Introduction
There has been a paradigm shift in treating acute promyelocytic leukemia (APL) in the past few decades. Differentiating agents, all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), now form the backbone of treatment for APL. Low and intermediate-risk APL is associated with excellent long-term outcomes when treated with ATO and ATRA. However, the treatment of high-risk APL remains debatable due to the high early mortality from respiratory failure due to differentiation syndrome and severe bleeding due to disseminated intravascular coagulation [Citation1]. Therefore, the current recommendations include adding anthracycline or gemtuzumab ozogamicin (GO) to ATO + ATRA to control leukocytosis in high-risk APL [Citation2]. Few studies have reported treating high-risk APL with only ATO + ATRA. One pilot study reported treating 20 high-risk APL patients with oral ATO + ATRA without anthracycline/GO, though cytarabine was used for cytoreduction in 80% of patients [Citation3]. Vaid et al. recently reported using ATO + ATRA to treat a subset (n = 13/21) of high-risk APL (WBC 10–50 × 109/L) due to the presence of infections at presentation. This cohort had a mortality rate of 43% [Citation4]. Even with anthracyclines, the mortality rate is 23–34% in the intention-to-treat analysis of high-risk APL patients in India [Citation4–7]. GO is unavailable in India. Our center protocol includes ATO + ATRA for all risk classes with additional hydroxyurea as cytoreductive therapy in high-risk APL patients [Citation8]. Here we describe the efficacy and safety of high-dose hydroxyurea for cytoreduction in ultra-high-risk APL [Citation9].
Methods
After obtaining the institutional ethics committee approval, we analyzed all consecutive ultra-high-risk APL patients presenting with a WBC count >30 × 109/L between 2016 and 2021. All patients were started on ATO at a dose of 0.15 mg/kg/day given intravenously and ATRA at a dose of 45 mg/m2/day in two divided doses orally at the suspicion of APL [Citation8]. The diagnosis was confirmed by polymerase chain reaction for PML-RARα. All patients also received oral prednisolone at a dose of 0.5–1 mg/kg/day for 14 days, followed by a taper over one week. Hydroxyurea 50–100 mg/kg/day in divided doses was used for cytoreduction during induction in all patients. High-dose hydroxyurea (HDH) (off-label, no dose limit) was given to a subset of patients at the physician’s discretion. HDH was defined as a daily dose of >100 mg/kg/day with a minimum cumulative dose of 15 g of hydroxyurea during the hospital stay. The maximum dose of hydroxyurea given to patients was 2 g per oral every 2 h for the initial 12–24 h (Vancouver protocol). Hydroxyurea dose was reduced to 50–100 mg/kg/day for a WBC count <30 × 109/L and stopped once the WBC count was <5 × 109/L. Post-induction therapy, patients underwent a bone marrow examination to document remission. After remission, patients received three cycles of consolidation, each one month apart. Each consolidation cycle constituted ATO at 0.15 mg/kg/day and ATRA at 45 mg/m2/day for four weeks. After the third consolidation cycle, patients received maintenance therapy with ATRA for 15 days every three months, daily 6-mercaptopurine, and weekly oral methotrexate for one year. Molecular monitoring for PML-RARα was done using quantitative polymerase chain reaction at periodic intervals. The records for all patients were reviewed for demographic details, presenting complaints, disease and treatment characteristics, maximum hydroxyurea dose utilized, cumulative hydroxyurea dose, and adverse effects, including liver dysfunction and mucositis. Information regarding relapse, death during follow-up, and cause of death were also noted. Early death was defined as death due to any cause occurring within seven days of presentation and therapy initiation. Patient, disease, and treatment outcomes were compared between patients who received HDH and no HDH. Categorical variables were compared using Chi-square or Fisher exact test. Continuous variables were compared using the independent t-test for normally distributed and the Mann-Whitney test for skewed variables. The Kaplan-Meier method was used to compare overall survival (OS). A p-value <0.05 was taken as significant.
Results
Thirty-two ultra-high-risk APL patients with WBC count >30 × 109/L (15 HDH and 17 no-HDH) were included in this analysis. Both the groups were matched for age (median 23 years (12–48) vs. 35 years (12–56), p = 0.2) and gender (). The median WBC count was 81.7 × 109/L (31.1–188.8) and 58.3 × 109/L (33.4–170.9) (p = 0.3) in both groups respectively, with an equal proportion of patients with WBC >50 × 10/L (73% vs. 53%, p = 0.3) and >100 × 10/L (47% vs. 24%, p = 0.3). The proportion of patients presenting with coagulopathy (53% vs. 77%, p = 0.2), major bleed (13% vs. 29%, p = 0.4) and intracranial bleed (13% vs. 18%, p = 1.0) were similar in both the groups. So was the spontaneous differentiation syndrome similar in both groups (33% vs. 29% p = 0.8).
Table 1. Comparison of high-risk APL patients treated with and without high-dose hydroxyurea.
