Abstract
Aim
Bowel dysfunction after colon cancer (CC) surgery is widely neglected in current follow up programmes. This study explored changes in bowel function and quality of life (QoL) from three (3 m) to twelve months (12 m) after surgery in CC patients undergoing right- or left-sided colon resection (RightSCR/LeftSCR) and investigated differences between the two groups 12 m after surgery.
Method
CC patients undergoing surgical resection in 2018–2020 at five surgical departments were included in this population-based prospective cohort study. Included patients completed electronic surveys consisting of a collection of validated scores 3 m and 12 m after surgery.
Results
A total of 708 CC patients (423 RightSCR, 285 LeftSCR) were included. In RightSCR, no improvement was observed from 3 m to 12 m in most scores/items, on the contrary, symptom worsening in flatus- and faecal incontinence and urgency was observed (p < 0.05). Also, the proportion of patients rating their bowel function as very good/good decreased (p < 0.05) in this group. In LeftSCR improvement was found in flatus and faecal incontinence, urgency and night-time defaecation (p < 0.02), while no improvement was observed in the remaining scores/items. At 12 m, higher proportions of RightSCR than LeftSCR reported loose stools, incontinence and urgency (all p < 0.001), whereas LeftSCR more often reported hard stools and flatus incontinence (p < 0.05). Among all CC patients 18.3% reported bowel-related impairment of QoL at 12 m with no differences between the two groups.
Conclusion
From 3 m to 12 m no significant change was observed in the majority of bowel function and QoL scores/items, however, some symptoms worsened in RightSCR, while a few improved in LeftSCR. Bowel dysfunction and impaired QoL were still common in both groups at 12 m, although the symptom pattern differed between the groups. These findings call for a systematic screening for bowel dysfunction to ensure early treatment of symptoms.
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide and the second most common cause of cancer-related death [Citation1]. Survival rates have increased significantly during the past decades [Citation2,Citation3], and a global trend towards an increasing number of young people being diagnosed with CRC has been observed [Citation4–6]. These trends have produced a rising number of CRC survivors.
Whereas the continuously improving oncological outcomes after colon cancer (CC) treatment have been analysed extensively in prior publications [Citation3,Citation7,Citation8], the long-term functional consequences of treatment have received less attention. Most studies of functionality following surgical CRC treatment have focused on rectal cancer (RC) patients because of a well-documented high prevalence of late sequelae and impaired quality of life (QoL) in this patient group [Citation9–15]. In contrast, functional results after surgical treatment of CC are still poorly documented in the literature. Most of the few previously published studies investigating late sequelae after CC are primarily cross-sectional, include < 100 patients, and often pool CRC patients, making it difficult to identify the specific problems experienced by the subgroup of CC patients [Citation16]. However, three recent studies including large patient numbers [Citation17–19] have demonstrated that a significant proportion of CC patients suffer from severe long-term bowel dysfunction and that the symptom pattern differs between patients undergoing right- and left-sided colon resection. Even so, the specific impact of surgery on CC patients’ bowel function and QoL over time remains unclear and needs to be further investigated.
In this study, we hypothesised that CC patients’ bowel function would improve spontaneously during the first year after surgery and that the symptoms still present at that time would differ between patients undergoing a right-sided colon resection (RightSCR) and a left-sided colon resection (LeftSCR).
Hence, the primary aim of the present study was to explore changes in bowel function and QoL from 3 months (3 m) to 12 months (12 m) after surgery in CC patients undergoing a RightSCR or a LeftSCR.
The secondary aim was to investigate differences in bowel function and QoL 12 m after surgery between these two groups of CC patients.
Methods
Data for the present study were extracted from the database of a large, ongoing, multicentre, population-based, prospective cohort study investigating late sequelae following CRC treatment via patient-reported outcome measures (PROMs). A detailed presentation of the study design has been published previously [Citation20].
