Abstract
Background
The net effect of menopausal hormone therapy on the risk of death is understudied, and current evidence is conflicting. Our aim was to investigate the association between menopausal hormones and risk of all-cause, cardiovascular, and cancer-specific mortality, based on the Swedish Prescribed Drug Registry and National Patient Registry.
Methods
This Swedish population-based matched cohort study included all women, 40 years or older, who had received at least one prescription of systemic menopausal hormone therapy between 2005–2014 (n = 290,186), group level matched 1:3 to non-users (n = 870,165). Multivariable conditional logistic regression models estimated the relative risk of all-cause and cause-specific mortality, adjusting for several clinical factors and comorbidities.
Results
Ever-use of menopausal hormones was associated with a slightly lower overall odds of all-cause (OR = 0.97, 95%CI 0.95–0.98) and cardiovascular (OR = 0.97, 95%CI 0.95–0.99) mortality, whilst 30% lower overall odds of cancer-related mortality (OR = 0.70, 95%CI 0.68–0.72) was shown. The odds of all-cause and cancer-related mortality were consistently reduced among women who began menopausal hormone therapy ≤60 years, whereas the association with cardiovascular mortality was inconsistent. In contrast, oestrogen-only therapy was associated with elevated odds of all-cause (OR = 1.14, 95%CI 1.11–1.16) and cardiovascular mortality (OR = 1.04, 95%CI 1.01–1.06) among women who began treatment at ≥70 years. Among current users, oestrogen-only therapy was associated with higher odds of all-cause (OR = 1.48, 95%CI 1.44–1.52) and cardiovascular mortality (OR = 1.24, 95%CI 1.20–1.28), whereas past use of oestrogen-only therapy suggested lower odds of mortality.
Conclusions
Our generalisable data suggest that early menopausal hormone treatment initiation does not increase the odds of mortality. However, the role of oestrogens in particularly cardiovascular mortality remains to be investigated.
Introduction
Menopausal hormone therapy (MHT) encompasses various treatments prescribed primarily to prevent vasomotor symptoms during menopause [Citation1]. MHT was one of the most commonly prescribed drugs in the United States during the 1990s. The decline in MHT prescriptions over the past two decades might have resulted in a whole generation of women missing the beneficial effects of MHT. Menopausal hormones are still to date the most effective licenced prescription drugs to alleviate menopausal symptoms, experienced by 50% up to 75% of women [Citation2–4]. Cumulative evidence indicates MHT use is associated with both favourable and deleterious outcomes, varying across different treatment types, formulations and regimens, [Citation5–9] also depending on the individual risk profile. Whereas several studies have focussed on the association between MHT use and the risk of various cancer types and cardiovascular events, [Citation10–15] evidence for the net effect of MHT on the risk of all-cause and cause-specific mortality remains inconclusive [Citation16–21]. Notably, the age of women at enrolment has varied in the previous studies, and direct comparisons are challenging considering that the formulations and doses have changed over time, and the prescription patterns.
Currently, the latest guidelines of the US Preventive Services Task Force advise against MHT use for primary prevention of chronic diseases associated with ageing, as current evidence is insufficient to outweigh potential health benefits from the potential risks clearly [Citation22]. The updated Swedish guidelines advocate using MHT primarily to treat vasomotor symptoms among women younger than 60 years or within 10 years from menopausal onset (median 51 years). A distinctive feature of Sweden is the availability of various MHT treatment options [Citation23,Citation24]. Therefore, we conducted this population-based matched cohort study including virtually all Swedish MHT ever-users, investigating the net effect of contemporary MHT use on the risk of all-cause and cause-specific mortality.
Material and methods
Study design
This nationwide population-based matched cohort has been used extensively to investigate the risk of breast, [Citation25] gastro-oesophageal, [Citation26] colorectal, [Citation27] biliary tracts, [Citation28] pancreatic, [Citation29] and ovarian cancer [Citation30], and as a source cohort for a study on colorectal cancer survival [Citation31]. The study design and the group-level matching procedure (1:3) have been thoroughly described elsewhere [Citation25]. In short, the Swedish Prescribed Drug Registry was used to identify virtually all Swedish MHT ever-users between 1 July 2005 and 31 December 2014. The unique Swedish personal identity number ensured a valid data linkage between other national registries supervised by the National Board of Health and Welfare [Citation32,Citation33]. The Regional Ethical Review Board in Stockholm granted the ethical approval (2014/1291-31/4) without a need for informed consent.
