelial surface and the number of the dermal papillae. *p value < .05
To analyze the presence of blood vessels, sections were immunohistochemically stained with antibodies against coagulation factor VIII von Willebrand, present in endothelial cells [Citation10]. The morphometrical analyses of blood vessels were made, by measuring the surface area per unit volume (mm2/mm3), as described elsewhere [Citation11,Citation12]. The specimens were also stained with antibodies against protein gene product 9.5 (PGP 9.5), for the detection of nerve fibers [Citation13]. The volume fraction of immunohistochemically stained nerves was estimated in percent of the volume of examined tissue [Citation14].
Radiation dose at biopsy site
For calculating the radiation dose at biopsy site, we collected the prescribed field margins, target definition, set-up images, portal image films, treatment protocol and dose per fraction, and the total prescribed dose for each patient. On portal image films, the vaginal introitus was identified. The distance from the lower border of the EBRT field was measured, and the EBRT dose at the biopsy site was calculated. For the BT, we used orthogonal x-ray images and made an estimation of the delivered dose based on isodose-curves from the actual treatment for each patient. We calculated the EQD2-doses for both the EBRT and the BT, and thus obtaining the total dose at the biopsy site. In order to correlate radiotherapy-induced histological findings, the radiation dose at the biopsy site was calculated in two different treatment groups; (I) pre-operative BT, (II) pre-operative BT + EBRT or primary chemoradiotherapy.
Questionnaire
The questionnaire was developed from a study-specific questionnaire, described and used by Bergmark et al, 1999 [Citation2]. The original questionnaire was based on in-depth interviews with cervical cancer survivors and face-to-face validated. In our questionnaire, we included 49 questions, concerning demographic data, assessment of sexual dysfunction, information on quality of life and psychological health. Thirty-nine questions were specifically related to sexuality, such as sexual interest, vaginal lubrication and genital swelling, vaginal length reduction and inelasticity, superficial and deep dyspareunia and frequency of intercourse and orgasm.
Statistical methods
The study is exploratory and the analysis of data from the survivors and controls were performed using Student’s t-test or Mann–Whitney U-test wherever appropriate. Pearson correlation coefficient R was used to test the correlation between hormonal levels and epithelial measurements variables. The responses to the questionnaire were dichotomized, and the results were presented as relative risks (95% confidence intervals), calculated as a proportion of the cancer survivors reported a particular problem divided by the proportion of the controls. Spearman’s rank correlation test was used for correlating vaginal atrophy and the responses of the questionnaires to the epithelial measurements. Significance level was set at p < .05 and non-significant values were marked (NS).
Results
Thirty-four out of 66 cancer survivors and 37 control women met the inclusion criteria and agreed to participate in the study. The majority who did not want to take part in the study, declined due to psychological reasons. Age, stage, and treatment did not differ significantly between participants and non-participants among the cancer survivors. There was no difference between the survivors and controls in mean age and the proportion of women having a partner (). The surgery performed was radical hysterectomy and pelvic lymph node extirpation (Piver type II), except for one patient with a history of occult cervical cancer. Regarding radiotherapy, five patients were treated with preoperative BT only and received minimal radiation dose at biopsy site in the distal part of the vagina, median 0.0 Gy (range 0–9.0). The other 29 patients who were treated with EBRT and BT received median 44.8 Gy (range 38.8–69) at the biopsy site. Two thirds of the patients received concomitant cisplatin. FIGO tumor stage and cancer treatment are shown in . The most common benign operations among the control women were abdominal surgery for myoma (hysterectomy or enucleation), hysteroscopy, procedures for contraception (sterilization or IUD) and conisation for cervical dysplasia. Endometriosis was diagnosed in four controls during laparoscopy.
Table 1. Demographics and clinical characteristics of cervical cancer survivors and control women.
Thirty-eight percent of the cervical cancer survivors used local estrogen therapy, where 8/13 had started the treatment after the symptoms of atrophy had occurred. Systemic hormonal therapy was more common, being used by 79% of the patients. Treatment with estradiol alone or combined with gestagen was depending on whether the cancer treatment included hysterectomy or not. Women treated with primary radiotherapy were substituted with combined systemic hormonal therapy. Among the control women 38% had hormonal contraceptives, most often gestagenic methods ().