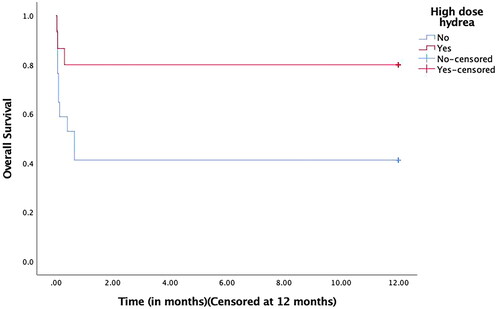
The median cumulative hydroxyurea dose was 57 g (15–112) and 13 g (5–51) in both groups (p < 0.001). The median number of days of hydroxyurea was similar in both patient groups (seven vs. three days, p = 0.3). Patients who received HDH had significantly lower early mortality (13% vs. 53%; p = 0.02). None of the patients in either group developed grade 3–4 mucositis. There appeared to be a higher incidence of grade 3–4 hepatotoxicity in the HDH group which may be attributed to high-dose hydroxyurea, but the difference was insignificant (27% vs. 6%, p = 0.1). The median number of days to achieve a complete hematological response was similar in the two groups (26 vs. 32 days, p = 0.4). The median follow-up for the cohort was 22 months. There have been no molecular relapses in either group till the last follow-up. The 30-day survival rate in the HDH group was 80% (95% CI- 60.4–99.6) in comparison to 41% (95% CI- 17.5–64.5) to the no-HDH group. The one-year OS was significantly higher in the HDH group (80% vs. 41%, p = 0.02) ().
Discussion
Our results show that high-dose hydroxyurea can be effectively and safely used for cytoreduction in patients with ultra-high-risk APL. The rationale for using a short period of high-dose hydroxyurea is near 100% oral bioavailability, short time to peak action (1–4 h) and half-life (2–4 h), and its concentration in leukocytes [Citation10]. These characteristics make it preferable to anthracyclines as a cytoreductive agent associated with a longer duration of neutropenia and a higher risk of infections. The off-label dose of hydroxyurea for cytoreduction in AML ranges from 50 to 100 mg/kg/day [Citation11]. The maximum tolerated dose of hydroxyurea using continuous infusion is 27 g/m2/day [Citation12]. Kim et al. recently described the safety and feasibility of hydroxyurea (no dose limit, maximum 17.5 g/day) for cytoreduction in patients with newly diagnosed acute myeloid leukemia (AML) before definitive therapy [Citation13]. In resource-challenged settings, HDH is an effective option with low mortality rates than those reported without its use [Citation4] or use of anthracyclines [Citation5–7] for the treatment of ultra-high-risk APL. With the limitation of small, retrospective analysis, our study suggests exploring the role of high-dose hydroxyurea for cytoreduction in combination with ATO + ATRA prospectively in managing ultra-high-risk APL.
Ethical approval
Ethical approval was obtained from Institute Ethics Committee prior to submission to the journal
Acknowledgements
The authors would like to acknowledge C-Heart-C for providing ATO and ATRA free of cost to the patients
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data will be made available on reasonable request by contacting the corresponding author.
Written consent was obtained from the patients for treatment and publication.
References
- Stahl M, Tallman MS. Acute promyelocytic leukemia (APL): remaining challenges towards a cure for all. Leuk Lymphoma. 2019;60(13):3107–3115.
- Yilmaz M, Kantarjian H, Ravandi F. Acute promyelocytic leukemia current treatment algorithms. Blood Cancer J. 2021;11(6):123.
- Zhu H-H, Liu Y-R, Jia J-S, et al. Oral arsenic and all-trans retinoic acid for high-risk acute promyelocytic leukemia. Blood. 2018;131(26):2987–2989.
- Vaid T, Aggarwal M, Dass J, et al. Shifting gears to differentiation agents in acute promyelocytic leukemia with resource constraints-a cohort study. Acta Oncol. 2022;2022:1–6.
- Kulkarni UP, Selvarajan S, Lionel S, et al. Real world data with concurrent retinoic acid and arsenic trioxide for the treatment of acute promyelocytic leukemia. Blood Cancer J. 2022;12(1):22.
- Kapoor J, Mirgh SP, Agrawal N, et al. High risk acute promyelocytic Leukemia – an enigma for hematologists: optimizing treatment with APML-4 protocol. Indian J Hematol Blood Transfus. 2022;38(2):394–402.
- Yedla RP, Bala SC, Pydi VR, et al. Outcomes in adult acute promyelocytic leukemia: a decade experience. Clin Lymphoma Myeloma Leuk. 2020;20(4):e158–e64.
- Varma S, Yanamandra U, Khadwal A, et al. High risk apml treated successfully with four cycles of ATO and ATRA combination in resource constrained settings. Blood. 2015;126(23):3322–3322.
- Lou Y, Ma Y, Sun J, et al. Effectivity of a modified Sanz risk model for early death prediction in patients with newly diagnosed acute promyelocytic leukemia. Ann Hematol. 2017;96(11):1793–1800.
- Rodriguez GI, Kuhn JG, Weiss GR, et al. A bioavailability and pharmacokinetic study of oral and intravenous hydroxyurea. Blood. 1998;91(5):1533–1541.
- Grund FM, Armitage JO, Burns P. Hydroxyurea in the prevention of the effects of leukostasis in acute leukemia. Arch Intern Med. 1977;137(9):1246–1247.
- Gandhi V, Plunkett W, Kantarjian H, et al. Cellular pharmacodynamics and plasma pharmacokinetics of parenterally infused hydroxyurea during a phase I clinical trial in chronic myelogenous leukemia. J Clin Oncol. 1998;16(7):2321–2331.
- Kim K, Konopleva M, DiNardo CD, et al. Urgent cytoreduction for newly diagnosed acute myeloid leukemia patients allows acquisition of pretreatment genomic data and enrollment on investigational clinical trials. Am J Hematol. 2022;97(7):885–894.