This population-based matched cohort study included all Swedish women, 40 years or older at first prescription, who received at least one dispensed prescription of systemic MHT between 1 July 2005 and 31 December 2013/2014, as ascertained from the Swedish Prescribed Drug Registry. The index date was the date of the first dispensed prescription of menopausal hormones. The Swedish Causes of Death Registry was used to ascertain the date and cause of death, defined by the International Classification of Diseases (ICD) 10th edition. Information on the cause of death was available until December 2013 and for all-cause mortality until December 2014. Clinical data were ascertained from the National Patient Registry (in- and outpatient care) [Citation34].
Exclusion criteria
All women with a history of any malignancy (apart from non-melanoma skin cancer, ICD-10 codes C43.0-9) were excluded before the matching. We also excluded MHT preparations with local effects (i.e., vaginal creams) because they are unlikely to have systemic effects [Citation35].
Exposure
The Swedish Prescribed Drug Registry, which is over 99% complete for all outpatient care drugs, was used for exposure ascertainment based on Anatomical and Therapeutic Chemical (ATC) classification codes (Supplementary Table 1). Notably, MHT is not sold over the counter in Sweden, and injectable MHT is not used [Citation3,Citation25]. Women who received one or more dispensed progestin prescriptions during the study period were classified as EP-MHT users. Oestradiol accounted for over 99% of the oestrogenic component in EP-MHT prescriptions. Furthermore, MHT users were classified as current versus past users. Current users were defined as women who had received at least one MHT prescription within six months before their death or end of the study period. All other women were classified as past users. Notably, one prescription in Sweden covers three months, allowing for a potential washout period.
Outcome
The primary outcome was the risk of all-cause mortality (ICD-10 codes: A00-Z99) (Supplementary Table 2). The secondary outcomes were the two most common causes of death in Sweden, [Citation36] cardiovascular mortality (ICD-10 codes: I00-99) and cancer mortality (ICD-10 codes: C00-C97 and D00-D09).
Statistical analyses
Multivariable conditional logistic regression models were used to estimate the relative risk of death due to all-cause and cause-specific mortality (i.e., cardiovascular and cancer mortality), providing adjusted odds ratios (ORs) with 95% confidence intervals (CIs). These analyses were adjusted for all the eight matching variables (i.e., year of birth, hysterectomy, delivery, thrombotic events, smoking- and alcohol-related diseases, obesity, diabetes), and osteoporosis (i.e., confounding by indication), comparing MHT ever-users with women who did not receive MHT during the study period. Because the group-level matching procedure counterbalances the effect of follow-up time between the groups, no substitute date was created for the non-exposed women to evaluate the duration of follow-up [Citation25,Citation30]. The analyses were stratified by women’s age at first prescription (categorised as <60 years, 60–69 years, and 70 years and older, based on the updated Swedish guidelines) [Citation23,Citation24], MHT types, and current versus past use. Subgroup analyses investigated the different oestrogen formulations and EP-MHT regimens separately. Women who switched therapy types during the study period were excluded from the subgroup analyses. Women were followed up until death or the end of the study period (December 2014), whichever occurred first. Interaction by age was modelled on an additive scale, with the youngest group constituting the comparison group. A p-value <0.05 was considered indicative of statistical significance. To assess the robustness of our results, we conducted sensitivity analyses excluding all women who died within the first year (i.e., between July 2005 and June 2006). All statistical analyses were performed on STATA MP4 15.1.
Patient and public involvement
Due to the registry-based nature of the study, no patients or the public were directly involved in the study.
Results
The results of this study are reported in line with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [Citation37]. A total of 1,160,351 women were included in this nationwide cohort, including 290,186 MHT ever-users and 870,165 group-level matched MHT non-users. During the study period, 33,650 (11.7%) of MHT ever-users died and 104,740 (12.0%) of non-users died (). The median age at death was 87 years among MHT ever-users and 86 years among MHT non-users.
Table 1. Descriptive characteristics of the study included women between 2005 and 2014.