Table 2. Hormone therapy, hormonal status and clinical vaginal characteristics of cervical cancer survivors and control women.
Clinical findings
The inspection of the vagina showed that 91% of the cancer survivors had signs of atrophy with pale and/or flat mucosa to various degrees. The atrophy was mostly graded as mild or moderate (). The survivors had a shorter vagina than the controls when measured at examination and telangiectasia were present in 82% of the survivors (). No women in the control group had visible or palpable vaginal changes at examination. The biopsy taking was well tolerated with almost no bleeding among patients, whereas in the control group a stitch was needed in a few cases for hemostasis. No other complications were observed.
Epithelial measurement
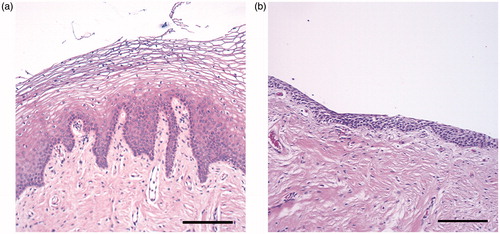
During the initial examination of the biopsies, we noticed marked differences in the composition of the tissue between cervical cancer survivors and control women. The survivors had a thinner epithelium and a dense connective tissue (). In the computer-assisted epithelial measurement, we found that the number of the dermal papillae was lower in the biopsies from the survivors, median 3.3 (IQ range 1.0–4.3), compared to 4.0 (IQ range 3.0–4.8), p < .05. The distance between the dermal papillae (DPD) was significantly longer in the biopsies from the cervical cancer survivors, median 381 µm (IQ range 293–1002) compared to 279 µm (IQ range 239–337) in the control women, p < .001. Vaginal biopsies with no dermal papillae were found in 19% of the cervical cancer survivors, compared to none in the control women. In the survivors, there was also a significantly shorter distance from basal layer to epithelial surface (BLS), median 339 µm (IQ range 220–419) compared to 382 µm (IQ range 317–464) in the control women, p < .05. There were no differences in the distance from dermal papilla top to epithelial surface (DPS) or dermal papilla width (DPW) between survivors and control women. shows schematic illustrations of the results. No differences in any of the epithelial measurements were found between patients with minimal radiation dose at the biopsy site (preoperatively BT only), compared to those who had received a high radiation dose (EBRT + BT).
Figure 2. Hematoxylin–eosin stained biopsies from the vaginal wall in healthy women with thick squamous cell epithelia with dermal papillae (A), and cervical cancer survivors with thin epithelia and dense connective tissue (B). Scale bar =200 μm.
The mean surface area fraction of blood vessels per tissue volume among the survivors was 106.2 mm2/mm3 (SD 26.6), while for the control women it was 101.6 mm2/mm3 (SD 25.6), NS. The volume fraction of nerves was low both among the survivors and the control women and we did not observe any differences. In 75% of both the survivors and control women, the nerve tissue constituted a maximum of 1% of the tissue volume (median 0%). The remaining 25% had a maximum of 4% nerve tissue.
Hormonal and correlation analyses
The results of the serum hormonal levels are presented in . There was no difference in serum estradiol between cancer survivors and control women, which implies that the survivors were sufficiently substituted. As expected, the serum progesterone level was significantly lower among survivors, due to ovarian insufficiency with no ovulations. The serum levels of FSH showed menopausal values in the cancer survivors and normal values in the control women. The levels of LH and SHBG were higher in patients, whereas there was no difference in levels of free testosterone ().
Table 3. Steroid hormone levels of cervical cancer survivors and control women.
In our correlation analyses, including both cancer survivors and control women, we saw a positive correlation between estradiol and the epithelial variables DPW (R .296, p = .015), DPS (R .308, p = .011) and BLS (R .304, p = .012), but not for DPD (R −.107, p > .05). This result means that the level of estradiol correlates to the thickness and volume of the epithelium. For progesterone, there was a positive correlation with DPW (R .287, p = .018), but not for the other epithelial variables. For the clinical finding of vaginal atrophy, there was a positive correlation to DPD (rs .385, p = .001) and a negative correlation to the number of dermal papillae (rs −.276, p < .05).