In- and exclusion criteria
Eligible for the present study were CC patients older than 18 years who underwent resectional surgery at the surgical departments at Aarhus, Viborg, Randers, Aalborg, and Hjoerring hospitals, which cover 23% of all CC cases in Denmark [Citation21]. Patients were included if they had a right-sided hemicolectomy, an extended right-sided hemicolectomy or a left-sided hemicolectomy which includes resection of the sigmoid colon according to Danish guidelines. Patients undergoing other types of colonic cancer surgery were excluded from the analyses. Patients with advanced tumours, defined as tumour growth beyond the mesocolic fascia, histopathological pT4 tumours with invasion of adjacent structures, or peritoneal carcinomatosis were also excluded, as these patients were enrolled in another scientific study with similar aims. Further exclusion criteria were cognitive impairment, linguistic limitations, participation in another scientific study running counter to the present one, or not living in the region of one of the participating centres. Also, patients were excluded if the project nurse considered it unethical to approach the patients due to late-stage disease/palliative treatment, severe comorbidity, or other major physical/mental limitations.
Patients who completed PROMs at both 3 and 12 months after surgery were included. We excluded patients from the analyses if the survey was completed outside the accepted time range, defined as less than two months or more than seven months after surgery for the 3 m survey, and less than ten months or more than 16 months after surgery for the 12 m-survey.
Data and data sources
Electronic PROMs were distributed via ‘e-Boks’, a secure public electronic mail system provided by the Danish government [Citation22]. A paper version of the PROMs was distributed by ordinary mail to patients not capable of/willing to respond electronically. Clinical variables were extracted from the national clinical database of the Danish Colorectal Cancer Group (DCCG) [Citation23]. In this database, > 95% of all patients with a first-time CRC are registered, and the database has proven to provide highly valid and complete data [Citation24]. Information on post-operative chemotherapy was extracted from the patient charts, whereas presence of a stoma was reported by the patients.
The PROM collection consisted of the Bristol Stool Form Scale [Citation25,Citation26], the Patient Assessment of Constipation Symptoms (PAC-SYM) questionnaire [Citation27,Citation28], the Low Anterior Resection Syndrome (LARS) score [Citation29], the St. Marks Faecal Incontinence/Vaizey score [Citation30], the EuroQoL 5 Dimensions-5 levels (EQ5D-5L) score [Citation31–33]. Furthermore, five ad hoc items not covered by the above-mentioned validated scores were added. All validated scores and ad hoc items are presented in detail in Appendix A ().
Statistical analyses
Type of surgery was dichotomised into two groups: RightSCR (right-sided hemicolectomies and extended right-sided hemicolectomies) and LeftSCR (left-sided hemicolectomies and sigmoid resections).
Clinical and sociodemographic characteristics of the included patients were presented separately for RightSCR and LeftSCR, with numbers (n) (%) for categorical variables and mean (Standard Deviation (SD)) for continuous variables.
Bowel function and QoL at 3 m and 12 m were presented separately for RightSCR and LeftSCR with n (%) for categorical variables and median (interquartile range (IQR)) for continuous variables. Differences between the two time points within the groups were tested using McNemar’s test or the Wilcoxon Signed Rank Test, depending on the type and distribution of the variables.
Differences between RightSCR and LeftSCR at 12 m were tested using the Wilcoxon Rank Sum Test and Fisher’s Exact test, as appropriate.
For categorical variables with three response options, each response option was tested against the total of the two other response options.
Differences in means and proportions were presented with a 95% confidence interval (CI).
The distribution of PAC SYM variables was right-skewed and dominated by zeros. Therefore, the median and IQR were reported in and . We reported the mean change of PAC SYM from 3 m to 12 m in along with their 95% CIs, which were calculated using the bootstrap method. The p-values were calculated using the sign-rank test. For comparison of RightSCR and LeftSCR of the PAC SYM variables, we used quantile regression for medians and differences between the medians and their 95% CI along with the p-value were reported.
Table 2. Change in bowel function from 3 m to 12 m after surgery, for patients undergoing a right-sided or a left-sided colon resection (n = 662).
Table 3. Bowel symptoms in right- and left-sided colon resections 12 months after surgery (n = 677).
Due to the high number of included variables, only symptoms showing statistically significant differences are presented in the text, while all results are shown in tables.