Due to a successful matching procedure age, clinical factors and comorbidities were equally distributed between the groups. Oestrogen combined progestin treatment was more common (n = 154,198, 53.1%) than E-MHT only (n = 117,996, 40.7%), and 6.2% (n = 17,992) of the women received tibolone. Of oestrogen formulations, oestradiol (n = 53,339, 45.2%) and oestriol (n = 55,653, 47.2%) were most prescribed, while 1.0% (n = 1161) received conjugated oestrogens, and 6.7% (n = 7843) received both. Among EP-MHT regimens, continuously administered testosterone-derived regimens were the most common (n = 53,360, 34.6%), followed by continuous progesterone-derived regimens (n = 30,123, 19.5%).
All-cause mortality
Compared with non-users, ever-use of MHT was associated with 3% lower odds of all-cause mortality (OR = 0.97, 95%CI 0.95–0.98) (, Supplementary Table 3). However, important differences were revealed by age at treatment initiation, MHT types, and current versus past use. Compared with non-users, higher all-cause mortality was shown among E-MHT users, particularly among women who began treatment at ≥70 years (OR = 1.14, 95%CI 1.11–1.16, p-interaction <0.0001). In contrast, E-MHT user among younger women was associated with lower odds of mortality, and this association was strongest among women ≤60 years (OR = 0.51, 95%CI 0.45–0.57, p-interaction <0.0001). Among current E-MHT users, the odds were 48% increased (OR = 1.48, 95%CI 1.44–1.52), while past E-MHT use was associated with lower odds of all-cause mortality (OR = 0.83, 95%CI 0.80–0.86) (). Considering all women, tibolone was associated with lower odds of mortality (OR = 0.60, 95%CI 0.55–0.66), although p-interaction is significant, suggesting a stronger inverse association in younger age groups. Similarly, the use of EP-MHT was associated with lower odds of all-cause mortality (OR = 0.76, 95%CI 0.74–0.79, p-interaction 0.069) (, Supplementary Table 3). Among all past MHT users, tibolone showed the strongest inverse association (OR = 0.62, 95%CI 0.55–0.69).
Figure 1. Association between menopausal hormone therapy use and risk of mortality. OR: odds ratio. Note: These odds ratios and other information is provided in more detail in the Supplementary Table 3.
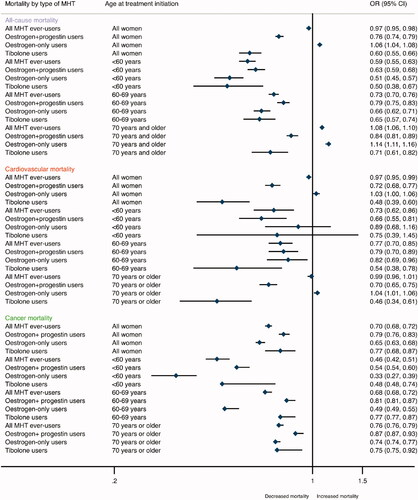
Table 2. The association of menopausal hormone therapy (MHT) use with all-cause and cause-specific mortality during the study period stratified by current versus past use of MHT.
Cardiovascular mortality
Of MHT ever-users 4.8% (n = 13,985) died due to cardiovascular events and respectively 5.0% (n = 43,328) of MHT non-users died due to cardiovascular cause (). Compared with non-users, ever-use of MHT was associated with 3% lower odds of cardiovascular mortality (OR = 0.97, 95%CI 0.95–0.99) (, Supplementary Table 3). However, differences were seen between age at treatment initiation and MHT types. Oestrogen-only therapy was associated with a slightly higher odds of cardiovascular mortality, particularly among women who began MHT treatment at ≥70 years (OR = 1.04, 95%CI 1.01–1.06, p-interaction <0.0001), whereas the odds of cardiovascular mortality were shown lower among E-MHT users who were 60–69 years at treatment initiation (OR = 0.82, 95%CI 0.69–0.96, p-interaction <0.0001). Overall, tibolone use (OR = 0.48, 95%CI 0.39–0.60) showed a more pronounced inverse association with the odds of cardiovascular mortality than EP-MHT use (OR = 0.72, 95%CI 0.68–0.77). When stratified by current and past users of MHT, current use of E-MHT was associated with higher odds of cardiovascular mortality (OR = 1.24, 95%CI 1.20–1.28), whereas no apparent association was found for tibolone or EP-MHT (). In contrast, past MHT use was inversely associated with cardiovascular mortality, and the association was stronger among tibolone (OR = 0.36, 95%CI 0.27–0.48) and EP-MHT users (OR = 0.64, 95%CI 0.59–0.68) than E-MHT users (OR = 0.86, 95%CI 0.83–0.89).