Epithelial measurements in patients using local estrogen therapy showed lower DPS median 154.3 µm, compared to patients without local substitution median 236.3 µm, p = .039. No differences were observed in epithelial measurement in patients with or without systemic estradiol.
Questionnaire
It was more common among the cancer survivors to report physical vaginal symptoms such as reduced vaginal lubrication and genital swelling, reduction of vaginal length and elasticity during intercourse. However, there were no differences between the groups in superficial and deep dyspareunia. The frequency of vaginal intercourse was the same, but it was much more common to regularly use lubricants among the cancer survivors who also reported significantly lower frequency of orgasm. The control women felt more sexually attractive compared to the cancer survivors, who also felt less satisfaction with their present sexuality. There were no differences in interest in sex and sexual thoughts, but it was less common among the cancer survivors to feel sexual desire at least once a week compared to control women. We found no differences between the cancer survivors and the control women in physical and psychological wellbeing and level of anxiety and depression (). In the correlation analyses, including both cancer survivors and control women, we saw a negative correlation between reported reduction of vaginal length during intercourse and the epithelial variables DPS, rs −.275 (p = .027) and number of papillae, rs −.257 (p = .038), but not for any other reported symptoms of sexual dysfunction.
Table 4. Sexual function, well-being, depression and anxiety in the cervical cancer survivors and the control women.
Discussion
Our study presents data on long-term cervical cancer survivors, an increasing group of women who will live for many years with vaginal symptoms affecting their sexual health and quality of life. We found morphological changes with reduced vaginal epithelial volume in cervical cancer survivors treated with radiotherapy. The survivors had thinner epithelium and sparse dermal papillae, the pathophysiological picture of atrophy, which was also a predominant clinical finding. The epithelial changes correlated partly to the reported sexual physical complaints among the survivors.
Vaginal atrophy was found in the morphological epithelial measurements and also during the clinical examination. Similar findings, using vaginoscopy, have previously been reported in cervical cancer survivors 3–24 months after radiotherapy by Kirchheiner et al. [Citation15]. The atrophic changes are considered to evolve overtime where hypo-vascularization and hypoxia are thought to play a major role [Citation16]. The immediate response to irradiation is loss of the whole vaginal epithelium, i.e., radiation vaginitis, which occurs within the first weeks after therapy [Citation3,Citation17]. The long-term effects vary in individuals but are often established after approximately two years when re-epithelialization of the basal layer and partial maturation of the epithelial cell is completed [Citation3]. Our patients were all examined more than two years after completed radiotherapy and their persistent vaginal symptoms must therefore be regarded as chronic.
The treatment of advanced cervical cancer with radiotherapy results in low serum levels of estradiol by destruction of the oocytes and complete cessation of the estrogen production. In our study, there was a positive correlation between serum levels of estradiol and the vaginal epithelial thickness. All the cancer survivors were sufficiently substituted with systemic hormonal therapy, but even so, 91% of the survivors showed signs of vaginal atrophy, indicating that the epithelial response to serum estradiol is impaired. These results support previous suggestions that radiotherapy make the vagina less responsive to systemic and topical estrogen [Citation18]. The interplay of the serum hormone levels and expression of steroid hormone receptors in the vaginal wall is important to maintain a functional mucosa. Our previous study has shown fibrosis in the vaginal wall after radiation [Citation7], which may hamper the systemic estradiol to reach the epithelial receptors. In that aspect, local estrogen therapy might play a larger role than previously thought, especially the timing when the treatment should start. In our study only 38% used local estrogen and the majority of these women had started the therapy after symptoms of atrophy had occurred. We found thinner epithelium in the group of patients that were treated with local estrogen. One might speculate if local estrogen therapy should start directly after the radiotherapy is completed and before the late effects on the vaginal mucosa have been established.
