Abstract
Background
With increasing interest in organ-preserving strategies for potentially curable esophageal cancer, real-world data is needed to understand the impact of pathological tumor response after neoadjuvant chemoradiotherapy (CRT) on patient outcome. The objective of this study is to assess the association between pathological tumor response following CROSS neoadjuvant CRT and long-term overall survival (OS) in a nationwide cohort.
Material and methods
All patients diagnosed in the Netherlands with potentially curable esophageal cancer between 2009 and 2017, and treated with neoadjuvant CRT followed by esophagectomy were included. Through record linkage with the nationwide Dutch Pathology Registry (PALGA), pathological data were obtained. The primary outcome was pathological tumor response based on ypTNM, classified into pathological complete response (ypT0N0) and incomplete responders (ypT0N+, ypT+N0, and ypT+N+). Multivariable logistic and Cox regression models were used to identify predictors of pathological complete response (pCR) and survival.
Results
A total of 4946 patients were included. Overall, 24% achieved pCR, with 19% in adenocarcinoma and 42% in squamous cell carcinoma. Patients with pCR had a better estimated 5-year OS compared to incomplete responders (62% vs. 38%, p< .001). Of the patients with incomplete response, ypT+N+ patients (32% of total population) had the lowest estimated 5-year OS rate, followed by ypT0N+ and ypT+ N0 (22%, 47%, and 49%, respectively, p< .001). Adenocarcinoma, well to moderate differentiation, cT3-4, cN+, signet ring cell differentiation and lymph node yield (≥15) were associated with lower likelihood of pCR.
Conclusion
In this population-based study, pathological tumor response based on the ypTNM-stage was associated with different prognostic subgroups. A quarter of patients achieved ypT0N0 with favorable long-term survival, while one-third had an ypT+N+ response with very poor survival. The association between pathological tumor response and long-term survival could help in more accurate assessments of individual prognosis and treatment decisions.
Introduction
Esophageal cancer accounts for over half a million new cases worldwide (572,000/year) and ranks sixth in cancer-related mortality rates [Citation1, Citation2]. In contrast to esophageal squamous cell carcinoma (ESCC), the incidence of esophageal adenocarcinoma (EAC) is estimated to rise in high-income countries with a sustained increase in the next years [Citation1,Citation2]. During the past decade, neoadjuvant chemoradiotherapy (CRT) has been successfully introduced in the treatment of potentially curable esophageal cancer [Citation3,Citation4]. Before that, high rates of locoregional and distant metastases were seen after primary surgical resection with 5-year overall survival (OS) rates varying between 20 and 30% [Citation5–7].
The almost 10-years ago published multicenter randomized controlled CROSS-trial comparing neoadjuvant CRT combined with esophagectomy to surgery alone showed a significant improvement in OS (median 49 vs. 24 months) [Citation6,Citation8]. Pathological complete response (pCR) was noted in 49% of patients with ESCC and in 23% with EAC [Citation6], which was associated with improved survival outcomes [Citation5,Citation9–11].
The increasing use of neoadjuvant CRT for potentially curable esophageal cancer has raised further discussions regarding the evaluation and classification of tumor response in relation to treatment optimization and organ-sparing approaches. In this regard, evidence of the long-term prognostic impact of different pathological tumor responses is highly relevant but requires further investigation. Previous multicenter and population-based studies were to some extent limited by insufficient data on the type of the neoadjuvant CRT regimen [Citation12–16], or by a relatively small sample size [Citation10,Citation17–19]. The aim of this population-based study was therefore to assess the association between pCR after neoadjuvant CRT and long-term survival in a large nationwide cohort of patients treated with the CROSS-regimen, and to identify clinicopathological predictors of pCR.
Material and methods
Study design and data collection
Data were obtained from the Netherlands Cancer Registry (NCR). This nationwide registry contains clinicopathological data on all newly diagnosed malignancies in The Netherlands. Specially trained data managers of the NCR routinely extract information on diagnosis, tumor stage and treatment directly from medical records. Tumor topography and morphology are coded according to the International Classification of Diseases for Oncology (ICD-O). Information on vital status is obtained through annual linkage with the Municipal Personal Records Database and was at the time of performing this study completed until January 2019. Additional or missing pathological data needed to determine pathological tumor response were obtained through record linkage with the nationwide network and registry of histo- and cytopathology in the Netherlands (PALGA). All data were handled confidentially and in accordance to privacy regulations for medical research.