Cancer-related mortality
Of MHT ever-users 1.9% (n = 5608) died due to cancer and respectively 2.8% (n = 23,956) of MHT non-users died (). Compared with non-users of MHT, ever-use was associated with 30% lower odds of cancer-related mortality (OR = 0.70, 95%CI 0.68–0.72) (, Supplementary Table 3). The association was strongest among women who began MHT treatment ≤60 years (OR = 0.46, 95%CI 0.42–0.51, p-interaction <0.0001). Compared with non-users, the inverse association was overall more pronounced for current than past users, particularly for current E-MHT users (OR = 0.53, 95%CI 0.50–0.56) in comparison to the odds among past E-MHT users (OR = 0.76, 95%CI 0.73–0.80) (). Whereas the odds of cancer-related mortality were reduced among all current MHT users (OR = 0.52, 95%CI 0.49–0.54), a marginal association was shown for past EP-MHT use (OR = 0.95, 95%CI 0.90–1.00).
Formulations and regimens
Among E-MHT users, the different oestrogen formulations were associated with varying odds of mortality (Supplementary Table 4). Compared with MHT non-users, oestradiol was associated with lower odds of mortality, and the association was strongest for cancer mortality (OR = 0.53, 95%CI 0.49–0.56). Oestriol use was associated with higher odds of all-cause (OR = 1.22, 95%CI 1.19–1.25) and cardiovascular mortality (OR = 1.08, 1.05–1.11), while lower odds of cancer-related mortality was noted (OR = 0.74, 95%CI 0.71–0.78). Among EP-MHT users, continuous regimens, both progesterone- and testosterone-derived, were associated with 26-31% lower odds of mortality overall. In contrast, sequential testosterone-derived regimens were associated with an increased odds of all-cause mortality (OR = 1.24, 95%CI 1.11–1.38) and higher odds of cardiovascular mortality (OR = 1.56, 95%CI 1.01–2.41).
Sensitivity analyses
The association remained similar in the sensitivity analyses, excluding women who died within the first year from the beginning of the study (Supplementary Table 5).
Discussion
In this nationwide and population-based study, we observed a minimally reduced overall odds of all-cause and cardiovascular mortality among MHT ever-users compared with MHT non-users. In contrast, we found a 30% likelihood of overall cancer-related mortality among MHT users. Furthermore, the overall all-cause and cancer-specific mortality risks were consistently lower among women who began treatment <70 years. However, no association with cardiovascular mortality was found among women who initiated oestrogen-only or tibolone treatment ≤60 years. The observed increased mortality appeared to be driven by oestrogen-only therapy and particularly by oestriol but not oestradiol which accounted for over 99% of the oestrogenic component in all EP-MHT treatments. Moreover, an increased odds of all-cause and cardiovascular mortality was shown among older women on E-MHT (≥70 years). Among current users of E-MHT, but not past users, 48% increased odds of all-cause mortality and a 24% higher cardiovascular mortality was shown. For tibolone and EP-MHT use, no overall association was found. Notably, continuous EP-MHT regimens were associated with lower odds of mortality, whereas sequential administration of testosterone-derivatives increased the odds of all-cause mortality by 24%.
This study has several strengths, including population-based and nationwide design, enhancing statistical power to detect differences, counteracting selection bias, and facilitating the generalisability of our results. The unique Swedish personal identity number ensured valid data linkage between the high-quality Swedish health data registries, with high coverage and completeness of follow-up [Citation33,Citation38]. Information on the exposure was ascertained from the virtually complete Swedish Prescribed Drug Registry, and systemic MHT is not available over the counter in Sweden, minimising the risk of misclassification of exposure. The outcome was ascertained from the Swedish Causes of Death Registry, which is 100% complete.
Our study has some limitations as well. Whereas the population-based methodology counteracts potential selection bias, we cannot eliminate the possibility of healthy user bias. A link between MHT use, socioeconomic status and potentially better survival and higher adherence to treatment has been described [Citation3,Citation39]. To alleviate these concerns, we stratified the analyses by the different MHT types and current versus past use. Nevertheless, clinicians may be more prone to prescribe MHT to healthier women. The use of menopausal hormones is contraindicated among women at high risk of breast cancer, with current or a history of cardiovascular disease or among women with severe liver disease [Citation23,Citation24]. A closer follow-up is favoured among women using MHT, which could potentially lead to earlier detection of diseases selectively among MHT users. A sensitivity analysis excluding women who died within the first year showed similar results.