All the cancer survivors in the study were treated with radiotherapy. We found no differences in epithelial changes in the survivors with high radiation dose at biopsy site compared to low dose. This finding is in contrast to our previous study showing a dose-dependent increase of fibrosis in the connective tissue, indicating that the changes in the connective tissue are a direct effect of the radiation. The result of this study indicates that the late side effect of atrophy seems to be secondary to radiation with the epithelium being less responsive to both systemic and local estrogen therapy. Our results must be interpreted with caution due to the small size of the study with only five patients treated with pre-operative BT only. However, the findings give rise to some interesting issues that might be of therapeutic importance. With fibrotic and vascular changes of the connective tissue, it might be difficult for the systemic estradiol to reach the epithelium. In addition, effective local estrogen therapy is dependent of present estrogen receptors in the mucosa. In a study surveying the estrogen receptor (ER) α expression in breast cancer cells after radiation, there was a reduction in mRNA level and protein synthesis 72 h post-radiation at 8 Gy [Citation19]. It is unknown whether this change in ERα expression is permanent and if it also occurs after radiation in other tissues.
Normal vessel function and vessel innervations are essential for a functional mucosa. Lack of lubrication could possibly be due to fibrotic changes affecting the vessel function in the connective tissue, which hinders fluid reaching the surface. We found no differences in the surface area fraction of blood vessels and nerve fibers in the superficial part of the vaginal wall between survivors and controls. In the biopsies, only the superficial part of the vaginal wall was represented omitting analyses of possible changes of deeper located blood vessel and nerve fibers regulating the microcirculation of the tissue. When taken the biopsies, the morphological changes of the mucosa were, however, further demonstrated with almost no bleeding in the survivors compared to controls. To study the physiological reaction on vasoconstriction in the vaginal wall at arousal, photoplethysmography could be a preferable method [Citation20].
The pathophysiology of sexual dysfunction in cervical cancer survivors is complex and multi-factorial. In the questionnaire the cervical cancer survivors reported more physical vaginal symptoms, with reduced vaginal lubrication and genital swelling, which partly can be caused by the decreased epithelial volume. They also reported reduction in length of the vagina and reduction in elasticity during intercourse, changes that most likely are due to the radiotherapy-induced vaginal fibrosis in the connective tissue previously reported by our group [Citation7]. Radical hysterectomy can also cause fibrosis and shortening of the vagina [Citation21]. Dyspareunia, both superficial and deep, is one of the most disturbing sequelae after treatment of cervical cancer [Citation2,Citation22]. In our results there were no differences between the two study groups regarding dyspareunia. One explanation might be the small size of the study. We must also take into account that the control group was possibly not optimal regarding analyzing sexual function. The differences in sexual function would probably have been larger using a control group of women without gynecological diseases and in need of gynecological surgery.
The strength of this study is the various methods that have been used. By combining clinical examination, epithelial measurements, hormonal analyses and the psycho-sexual questionnaire, more detailed information of the consequences of radiotherapy in cervical cancer treatment is obtained. The sample size is, however, a limitation together with the heterogeneous material concerning treatment. When discussing the results one should consider that the patients were treated with traditional CT-based EBRT and brachytherapy, before more modern radiotherapy modalities like intensity modulated radiation therapy (IMRT) and MRI-based image-guided adaptive brachytherapy (IGABT) were introduced at our institution. Our biopsies were taken from the distal part of the vagina where the radiation dose is similar with modern radiotherapy, in contrast to the upper part of the vagina where the doses are likely to be even higher with IMRT and IGABT. Analysis from the EMBRACE Study [Citation23] showed that severe vaginal morbidity after IGABT (grade 3-4, i.e., fistulas and total vaginal stenosis) was significantly less compared to data from studies using earlier technique. However, mild to moderate vaginal morbidity (grade 1-2) was still pronounced after IGABT [Citation24]. All the survivors were treated with radiotherapy with a definitive dose to the ovaries, which results in destruction of the oocytes and complete cessation of the estrogen production. More than half of the survivors also had chemotherapy. Previous studies have shown more severe late toxicities following concomitant chemoradiotherapy, compared with radiotherapy alone. However, this effect was only seen in postmenopausal women [Citation25].
As the main result of this study, we found pronounced atrophic vaginal epithelium in the cervical cancer survivors. The epithelial volume correlated to serum levels of estradiol rather than radiation dose. The results indicate that the vaginal epithelium is less responsive to systemic estradiol after radiation, at least in the chronic state. This information is relevant concerning the treatment and care of long-term survivors. To hamper the atrophic process affecting sexual function, an early start of local estrogen after therapy might be of importance.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Jensen PT, Froeding LP. Pelvic radiotherapy and sexual function in women. Transl Androl Urol. 2015;4:186–205.