Study population
All patients with potentially curable cancer (cT1b-4N0-3M0) of the esophagus or cardia (C15.0-C16.0) diagnosed in the period 2009–2017 and who were treated with trimodality treatment were identified in the NCR. Only patients with adenocarcinoma, squamous cell carcinoma, or large-cell undifferentiated carcinoma were selected. If pathological tumor response could not be determined based on PALGA records, patients were excluded from analysis (N = 6). Pretreatment clinical staging in the Netherlands typically consists of endoscopy with diagnostic biopsies, computer tomography (CT) of the neck, thorax, and abdomen or integrated positron emission tomography-CT (PET-CT). When indicated and available endoscopic ultrasound and ultrasound of the neck were performed. For uniformity, TNM-staging was recorded according to the 7th edition of the Union for International Cancer Control. For patients diagnosed during 2015–2017 more detailed information on administered chemotherapy and radiotherapy was available.
Treatment
According to the Dutch guideline (v3.0, 2010, update 2014), trimodality treatment consisting of neoadjuvant CRT combined with esophagectomy is currently standard of care for all patients with potentially curable esophageal cancer. Neoadjuvant CRT based on the CROSS-trial, i.e., five cycles of carboplatin and paclitaxel with concurrent radiotherapy (41.4 Gy in 23 fractions), is the recommended regimen.
Definitions
The absence of viable residual tumor cells at the primary tumor site and in resected lymph nodes was defined as ypT0N0. Any extent of residual invasive tumor at the primary site (T1–4) was classified as ypT+ and as ypN+ if lymph nodes were involved (ypN1–3). Pathological tumor response in the surgical resection specimen was categorized according to ypTNM stage: pCR (ypT0N0) and incomplete responders (ypT0N+, ypT+N0, and ypT+N+). Lymph node yield was grouped as <15 and ≥15 resected nodes according to the recommendations of the Dutch Institute for Clinical Auditing. Presence of signet ring cell (SRC) differentiation was scored independently in pretreatment biopsies and the resection specimen.
Statistical analyses
Baseline characteristics were assessed overall and according to pathologic tumor response. Survival time was defined as time from surgical resection to death or end of follow-up. The OS was calculated with the Kaplan–Meier method and compared with log-rank test. Logistic regression and Cox models were performed according to histology type to identify clinicopathological variables associated with the likelihood of pCR and survival. For multivariable regression analyses, first covariates with a p value <.2 in the univariable analyses were preselected. Then, backward elimination was performed by manually omitting nonsignificant covariates one-by-one until all variables remaining in the model had a p value <.05. The predictors in the model were also validated by an automated backward selection based on log-likelihood ratio tests, which resulted in comparable estimates. Comparison of the cases with missing values to the complete cases with regard to baseline characteristics did not result in additional covariates to be adjusted for in the regression analyses. Additionally, subgroup analyses were performed in patients from the years 2015–2017, for whom more detailed clinical variables, such as body weight, tumor length, and length of the neoadjuvant CRT scheme, were available. Odds ratios (ORs) and hazard ratios (HRs) are presented with 95% confidence interval (CI). Statistical analysis was performed using SPSS Statistics v25.0 (IBM Corp., Armonk, NY, USA).
Results
Study population
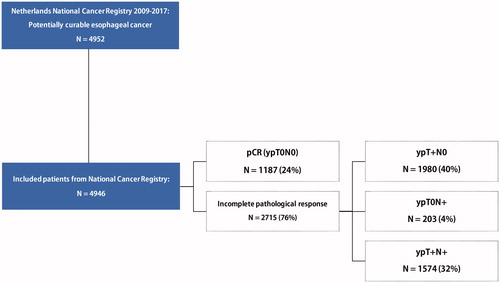
A total of 4946 patients diagnosed with potentially curable carcinoma of the esophagus or cardia was included (). The number of centers performing surgery declined from 26 in 2010 to 18 in 2017 due to centralization. Since 2015, at least 94% of included patients were registered to have been treated with the CROSS-regimen.