Another concern is potential left censoring, as the Swedish Prescribed Drug Registry was established in July 2005, and we lacked information on potential MHT use before the index date. A large proportion of the cohort (59.8%) was already on MHT from the start of the study; [Citation25] however, any exposure misclassification should be at random between the groups. Whereas duration of treatment should be considered, it was more informative to compare current versus past users of menopausal hormones, given the concern of left censoring.
Although we could adjust for several potential confounding factors, we lacked data on oral contraceptive use. However, this group of women would be very young concerning the outcomes considering most Swedish oral contraceptive users are young adults [Citation40]. Furthermore, whereas we lacked data on BMI, our analyses were adjusted for obesity. However, it should be acknowledged that whereas the function of ovaries ceases at menopause, heavier women with more adipose tissues might have different circulating hormone levels, which could influence the cumulative exposure to endo- and exogenous oestrogens [Citation41]. Our analyses were not adjusted for unilateral/bilateral oophorectomies without concomitant hysterectomy because hysterectomy has a significant influence on deciding the type of menopausal hormones women will receive – which is not the case for oophorectomies. Whereas unilateral/bilateral oophorectomies without a concomitant hysterectomy were rarely recorded during the study period, [Citation42] it is also less likely that ovaries are left in among women who are already menopausal. Additionally, several indications for oophorectomies are single-sided, yet the Swedish codes for laterality are inconsistent and inadequate to determine with certainty whether both ovaries were removed [Citation43].
The results of this nationwide and population-based cohort study are in line with the previous two meta-analyses, including 31 randomised controlled trials, [Citation17] and another meta-analysis pooling together 30 studies [Citation19]. Both of these systematic reviews and meta-analyses revealed a 28% lower mortality risk (n = 16 studies) [Citation17], respectively, 39% lower risk of mortality (n = 13 studies) [Citation19] among women younger than 60 years, while no association was found among older women (the mean age at treatment initiation was 66.9 years, [Citation17] respectively >60 years) [Citation19]. Our results suggest that oestrogen-only therapy among women ≥70 years or older could be associated with higher odds of all-cause and cardiovascular mortality. In the extended analysis of the Women’s Health Initiative trial, no impact of either combination therapy (HR = 1.02, 95%CI 0.96–1.08) nor unopposed oestrogens (HR = 0.94, 95%CI 0.88–1.01) were observed compared to placebo on the risk of all-cause mortality. The results were similar for cardiovascular and cancer mortality during a cumulative 18 years of follow-up [Citation44]. In contrast, in a population-based study including 14,361 women (aged ≥45 years and enrolled between 2003−2007), MHT ever-use initiated at an early menopausal age was associated with a higher risk of all-cause mortality (HR = 1.31, 95%CI 1.10–1.56), whereas no association was shown among MHT never-users (HR = 1.01, 95%CI 0.85–1.20), without consideration of the smoking status of ethnicity in the analyses.Citation20
In a prospective Danish study including fewer than 30,000 women (aged 50–64 years), the effect of lower cardiovascular mortality was evident only after five years of follow-up [Citation21]. In our data, current oestrogen-only use, but not past use, was associated with a higher risk of all-cause and cardiovascular mortality. Timing of treatment initiation could explain the observed increased odds among current users, as an increase in cardiovascular mortality has been described shortly following MHT treatment initiation, only to flatten out after a few years of MHT use [Citation45]. This could partly explain the lower cardiovascular mortality found among past users, who may have passed the time window of increased risk, and therefore, our results should be interpreted cautiously. Another possibility is that oestrogens may reduce the risk of coronary disease, [Citation46] and past users may have received MHT for a longer duration. Currently, the primary indication of MHT is the alleviation of the vasomotor symptoms of menopause [Citation22]. Yet, it is plausible that women prescribed MHT are experiencing more severe symptoms. These women may have different circulating hormone levels or changes in levels over the menopausal transition, influencing the cumulative exposure to endo- and exogenous oestrogens [Citation47]. Whereas this study cannot determine the underlying pathophysiological mechanisms, our findings highlight the need for more mechanistic studies, particularly on the role of oestrogens in cardiovascular mortality.