A multivariate logistic regression analysis was conducted to investigate the association of selected variables with the ad hoc item ‘Overall, how much does your bowel function affect your quality of life?’ at 12 m. For this purpose, the response options were dichotomised into ‘not at all/a little’ versus ‘somewhat/very much’. Age, gender, body mass index, type of surgery, surgical approach, chemotherapy, pathological Union for International Cancer Control (pUICC) stage, WHO performance status, Charlson comorbidity index and presence of a stoma at 12 m were included as independent/explanatory variables. Since bowel function is a part of the binary outcome of the regression analyses (‘How much does bowel function affect your QoL’) bowel function was not included among the explanatory variables.
Ethical approvals
Participants signed an informed consent form after receiving information about the study. This survey-based study did not require consent from the National Committee on Health Research Ethics in Denmark. It was registered with the Danish Data Protection Agency as Health Science Research of the Central Denmark Region.
Results
In- and exclusions
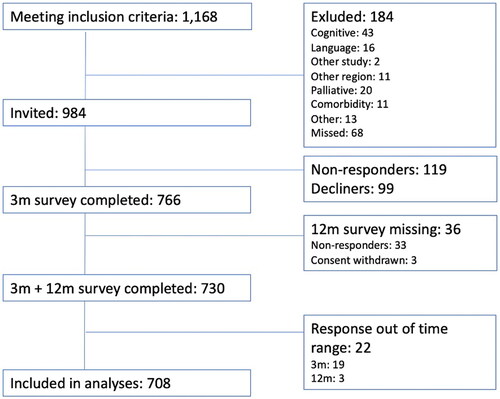
A flow chart of included and excluded patients is presented in .
Among 1,168 CC patients undergoing a RightSCR or a LeftSCR at the participating centres during the inclusion period, 184 were excluded for reasons shown in . Among the invited 984 CC patients, 766 accepted to participate and completed the survey at 3 m, corresponding to an inclusion rate of 77.8%. After having completed the 3 m survey, another 58 patients were excluded (). Hence, a total of 708 patients of the initially eligible patients were included in the final analyses (72.0%).
Patient characteristics
shows the clinical and sociodemographic characteristics of the 708 patients, among whom 423 (59.7%) had undergone a RightSCR and 285 (40.3%) a LeftSCR.
Table 1. Patient characteristics (n = 708).
The median (IQR) time between surgery and completion of the 3 m survey was 105 (97–119) days; for the 12 m survey, 373 (366–384) days.
Change in bowel function and QoL from 3 m to 12 m
A total of 46 patients with a stoma at 3 m and/or 12 m were excluded from these paired analyses. The results for the remaining 662 patients are presented in .
RightSCR: no change or symptom worsening
In RightSCR, no changes were observed in most scores/items from 3 m to 12 m. However, patients having undergone RightSCR experienced a statistically significant symptom worsening from 3 m to 12 m in the rectal subscale of the PAC-SYM (mean diff (95% CI) 0.07 (0.02;0.13), p = 0.01). However, as the minimal important difference (MID) for PAC-SYM has previously been established to be 0.6 points [Citation28] the size of the difference is considered clinically irrelevant.
A worsening of three other symptoms was also observed among patients having undergone RightSCR. The proportion of patients never experiencing flatus incontinence decreased from 3 m to 12 m (38.6% versus 33.1%, p = 0.03. The proportion of patients never experiencing incontinence for liquid stool declined from 62.3% at 3 m to 54.9% at 12 m (p = 0.01). The proportion of patients who never experienced faecal urgency decreased from 35.2% at 3 m to 30.2% at 12 m (p = 0.048). As shown in , it seems that the patients are ‘moving down the scale’, which corresponds to a worsening of symptoms.
Also, the proportion of patients who rated their bowel function as ‘Very good/good’ decreased from 68.8% to 63.7% (p = 0.047).
LeftSCR: no change or symptom improvement
In patients who had received LeftSCR, no changes were observed in most scores/items from 3 m to 12 m. However, from 3 m to 12 m, an improvement was observed in five bowel function scores/items. A decrease from 3 m to 12 m was observed in the proportion of patients reporting weekly flatus incontinence (47.2% versus 39.0%, p = 0.02). Likewise, the proportion reporting weekly liquid stool incontinence decreased from 11.4% to 2.9% (p < 0.001), weekly urgency decreased from 33.5% to 22.2% (p < 0.001), and weekly night-time defaecation from 13.0% to 7.7% (p = 0.01). As shown in , it seems that the patients are ‘moving up the scale’, which corresponds to an improvement of symptoms.