Baseline characteristics
Mean age was 64 years ± SD 8.7 (). Overall, the majority of patients were men (78%), diagnosed with cT2-3 (93%), EAC (79%) of the lower third of the esophagus (76%). Clinical suspicion of lymph node metastasis (cN+) was present in 60% of the cases. Transthoracic esophagectomy was the most frequently performed surgical approach (66%), increasing from 52% in 2009 to 87% in 2017. The R0-resection rate was 95.5% with a median lymph node yield of 18 (IQR: 13–24).
Table 1. Baseline characteristics of study population according to pathological tumor response.
Pathological outcome
A total of 1187 (24%) patients had pCR (ypT0N0) following neoadjuvant CRT, with 19% in EAC and 42% in ESCC. With the increasing incidence of EAC over the years, the overall pCR rate declined from 29% in 2010 to 23% in 2017. Most patients had incomplete pathological responses, 40% ypT+N0, 32% ypT+N+, and 4% ypT0N+ (). Overall, ypT0 and ypN0 were seen in 28% and 64% of all cases. Of the cN0-staged tumors at base line, 24% were eventually staged as ypN+.
In multivariable logistic regression analyses, histology type, differentiation grade, cT-category, and cN-category were associated with the likelihood of pCR (; Supplementary Table 1). In subgroup analysis, presence of a SRC component and lymph node yield (≥15 vs. <15) were associated with pCR in EAC. If an SRC component was present in the pretreatment biopsies or the resection specimen (16% of EAC), the pCR rate was 13% compared to 26% in patients without an SRC component (p < .001).
Table 2. Factors associated with pCR in multivariable logistic regression analysis.
Cohort 2015–2017
The distribution of the pathological response was comparable with the overall cohort (23% pCR, 39% ypT+ N0, 33% ypT+N+, and 4% ypT0N+). Patient-related factors, such as smoking, alcohol use, and body weight did not affect likelihood of pCR. Notably, noncompletion of the CROSS-regimen (less than five cycles) seen in 10% was not significantly associated with likelihood of pCR in multivariable analysis (OR 1.2, 95% CI 0.9–1.6).
Overall survival
Median follow-up after esophagectomy was 43 months (IQR 12–50). Patients with pCR had a better OS compared to incomplete responders, with estimated 5-years survival rates of 62% vs. 38% (p = .006; ). Patients categorized as ypT+N+ had the lowest estimated 5-year OS, followed by ypT0N+ and ypT+N0 (22%, 47%, and 49%, respectively, p < .001; ). For EAC and ESCC, similar survival rates were seen (Supplementary Table 2). Compared to the pCR group, patients with an ypT+N+ response were predominantly men with more advanced disease stage. Notably, the proportion of cardia tumors was highest in the ypT+N+ group (6% vs. 12%, p < .001). Excluding the cardia tumors did not affect the results of survival analyses.
Figure 2. Kaplan–Meier’s curves of overall survival according to (A) pathological tumor response, (B) ypTN tumor response groups, (C) ypT category, and (D) ypN category.
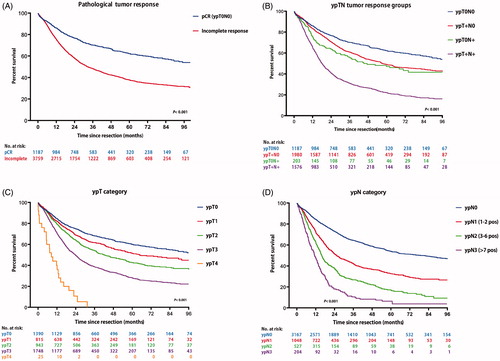
The prognostic significance of the ypT- and ypN-category in adjusted multivariable analyses is shown in ; Supplementary Table 4. Both categories were good predictors of survival time for EAC and ESCC (; ; Supplementary Table 2). With increasing ypT or ypN stage, there was an increasing risk of death. Adding the number of positive lymph nodes (ypN0-3) in the multivariable model resulted in additional prognostic differentiation within the ypT stage (Supplementary Table 3).
Table 3. Prognostic significance of ypT and ypN category in multivariable analysis.
Time to surgery
The median time from end of CRT to surgery was 8.6 weeks (IQR 6.7–9.9), increasing over time from 7 weeks in 2009 to 10 weeks in 2017 (p < .001). The highest pCR rate was observed 13–14 weeks after the end of neoadjuvant CRT in both EAC and ESCC, 21% and 46%, respectively. However, time to surgery as a continuous and categorical variable was not associated with the likelihood of pCR or long-term OS in multivariable analyses.