Conclusions
In conclusion, our results indicate that MHT use initiated before 60 years is not associated with higher odds of mortality. Notably, current use of oestrogen therapy, but not past use, and oestrogen-only therapy initiation at an older age were associated with higher odds of all-cause and cardiovascular mortality.
| Abbreviations | ||
| CI | = | Confidence interval |
| E-MHT | = | Oestrogen-only treatment |
| EP-MHT | = | Oestrogen combined therapy |
| MHT | = | Menopausal hormone therapy |
| OR | = | Odds ratio |
| RR | = | Relative risk |
Supplemental Material
Download MS Word (151 KB)Acknowledgements
We wish to express our sincere gratitude towards all the thousands of women, clinicians, healthcare staff members who contributed to the data collection, and the Swedish National Board of Health and Welfare to collect the data. We also wish to thank Dr Zangin Zeebari, Assistant Professor in Statistics, for his valuable comments on the revision.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The dataset for this study is held securely in a coded form at Karolinska Institutet, yet it belongs to the National Board of Health and Welfare (Socialstyrelsen). Data sharing agreements prohibit publicly making the dataset available, but the data will be made available upon reasonable request to the corresponding author (JS) after obtaining relevant ethical and data-sharing approval. The underlying analysis plan is available from the corresponding author (JS) upon request.
STROBE Statement—Checklist of items that should be included in reports of cohort studies.
Additional information
Funding
References
- Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: a meta-analysis. J Gen Intern Med. 2008;23(9):1507–1513.
- Lindh-Åstrand L, Hoffmann M, Hammar M, et al. Hot flushes, hormone therapy and alternative treatments: 30 years of experience from Sweden. Climacteric. 2015;18(1):53–62.
- Järvstråt L, Spetz Holm AC, Lindh-Åstrand L, et al. Use of hormone therapy in Swedish women aged 80 years or older. Menopause. 2015;22(3):275–278.
- Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47–53.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the women's health initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Kanaya AM, Herrington D, Vittinghoff E, et al. Glycemic effects of postmenopausal hormone therapy: the heart and estrogen/progestin replacement study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138(1):1–9.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873.
- Johnson JR, Lacey JV, Jr., Lazovich D, et al. Menopausal hormone therapy and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(1):196–203.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
- Green J, Czanner G, Reeves G, et al. Menopausal hormone therapy and risk of gastrointestinal cancer: nested case-control study within a prospective cohort, and Meta-analysis. Int J Cancer. 2012;130(10):2387–2396.
- Greiser CM, Greiser EM, Dören M. Menopausal hormone therapy and risk of breast cancer: a meta-analysis of epidemiological studies and randomized controlled trials. Hum Reprod Update. 2005;11(6):561–573.
- Beral V, Gaitskell K, Hermon C, et al. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet. 2015;385(9980):1835–1842.
- Lagergren K, Lagergren J, Brusselaers N. Hormone replacement therapy and oral contraceptives and risk of oesophageal adenocarcinoma: a systematic review and meta-analysis. Int J Cancer. 2014;135(9):2183–2190.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Estradiol-based postmenopausal hormone therapy and risk of cardiovascular and all-cause mortality. Menopause. 2015;22(9):976–983.
- Simin J, Tamimi R, Lagergren J, et al. Menopausal hormone therapy and cancer risk: an overestimated risk? Eur J Cancer. 2017;84:60–68.
- Tuomikoski P, Mikkola TS. Postmenopausal hormone therapy and coronary heart disease in early postmenopausal women. Ann Med. 2014;46(1):1–7.
- Nudy M, Chinchilli VM, Foy AJ. A systematic review and Meta-regression analysis to examine the 'timing hypothesis' of hormone replacement therapy on mortality, coronary heart disease, and stroke. Int J Cardiol Heart Vasc. 2019;22:123–131.
- Mudhune GH, Armour M, McBride KA. Safety of menopausal hormone therapy in breast cancer survivors older than fifty at diagnosis: a systematic review and Meta-analysis. Breast. 2019;47:43–55.
- Salpeter SR, Walsh JM, Greyber E, et al. Mortality associated with hormone replacement therapy in younger and older women: a meta-analysis. J Gen Intern Med. 2004;19(7):791–804.