Also, the St. Mark’s Faecal Incontinence Score decreased (mean difference (95% CI) −0.61 (−1.10;−0.13), (p = 0.01).
Differences between RightSCR and LeftSCR at 12 m
A total of 31 patients with a stoma at 12 m were not included in these comparative analyses. Among the remaining 677 patients, differences between RightSCR and LeftSCR were found in some bowel symptoms at 12 m. These symptoms are highlighted in the last column of .
Symptoms more prevalent in RightSCR than in LeftSCR at 12 m
At 12 m, a higher proportion of RightSCR than LeftSCR patients had Bristol Stool Type 6–7 (loose stools) (20.1% versus 5.1%) (p < 0.001). Also, RightSCR patients had a poorer function than LeftSCR patients had with regards to the two LARS Score items a) incontinence for liquid stool (‘never’ in 55.0% versus 68.7%, p < 0.001) and b) urgency (‘never’ in 29.8% versus 46.9%, p < 0.001) (). Despite these differences, the proportion of patients using constipating agents was similar in the two groups (5.5% versus 5.0%, p = 0.81).
Symptoms more prevalent in LeftSCR than in RightSCR at 12 m
At 12 m, hard stool consistency (Bristol Stool Chart Type 1–2) was reported by 10.2% of LeftSCR patients and by 6.0% of RightSCR patients (p = 0.046).
LeftSCR patients scored higher (poorer function) in the Abdominal subscale of the PAC-SYM, with a mean difference (95% CI) of −0.25 (−0.34; −0.16) (p < 0.001) and higher in the Stool subscale of the PAC-SYM, with a mean difference (95%CI) −0.40 (−0.50; −0.30) (p = < 0.001). However, these small differences are considered clinically irrelevant.
Finally, a smaller proportion of LeftSCR patients (24.0%) than RightSCR patients (32.9%) reported never experiencing flatus incontinence (p = 0.01).
For the remaining scores and items, no differences were found between RightSCR and LeftSCR patients at 12 m.
Factors associated with impact of bowel function on QoL at 12 m
Among all patients, seven did not respond to the item ‘Overall, how much does your bowel function affect your quality of life?’ at 12 m. Hence, they were not included in the logistic regression analyses exploring the association between selected clinical variables and impact on QoL. Among the remaining 701 patients, 128 (18.3%) responded ‘Somewhat’ or ‘Very much’ to this item at 12 m.
The multivariate logistic regression analysis revealed that age younger than 64 years and having a stoma at 12 m were the only two independent risk factors for reporting ‘Somewhat’ or ‘Very much’ impact of bowel function on QoL at 12 m ().
Table 4. Associations between demographic/clinical variables and QoL (N = 701).
Discussion
This is the first large population-based prospective cohort study using validated PROMs to investigate changes in bowel function and QoL during the first year after CC surgery. An inclusion rate of 72% was obtained, comprising 708 CC patients finally included in the analyses.
Main findings; change from 3 m to 12 m
We found that the majority of RightSCR patients’ and LeftSCR patients’ symptoms remained stable from 3 m to 12 m after surgery. However, some symptoms worsened in RightSCR patients, whereas some symptoms improved in LeftSCR patients. This might be explained by the fact that many RightSCR patients develop bile acid malabsorption and/or small intestinal bacterial overgrowth, which may be the driver of their symptoms [Citation34]. Resection of the ileocecal valve may allow colonic bacteria to colonise the distal small intestine. Typical symptoms of small intestinal bile acid malabsorption are diarrhoea, bloating, pain or, in severe cases, malabsorption. Additionally, resection of even a few centimetres of the terminal ileum may cause bile acid malabsorption from lack of resorptive capacity for bile acids or impaired small intestinal production of the enteric hormone FGF-19. This may be further aggravated by bacterial deconjungation, and thereby reduced resorption, of bile acids. Usually almost 98% of bile acids are resorbed in the distal ileum. When excessive amounts of bile acids reach the colon, they cause secretion and enhance peristalsis resulting in bile acid diarrhoea. In a recent study, we found that bile acid diarrhoea was the most common cause of diarrhoea after surgery for RightSCR. The condition is unlikely to improve without a specific diagnosis and treatment. LeftSCR patients, on the other hand, are more often bothered by ‘LARS-like’ symptoms, which may possibly improve over time with regeneration and healing of the nervous supply to the defaecation reflexes and by patients changing their diet and toilet habits (timing and positioning) to cope with their new situation [Citation35,Citation36].