Discussion
With 94% of all included cases of potentially curable esophageal cancer treated with CROSS, this neoadjuvant CRT regimen has been widely adopted in the past 5–10 years in The Netherlands. Our data show that pCR after CROSS was achieved in 24% of all patients. As expected, the pCR rate was lower in EAC (19%) than in ESCC (42%).
Our results are similar to the original data of the CROSS-trial and other studies focusing on pCR (rate: 16–32%) [Citation6,Citation10,Citation12–19]. Evidence from real-world data, complementary to evidence from conventional clinical trials, is essential to understand the impact of pathological tumor response on patient outcome and to identify high- and low-risk subgroups. To our knowledge, the current study includes the largest homogeneously treated group with regard to neoadjuvant CRT regimen. The majority of previously published population-based studies were limited by missing details on the neoadjuvant chemotherapy regimen or radiotherapy dose [Citation10,Citation12–17], or had smaller sample sizes compared to our study [Citation18,Citation19].
None of the studies have investigated predictive factors for pCR in a population-based setting. We found that well to moderately differentiated EAC, higher lymph node yield, higher T-stage, cN+, and presence of SRCs were associated with a lower probability of pCR. Poorly differentiated tumors were more likely to achieve pCR. One explanation could be the higher chemosensitivity due to increased proliferative activity and therefore higher susceptibility to DNA-damage and apoptosis [Citation20,Citation21]. Except for lymph node yield, all predictors were pretreatment variables that are routinely available prior to start of neoadjuvant CRT. Predictors and biomarkers of minor or major tumor response to neoadjuvant CRT obtained at the pretreatment stage are essential as they could potentially guide more individualized treatment decisions, such as directly proceeding to surgery without further delay.
In our dataset, the mean number of resected lymph nodes increased over the years from 13 to 20 in the period 2009–2015. In the Netherlands, a lymph node yield of ≥15 nodes is recommended since 2016 and is considered a quality indicator [Citation22]. The rationale is that an extended lymphadenectomy is needed for optimal nodal staging. Naturally, the association between a lower yield (<15) and a higher likelihood of pCR is due to the fact that finding positive lymph nodes is less likely if fewer lymph nodes are investigated. Occult micro-metastases, which have been suggested to be present in 10–50% of solid tumors of the upper digestive tract, will also likely be removed with an extended lymphadenectomy, affecting long-term outcome [Citation23–26].
The presence of an SRC component, seen in 16% of EACs was also associated with a lower likelihood of pCR. The proportion of EAC with SRC differentiation ranges from 1 to 26% in studies and is thought to be associated with poor prognosis [Citation27]. Even a small SRC component was associated with a lower likelihood of pCR and radical resection [Citation28,Citation29]. In one study, the presence of SRC was a negative predictor of pCR in a multivariable model [Citation9]. Nonetheless, the prognostic significance of SRC differentiation in EAC is still debated. Several studies have reported significantly poorer survival rates in SRC EAC after neoadjuvant CRT compared to non-SRC EAC, whereas in a recent study SRC differentiation was not an independent predictor of survival [Citation27,Citation30,Citation31]. Reporting of SRC differentiation in histopathologic examinations is however recommended as it may have implications for patient stratification and treatment strategies in the future.
The association between different pathological tumor responses and long-term survival could support more accurate assessments of individual prognosis. Several studies have demonstrated that patients with pCR have better survival outcomes than patients with residual disease [Citation6,Citation9,Citation14]. In our survival analysis, EAC patients with pCR had a three times lower risk of death compared to ypT+N+. This might be one of the reasons for the increased time to surgery in the Netherlands, as a prolonged interval after completion of CRT has been suggested to increase the likelihood of pCR [Citation32]. However, other possible reasons for this trend are a longer recovery time from the cytotoxic effects of chemotherapy without compromise on intra- and short-term postoperative outcomes [Citation33], and operational factors, such as access to and planning of PET-CT and surgery [Citation34].