- Malek AM, Vladutiu CJ, Meyer ML, et al. The association of age at menopause and all-cause and cause-specific mortality by race, postmenopausal hormone use, and smoking status. Prev Med Rep. 2019;15:100955.
- Holm M, Olsen A, Au Yeung SL, et al. Pattern of mortality after menopausal hormone therapy: long-term follow up in a population-based cohort. BJOG. 2019;126(1):55–63.
- Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US preventive services task force recommendation Statement. Jama. 2017;318(22):2224–2233.
- SFfOo G, Endokrinologi A-O. Preliminär SFOG-råd för menopausal hormonbehandling 2019, bakgrundsdokument. SFOG-Råd För Menopausal Hormonbehandling. 2019;37.
- Gynekologi SFfOo, endokrinologi A-orf. Preliminära SFOG-råd för menopausal hormonbehandling 2019. 2019. [cited 2020 Apr 11]. Available from: https://www.sfog.se/media/336474/mht-sfog-raad-preliminar-hemsida.pdf.
- Brusselaers N, Tamimi RM, Konings P, et al. Different menopausal hormone regimens and risk of breast cancer. Ann Oncol. 2018;29(8):1771–1776.
- Brusselaers N, Maret-Ouda J, Konings P, et al. Menopausal hormone therapy and the risk of esophageal and gastric cancer. Int J Cancer. 2017;140(7):1693–1699.
- Liu S. Debelius Fall Sadr-Azodi Engstrand Williams, Brusselaers Different menopausal hormone therapies and risk of colorectal cancer: a Swedish matched-cohort study. Under review 2020.
- Kilander C, Lagergren J, Konings P, et al. Menopausal hormone therapy and biliary tract cancer: a population-based matched cohort study in Sweden. Acta Oncol. 2019;58(3):290–296.
- Sadr-Azodi O, Konings P, Brusselaers N. Menopausal hormone therapy and pancreatic cancer risk in women: a population-based matched cohort study. United European Gastroenterol J. 2017;5(8):1123–1128.
- Simin J, Rulla MT, Callens S, et al. Menopausal hormone therapy treatment options and ovarian cancer risk: a Swedish prospective population-based matched-cohort study. Int J Cancer. 2020;147(1):33–44.
- Simin J, Liu Q, Wang X, et al. Prediagnostic use of oestrogen-only therapy is associated with improved colorectal cancer survival in menopausal women: a Swedish population-based cohort study. Acta Oncologica. 2021;60(7):881–887.
- Wettermark B, Hammar N, Fored CM, et al. The new swedish prescribed drug register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–735.
- Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667.
- Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450.
- Krause M, Wheeler TL, 2nd, Richter HE, et al. Systemic effects of vaginally administered estrogen therapy: a review. Female Pelvic Med Reconstr Surg. 2010;16(3):188–195.
- Socialstyrelsen. Statistik om dödsorsaker. In: Socialstyrelsen, editor. Hälso- och sjukvård, publiceringsår. Sweden: Socialstyrelsen; 2021.
- Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (ST ROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297.
- Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish cancer register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33.
- Ji JG, Sundquist J, Sundquist K. Use of hormone replacement therapy improves the prognosis in patients with colorectal cancer: a population-based study in Sweden. Int J Cancer. 2018;142(10):2003–2010.
- Lindh I, Skjeldestad FE, Gemzell-Danielsson K, et al. Contraceptive use in the Nordic countries. Acta Obstet Gynecol Scand. 2017;96(1):19–28.
- Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394(10204):1159–1168.
- Simin J, Tamimi RM, Callens S, et al. Menopausal hormone therapy treatment options and ovarian cancer risk: a Swedish prospective population-based matched-cohort study. Int J Cancer. 2020;147(1):33–44.
- Falconer H, Yin L, Grönberg H, et al. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2):dju410–dju410.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the women's health initiative randomized trials. Jama. 2017;318(10):927–938.
- Vandenbroucke JP. The HRT controversy: observational studies and RCTs fall in line. Lancet. 2009;373(9671):1233–1235.
- Fortini F, Vieceli Dalla Sega F, Caliceti C, et al. Estrogen-mediated protection against coronary heart disease: the role of the notch pathway. J Steroid Biochem Mol Biol. 2019;189:87–100.
- Randolph JF Jr, Sowers M, Bondarenko I, et al. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab. 2005;90(11):6106–6112.