Main findings; differences between groups at 12 m
In general, a high prevalence of bowel symptoms was found at 12 m in both groups, but with inter-group differences in the prevalence of the individual symptoms. A significantly larger proportion of RightSCR patients reported loose stools, liquid stool incontinence, and urgency, whereas a larger proportion of LeftSCR patients reported hard stools and flatus incontinence. Although symptom patterns differed between the two groups at 12 m, no statistically significant differences were found in self-rated bowel function or impact of bowel function on QoL. This might indicate that any kind of severe bowel problems may affect QoL, independently of the specific type of symptoms.
Of major interest, we found that 18.3% of all patients reported bowel-related impaired QoL 12 m after surgery; a result which corresponds well with previous publications, reporting that QoL was impaired in one of every five patients [Citation18,Citation37]. Whereas the multivariate logistic regression analyses showed that type of surgery was not associated with impaired QoL, we found that younger age and having a stoma were highly associated with bowel-related impaired QoL 12 m after surgery. Again, our results are in line with those of several other studies showing that these factors are closely associated [Citation38–40], although firm conclusions regarding the association between having a stoma and generic cancer-related QoL measures like the FACT-C and the EORTC QOL tools have previously proven to be difficult to draw [Citation41].
Another finding is that although a significantly higher proportion of RightSCR patients than LeftSCR patients have loose stools, no differences were observed between the groups regarding the use of constipating agents or fibre supplements. This may possibly be explained by bowel dysfunction being underdiagnosed, and the finding highlights the importance of routine screening and treatment of identified late sequelae not only in RC patients but also in CC patients. Although fewer CC patients than RC patients are affected by bowel dysfunction, the incidence of CC is approximately twice as high as RC, i.e., the total number of CC patients affected by bowel dysfunction is likely to be as high as the total number of affected RC patients.
Possible consequences of bowel dysfunction after CC
Previous studies have demonstrated that bowel dysfunction may have a significant impact on patients’ overall QoL [Citation17,Citation42,Citation43]. In addition, recent studies have shown that CC patients’ affiliation with the labour market is negatively affected up to ten years after surgery [Citation44,Citation45]. Although the underlying causes for this remain unclear, it seems reasonable to assume that severe bowel dysfunction may play a role; a hypothesis that must be explored in future, appropriately designed studies. Such studies are increasingly relevant as the prevalence of working-age CRC survivors is growing because of an increase in early-onset CRC, improved survival and rising retirement age in many countries.
Future perspectives
Whereas late sequelae after RC have gained increasing attention in recent decades, CC patients’ late sequelae remain largely neglected. The present and previous studies [Citation16–18] have shown that many CC patients suffer from long-term bowel dysfunction and that most bowel symptoms, particularly after right-sided resections, do not seem to resolve spontaneously. Since many bowel symptoms may be treated effectively by simple measures readily available in modern healthcare systems [Citation46,Citation47] a cost-effective systematic screening for bowel dysfunction via electronic PROMs and early intervention in the affected patients are advisable to improve patients’ QoL [Citation48]. Frequently, diarrhoea after RightSCR may be treated effectively once the correct diagnosis has been established, which is often bile acid malabsorption or small bowel bacterial overgrowth [Citation37,Citation46,Citation49]. Also, ‘LARS-like’ symptoms, chronic constipation, and obstructed defaecation, which are common after LeftSCR, may be managed effectively in dedicated late sequelae clinics [Citation50].