Different grading systems exist to assess the extent of pathological tumor response [Citation35,Citation36]. Yet, there is no generally accepted tumor regression grading system for prognostication in esophageal cancer, mainly due to considerable variations in surgical specimen handling and interobserver agreement between pathologists [Citation35,Citation37]. This study confirms that the ypTNM staging system, which is simple, widely used and routinely integrated into the pathology reports, is an excellent prognostic system to discriminate between good, intermediate, and poor survival groups. Most likely due to an insufficient sample size, the prognostic value of ypTNM staging was found to be only weak or even absent in previous studies [Citation35,Citation38,Citation39].
In the European Society for Medical Oncology (ESMO) and the National Comprehensive Cancer Network (NCCN) guidelines, trimodality treatment is considered the standard of care for potentially curable esophageal cancer [Citation3,Citation4]. Theoretically, some patients in the pCR group may well be overtreated with surgery. The results from the ongoing phase III, randomized, controlled SANO-trial, and ESOSTRATE-trial, comparing active surveillance after neoadjuvant CRT to standard surgery, are eagerly awaited and are expected to shed more light on the management of patients with clinical complete response.
After standard trimodality treatment, the majority of patients (76%) still have residual disease at the primary site and/or in the lymph nodes. Patients with an ypT+N+ response after CROSS have the worst survival rates. This implicates that current standards of care need further improvement. The optimal post-operative management of patients with a ypT+N+ response, however, is still unknown. There has been an increasing interest in post-operative therapies, such as adjuvant chemotherapy, which retrospective analyses have suggested to prolong survival in cN+ patients [Citation40,Citation41]. Other opportunities might also come from different treatment algorithms including modified (neo)adjuvant regimens or combinations with novel immunotherapeutic agents. Results from ongoing studies examining the feasibility of adjuvant chemotherapy, such as the SOX-trial (NCT02347904), are soon to be expected. In phase II and III clinical trials, addition of trastuzumab and pertuzumab to the CROSS-regimen for HER2+ EAC and nivolumab to PD-L1 positive EAC, was shown to be feasible and resulted in promising survival benefit in exploratory analyses [Citation42,Citation43].
One of the strengths of this analysis is the large sample size. This study is to our knowledge the largest cohort of patients treated with the CROSS-regimen. Furthermore, we used recent data from the NCR, known for its reliable and objective data collection in the Netherlands. Some extent of missing data is inevitable in population-based registries. Through linkage with the national pathology database we were able to add substantial missing data and include additional pathological data. Another limitation was that we could not estimate disease-specific survival, because the NCR does not register cause of death.
Conclusion
This nationwide population homogenously treated with the neoadjuvant CROSS-regimen showed that a quarter of the patients achieved pCR. Histology type, differentiation grade, cT-category, cN-category, lymph node yield, and SRC differentiation were found to be predictors of pCR. The ypTNM staging system seemed to have an excellent potential to discriminate between different prognostic subgroups. Patients with ypT0N0 had a significantly better long-term OS compared to incomplete responders. Overall, one-third of the population had an ypT+N+ response, which was associated with a poor long-term OS. Future prospective studies should evaluate alternative treatment modalities and surveillance strategies for this large subgroup, establishing a paradigm shift from ‘one size fits all’ to more personalized therapy.
| Abbreviations | ||
| CI | = | confidence interval |
| cN | = | clinical N stage |
| CT | = | computer tomography |
| CRT | = | chemoradiotherapy |
| cT | = | clinical T stage |
| HR | = | hazard ratio |
| IQR | = | interquartile range |
| EAC | = | esophageal adenocarcinoma |
| OR | = | odds ratio |
| ESCC | = | esophageal squamous cell carcinoma |
| pCR | = | pathological complete response |
| PET | = | positron emission tomography |
| SD | = | standard deviation |
| SCC | = | squamous cell carcinoma |
| SRC | = | signet ring cell |
| THE | = | transhiatal esophagectomy |
| TTE | = | transthoracic esophagectomy |
| UC | = | undifferentiated carcinoma |
| ypTNM | = | TNM staging assessed in the resection specimen after neoadjuvant therapy |
Supplemental Material
Download MS Word (64.8 KB)Disclosure statement
Rob Verhoeven has received research grants from Roche and Bristol-Myers Squibb. Hanneke van Laarhoven has served as a consultant for BMS, Celgene, Lilly, Nordic, Philips, and Servier and has received unrestricted research funding from Bayer, BMS, Celgene, Lilly, Merck Serono, MSD, Nordic, Philips, Roche, and Servier. Peter D. Siersema has received research grants from Boston Scientific, Pentax Medical, and The eNose company.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Arnold M, Laversanne M, Brown LM, et al. Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030. Am J Gastroenterol. 2017;112(8):1247–1255.
- Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl. 5):v50–v57.
- Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(7):855–883.
- Nygaard K, Hagen S, Hansen HS, et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg. 1992;16(6):1104–1109.
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084.
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6(9):659–668.
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098.
- Blum Murphy M, Xiao L, Patel VR, et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: the association with baseline variables and survival—The University of Texas MD Anderson Cancer Center experience. Cancer. 2017;123(21):4106–4113.
- Chao YK, Chen HS, Wang BY, et al. Factors associated with survival in patients with oesophageal cancer who achieve pathological complete response after chemoradiotherapy: a nationwide population-based study. Eur J Cardiothorac Surg. 2017;51(1):155–159.
- Francoual J, Lebreton G, Bazille C, et al. Is pathological complete response after a trimodality therapy, a predictive factor of long-term survival in locally-advanced esophageal cancer? Results of a retrospective monocentric study. J Visc Surg. 2018;155(5):365–374.
- Al-Sukhni E, Gabriel E, Attwood K, et al. No survival difference with neoadjuvant chemoradiotherapy compared with chemotherapy in resectable esophageal and gastroesophageal junction adenocarcinoma: results from the National Cancer Data Base. J Am Coll Surg. 2016;223(6):784–792e1.
- van der Werf LR, Dikken JL, van der Willik EM, et al. Time interval between neoadjuvant chemoradiotherapy and surgery for oesophageal or junctional cancer: a nationwide study. Eur J Cancer. 2018;91:76–85.
- Groth SS, Burt BM, Farjah F, et al. Prognostic value of neoadjuvant treatment response in locally advanced esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2019;157(4):1682–1693.e1.
- Lee A, Wong AT, Schwartz D, et al. Is there a benefit to prolonging the interval between neoadjuvant chemoradiation and esophagectomy in esophageal cancer? Ann Thorac Surg. 2016;102(2):433–438.
- Azab B, Amundson JR, Picado O, et al. Impact of chemoradiation-to-surgery interval on pathological complete response and short- and long-term overall survival in esophageal cancer patients. Ann Surg Oncol. 2019;26(3):861–868.
- Klevebro F, Lindblad M, Johansson J, et al. Outcome of neoadjuvant therapies for cancer of the oesophagus or gastro-oesophageal junction based on a national data registry. Br J Surg. 2016;103(13):1864–1873.
- Luc G, Gronnier C, Lebreton G, et al. Predictive factors of recurrence in patients with pathological complete response after esophagectomy following neoadjuvant chemoradiotherapy for esophageal cancer: a multicenter study. Ann Surg Oncol. 2015;22(S3):1357–1364.
- Vallbohmer D, Holscher AH, DeMeester S, et al. A multicenter study of survival after neoadjuvant radiotherapy/chemotherapy and esophagectomy for ypT0N0M0R0 esophageal cancer. Ann Surg. 2010;252(5):744–749.
- Yang C, Zhang J, Ding M, et al. Ki67 targeted strategies for cancer therapy. Clin Transl Oncol. 2018;20(5):570–575.
- Huang JX, Yan W, Song ZX, et al. Relationship between proliferative activity of cancer cells and clinicopathological factors in patients with esophageal squamous cell carcinoma. World J Gastroenterol. 2005;11(19):2956–2959.
- Auditing DIfC. Factsheet indicatoren Slokdarm-en maagcarcinoom (DUCA); 2019; [cited 2019 Mar 1]. Available from: https://dica.nl/duca/documenten
- Izbicki JR, Hosch SB, Pichlmeier U, et al. Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med. 1997;337(17):1188–1194.
- Komukai S, Nishimaki T, Suzuki T, et al. Significance of immunohistochemical nodal micrometastasis as a prognostic indicator in potentially curable oesophageal carcinoma. Br J Surg. 2002;89(2):213–219.
- Vazquez-Sequeiros E, Wang L, Burgart L, et al. Occult lymph node metastases as a predictor of tumor relapse in patients with node-negative esophageal carcinoma. Gastroenterology. 2002;122(7):1815–1821.