- Bergmark K, Avall-Lundqvist E, Dickman PW, et al. Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med. 1999;340:1383–1389.
- Grigsby PW, Russell A, Bruner D, et al. Late injury of cancer therapy on the female reproductive tract. Int J Radiat Oncol Biol Phys. 1995;31:1281–1299.
- Huffman LB, Hartenbach EM, Carter J, et al. Maintaining sexual health throughout gynecologic cancer survivorship: a comprehensive review and clinical guide. Gynecol Oncol. 2016;140:359–368.
- Vale CTJ, Stewart LA. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–5812.
- Andreyev HJ, Wotherspoon A, Denham JW, et al. “Pelvic radiation disease”: new understanding and new solutions for a new disease in the era of cancer survivorship. Scand J Gastroenterol. 2011;46:389–397.
- Hofsjo A, Bohm-Starke N, Blomgren B, et al. Radiotherapy-induced vaginal fibrosis in cervical cancer survivors. Acta Oncol. 2017;1–9.
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181.
- Blomgren B, Johannesson U, Bohm-Starke N, et al. A computerised, unbiased method for epithelial measurement. Micron. 2004;35:319–329.
- Turesson I. Individual variation and dose dependency in the progression rate of skin telangiectasia. Int J Radiat Oncol Biol Phys. 1990;19:1569–1574.
- Blomgren B, Bohm-Starke N, Falconer C, et al. A computerised stereological method for quantitative estimation of surface area of blood vessels. Image Anal Stereol. 2001;20:129–132.
- Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–276.
- Campbell LK, Thomas JR, Lamps LW, et al. Protein gene product 9.5 (PGP 9.5) is not a specific marker of neural and nerve sheath tumors: an immunohistochemical study of 95 mesenchymal neoplasms. Modern Pathol. 2003;16:963–969.
- Gundersen HJG, Boysen M, Reith A. Comparison of semiautomatic digitizer-tablet and simple point counting performance in morphometry. Virchows Archiv B Cell Pathol. 1981;37:317–325.
- Kirchheiner K, Fidarova E, Nout RA, et al. Radiation-induced morphological changes in the vagina. Strahlenther Onkol. 2012;188:1010–1017.
- Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–713.
- Pitkin RM, Bradbury JT. The effect of topical estogen on irradiated vaginal epithelium. Am J Obstet Gynecol. 1965;92:175–182.
- Abitbol MM, Davenport JH. The irradiated vagina. Obstet Gynecol. 1974;44:249–256.
- Toillon RA, Magne N, Laios I, et al. Interaction between estrogen receptor alpha, ionizing radiation and (anti-) estrogens in breast cancer cells. Breast Cancer Res Treat. 2005;93:207–215.
- Laan E, Everaerd W, Evers A. Assessment of female sexual arousal: response specificity and construct validity. Psychophysiology. 1995;32:476–485.
- Lammerink EA, de Bock GH, Pras E, et al. Sexual functioning of cervical cancer survivors: a review with a female perspective. Maturitas. 2012;72:296–304.
- Jensen PT, Groenvold M, Klee MC, et al. Longitudinal study of sexual function and vaginal changes after radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2003;56:937–949.
- Nkiwane KS, Potter R, Tanderup K, et al. Single line source with and without vaginal loading and the impact on target coverage and organ at risk doses for cervix cancer Stages IB, II, and IIIB: treatment planning simulation in patients treated with MRI-guided adaptive brachytherapy in a multicentre study (EMBRACE). Brachytherapy. 2013;12:317–323.
- Kirchheiner K, Nout RA, Tanderup K, et al. Manifestation pattern of early-late vaginal morbidity after definitive radiation (chemo)therapy and image-guided adaptive brachytherapy for locally advanced cervical cancer: an analysis from the EMRACE study. Int J Radiat Oncol Biol Phys. 2014;89:88–95.
- Gondi V, Bentzen SM, Sklenar KL, et al. Severe late toxicities following concomitant chemoradiotherapy compared to radiotherapy alone in cervical cancer: an inter-era analysis. Int J Radiat Oncol Biol Phys. 2012;84:973–982.