Limitations
This study has some limitations. Firstly, for various reasons, 28% of the initially eligible patients were not included in the analyses. Consequently, the results might not be representative of the whole CC population. Secondly, the included patients were all from Denmark, a country with highly centralised care of cancer patients. Hence, all patients were operated in high-volume, specialised tertiary referral centres. This might affect the generalisability, making it difficult to extrapolate the rates of bowel dysfunction of our patients to those from countries with differently organised healthcare systems. Also, the impact on QoL of a stoma or severe bowel dysfunction may differ significantly across cultures [Citation39,Citation40]. However, we believe that the results of this study are largely generalisable to countries managing cancer treatment in a healthcare system similar to the Danish.
Another limitation is the lack of information on level of division of the vessels and central lymph node resection (D2/D3). Currently, we do not have a clear-cut definition of how to perform D3 resection, and hence, this information has not been registered. Therefore, we could not distinguish between the groups. Nevertheless, it would be relevant to investigate in future studies.
Conclusion
Among CC patients undergoing a RightSCR, bowel symptoms and QoL did not improve spontaneously during the first year after surgery. Among patients undergoing a LeftSCR, some symptoms improved. Still, 12 m after surgery both RightSCR patients and LeftSCR patients were affected by bowel dysfunction, and 18% reported that it impacted their QoL negatively. These findings warrant implementation of an early systematic screening for and treatment of bowel dysfunction in standard CC follow up programmes.
Ethical approval
This survey-based study did not require consent from the National Committee on Health Research Ethics in Denmark. It was registered with the Danish Data Protection Agency as Health Science Research of the Central Denmark Region.
Patient consent
Participants signed an informed consent form after receiving information about the study.
Acknowledgements
The authors wish to acknowledge the nurses Bibi Damsgaard, Rikke Krogh Demming, Gitte Kjær Sørensen, Margit Majgaard, Karen Irene Jacobsen, Dorte Kløve Kjær and Helle Vindfeldt Rasmussen for their great efforts in collecting data for this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, TJ, upon reasonable request.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492.
- NORDCAN. 2022. [cited 2021 Sep 15]. https://nordcan.iarc.fr/en/dataviz/trends?mode=cancer&group_populations=1&multiple_cancers=1&sexes=1_2&cancers=520&types=0_1.
- Araghi M, Arnold M, Rutherford MJ, et al. Colon and rectal cancer survival in seven high-income countries 2010-2014: variation by age and stage at diagnosis (the ICBP SURVMARK-2 project). Gut. 2021;70(1):114–126. doi: 10.1136/gutjnl-2020-320625.
- Chambers AC, Dixon SW, White P, et al. Demographic trends in the incidence of young-onset colorectal cancer: a population-based study. Br J Surg. 2020;107(5):595–605. doi: 10.1002/bjs.11486.
- Vuik FE, Nieuwenburg SA, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68(10):1820–1826. doi: 10.1136/gutjnl-2018-317592.
- Saad El Din K, Loree JM, Sayre EC, et al. Trends in the epidemiology of young-onset colorectal cancer: a worldwide systematic review. BMC Cancer. 2020;20(1):288. doi: 10.1186/s12885-020-06766-9.
- Bertelsen CA, Neuenschwander AU, Jansen JE, et al. 5-Year outcome after complete mesocolic excision for right-sided Colon cancer: a population-based cohort study. Lancet Oncol. 2019;20(11):1556–1565. doi: 10.1016/S1470-2045(19)30485-1.
- van den Berg I, Coebergh van den Braak RRJ, van Vugt JLA, et al. Actual survival after resection of primary colorectal cancer: results from a prospective multicenter study. World J Surg Oncol. 2021;19(1):96. doi: 10.1186/s12957-021-02207-4.
- Juul T, Ahlberg M, Biondo S, et al. Low anterior resection syndrome and quality of life: an international multicenter study. Dis Colon Rectum. 2014;57(5):585–591. doi: 10.1097/DCR.0000000000000116.
- Feddern ML, Emmertsen KJ, Laurberg S. Quality of life with or without sphincter preservation for rectal cancer. Colorectal Dis. 2019;21(9):1051–1057. doi: 10.1111/codi.14684.
- Feddern M-L, Jensen TS, Laurberg S. Chronic pain in the pelvic area or lower extremities after rectal cancer treatment and its impact on quality of life: a population-based cross-sectional study. Pain. 2015;156(9):1765–1771. doi: 10.1097/j.pain.0000000000000237.