- Heeren PA, Kelder W, Blondeel I, et al. Prognostic value of nodal micrometastases in patients with cancer of the gastro-oesophageal junction. Eur J Surg Oncol. 2005;31(3):270–276.
- Bleaney CW, Barrow M, Hayes S, et al. The relevance and implications of signet-ring cell adenocarcinoma of the oesophagus. J Clin Pathol. 2018;71(3):201–206.
- Patel VR, Hofstetter WL, Correa AM, et al. Signet ring cells in esophageal adenocarcinoma predict poor response to preoperative chemoradiation. Ann Thorac Surg. 2014;98(3):1064–1071.
- Chen L, Liu X, Gao L, et al. The clinicopathological features and prognosis of signet ring cell carcinoma of the esophagus: a 10-year retrospective study in China. PLoS One. 2017;12(5):e0176637.
- van Hootegem SJM, Smithers BM, Gotley DC, et al. The impact of signet ring cell differentiation on outcome in patients with esophageal and gastroesophageal junction adenocarcinoma. Ann Surg Oncol. 2019;26(8):2375–2384.
- Machlowska J, Pucułek M, Sitarz M, et al. State of the art for gastric signet ring cell carcinoma: from classification, prognosis, and genomic characteristics to specified treatments. Cancer Manag Res. 2019;11:2151–2161.
- Qin Q, Xu H, Liu J, et al. Does timing of esophagectomy following neoadjuvant chemoradiation affect outcomes? A meta-analysis. Int J Surg. 2018;59:11–18.
- Nilsson K, Klevebro F, Rouvelas I, et al. Surgical morbidity and mortality from the multicenter randomized controlled NeoRes II Trial: standard versus prolonged time to surgery after neoadjuvant chemoradiotherapy for esophageal cancer. Ann Surg. 2020;272(5):684–689.
- Koëter M, van Steenbergen LN, Lemmens VE, et al. Hospital of diagnosis and probability to receive a curative treatment for oesophageal cancer. Eur J Surg Oncol. 2014;40(10):1338–1345.
- Puetz K, Bollschweiler E, Semrau R, et al. Neoadjuvant chemoradiation for patients with advanced oesophageal cancer – which response grading system best impacts prognostic discrimination? Histopathology. 2019;74(5):731–743.
- Klevebro F, Tsekrekos A, Low D, et al. Relevant issues in tumor regression grading of histopathological response to neoadjuvant treatment in adenocarcinomas of the esophagus and gastroesophageal junction. Dis Esophagus. 2020;33(6).
- Karamitopoulou E, Thies S, Zlobec I, et al. Assessment of tumor regression of esophageal adenocarcinomas after neoadjuvant chemotherapy: comparison of 2 commonly used scoring approaches. Am J Surg Pathol. 2014;38(11):1551–1556.
- Barbour AP, Jones M, Gonen M, et al. Refining esophageal cancer staging after neoadjuvant therapy: importance of treatment response. Ann Surg Oncol. 2008;15(10):2894–2902.
- Tong DK, Law S, Kwong DL, et al. Histological regression of squamous esophageal carcinoma assessed by percentage of residual viable cells after neoadjuvant chemoradiation is an important prognostic factor. Ann Surg Oncol. 2010;17(8):2184–2192.
- Burt BM, Groth SS, Sada YH, et al. Utility of adjuvant chemotherapy after neoadjuvant chemoradiation and esophagectomy for esophageal cancer. Ann Surg. 2017;266(2):297–304.
- Mokdad AA, Yopp AC, Polanco PM, et al. Adjuvant chemotherapy vs postoperative observation following preoperative chemoradiotherapy and resection in gastroesophageal cancer: a propensity score-matched analysis. JAMA Oncol. 2018;4(1):31–38.
- Stroes CI, Schokker S, Creemers A, et al. Phase II feasibility and biomarker study of neoadjuvant trastuzumab and pertuzumab with chemoradiotherapy for resectable human epidermal growth factor receptor 2-positive esophageal adenocarcinoma: TRAP study. J Clin Oncol. 2019;38(5):462–471.
- Kelly RJ, Lockhart AC, Jonker DJ, et al. CheckMate 577: a randomized, double-blind, phase 3 study of nivolumab (Nivo) or placebo in patients (Pts) with resected lower esophageal (E) or gastroesophageal junction (GEJ) cancer. J Clin Oncol. 2017;35(4_Suppl.):TPS212.