- Bregendahl S, Emmertsen KJ, Lindegaard JC, et al. Urinary and sexual dysfunction in women after resection with and without preoperative radiotherapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis. 2015;17(1):26–37. doi: 10.1111/codi.12758.
- Sun R, Dai Z, Zhang Y, et al. The incidence and risk factors of low anterior resection syndrome (LARS) after sphincter-preserving surgery of rectal cancer: a systematic review and meta-analysis. Support Care Cancer. 2021;29(12):7249–7258. doi: 10.1007/s00520-021-06326-2.
- Thyo A, Laurberg S, Emmertsen KJ. Impact of bowel and stoma dysfunction on female sexuality after treatment for rectal cancer. Colorectal Dis. 2020;22(8):894–905. doi: 10.1111/codi.14987.
- Pappou EP, Temple LK, Patil S, et al. Quality of life and function after rectal cancer surgery with and without sphincter preservation. Front Oncol. 2022;12:944843. doi: 10.3389/fonc.2022.944843.
- Verkuijl SJ, Jonker JE, Trzpis M, et al. Functional outcomes of surgery for Colon cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2021;47(5):960–969. doi: 10.1016/j.ejso.2020.11.136.
- Elfeki H, Larsen HM, Emmertsen KJ, et al. Bowel dysfunction after sigmoid resection for cancer and its impact on quality of life. Br J Surg. 2019;106(6):805. doi: 10.1002/bjs.10979.
- Larsen HM, Elfeki H, Emmertsen KJ, et al. Long-term bowel dysfunction after right-sided hemicolectomy for cancer. Acta Oncol. 2020;59(10):1240–1245. doi: 10.1080/0284186X.2020.1772502.
- Buchli C, Martling A, Sjovall A. Low anterior resection syndrome after right- and left-sided resections for colonic cancer. BJS Open. 2019;3(3):387–394. doi: 10.1002/bjs5.50128.
- Juul T, Brauner AB, Drewes AM, et al. Systematic screening for late sequelae after colorectal cancer-a feasibility study. Colorectal Dis. 2021;23(2):345–355. doi: 10.1111/codi.15519.
- DCCG. Danish Colorectal Cancer Group (DCCG) National annual report 2020. 2022. [cited 2022 Jun 30]. https://dccg.dk/wp-content/uploads/2021/10/DCCG-Aarsrapport-2020-publiceret.pdf.
- e-Boks.dk. 2022. [cited 2022 Jun 25]. https://www.e-boks.com/danmark/en/.
- Ingeholm P, Gogenur I, Iversen LH. Danish colorectal cancer group database. Clin Epidemiol. 2016;8:465–468. doi: 10.2147/CLEP.S99481.
- Klein MF, Gogenur I, Ingeholm P, et al. Validation of the Danish colorectal cancer group (dccg.dk) database - on behalf of the Danish colorectal cancer group. Colorectal Dis. 2020;22(12):2057–2067. doi: 10.1111/codi.15352.
- O'Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ. 1990;300(6722):439–440. doi: 10.1136/bmj.300.6722.439.
- Blake MR, Raker JM, Whelan K. Validity and reliability of the bristol stool form scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44(7):693–703. Oct doi: 10.1111/apt.13746.
- Frank L, Kleinman L, Farup C, et al. Jr. Psychometric validation of a constipation symptom assessment questionnaire. Scandinavian Journal of Gastroenterology. 1999;34(9):870–877.
- Yiannakou Y, Tack J, Piessevaux H, et al. The PAC-SYM questionnaire for chronic constipation: defining the minimal important difference. Aliment Pharmacol Ther. 2017;46(11–12):1103–1111. doi: 10.1111/apt.14349.
- Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012;255(5):922–928. doi: 10.1097/SLA.0b013e31824f1c21.
- Vaizey CJ, Carapeti E, Cahill JA, et al. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44(1):77–80. doi: 10.1136/gut.44.1.77.
- Jensen MB, Jensen CE, Gudex C, et al. Danish population health measured by the EQ-5D-5L. Scand J Public Health. 2023;51(2):241–249. 14034948211058060. doi: 10.1177/14034948211058060.
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14(6):1523–1532. doi: 10.1007/s11136-004-7713-0.
- Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70.
- Larsen HM, Krogh K, Borre M, et al. Chronic loose stools following right-sided hemicolectomy for Colon cancer and the association with bile acid malabsorption and small intestinal bacterial overgrowth. Colorectal Dis. 2023;25(4):600–607. doi: 10.1111/codi.16409.
- Varghese C, Wells CI, O'Grady G, et al. The longitudinal course of low-anterior resection syndrome: an individual patient meta-analysis. Ann Surg. 2022;276(1):46–54. doi: 10.1097/SLA.0000000000005423.
- Laursen BS, Sorensen GK, Majgaard M, et al. Coping strategies and considerations regarding low anterior resection syndrome and quality of life among patients with rectal cancer; a qualitative interview study. Front Oncol. 2022;12:1040462. doi: 10.3389/fonc.2022.1040462.
- Hope C, Reilly J, Lund J, et al. Systematic review: the effect of right hemicolectomy for cancer on postoperative bowel function. Support Care Cancer. 2020;28(10):4549–4559. doi: 10.1007/s00520-020-05519-5.
- Vonk-Klaassen SM, de Vocht HM, den Ouden ME, et al. Ostomy-related problems and their impact on quality of life of colorectal cancer ostomates: a systematic review. Qual Life Res. 2016;25(1):125–133. doi: 10.1007/s11136-015-1050-3.
- Krogsgaard M, Kristensen HO, Furnee EJB, et al. Life with a stoma across five european countries-a cross-sectional study on long-term rectal cancer survivors. Support Care Cancer. 2022;30(11):8969–8979. doi: 10.1007/s00520-022-07293-y.
- Kristensen HO, Thyo A, Emmertsen KJ, et al. Surviving rectal cancer at the cost of a colostomy: global survey of long-term health-related quality of life in 10 countries. BJS Open. 2022;6(6):zrac085. doi: 10.1093/bjsopen/zrac085.
- Lawday S, Flamey N, Fowler GE, et al. Quality of life in restorative versus non-restorative resections for rectal cancer: systematic review. BJS Open. 2021;5(6):zrab101. doi: 10.1093/bjsopen/zrab101.
- Pieniowski EHA, Nordenvall C, Palmer G, et al. Prevalence of low anterior resection syndrome and impact on quality of life after rectal cancer surgery: population-based study. BJS Open. 2020;4(5):935–942. doi: 10.1002/bjs5.50312.
- Pieniowski EHA, Palmer GJ, Juul T, et al. Low anterior resection syndrome and quality of life after Sphincter-Sparing rectal cancer surgery: a long-term longitudinal follow-up. Dis Colon Rectum. 2019;62(1):14–20. doi: 10.1097/DCR.0000000000001228.
- Pedersen P, Laurberg S, Andersen NT, et al. Differences in work participation between incident Colon and rectal cancer patients-a 10-year follow-up study with matched controls. J Cancer Surviv. 2022;16(1):73–85. doi: 10.1007/s11764-021-01005-x.
- Juul T, Laurberg S, Andersen NT, et al. Labor market attachment 2 years after colorectal cancer Surgery - A population-based study. Annals of Surgery Open. 2022;3(1)(1):e134. doi: 10.1097/AS9.0000000000000134.
- Larsen HM, Borre M, Christensen P, et al. Clinical evaluation and treatment of chronic bowel symptoms following cancer in the Colon and pelvic organs. Acta Oncol. 2019;58(5):776–781. doi: 10.1080/0284186X.2018.1562211.
- Jackson A, Lalji A, Kabir M, et al. The efficacy of a low-fat diet to manage the symptoms of bile acid malabsorption - outcomes in patients previously treated for cancer. Clin Med. 2017;17(5):412–418. doi: 10.7861/clinmedicine.17-5-412.
- Laurberg S, Juul T, Christensen P, et al. Time for a paradigm shift in the follow-up of colorectal cancer. Colorectal Dis. 2021;23(2):341–344. doi: 10.1111/codi.15401.
- Yde J, Larsen HM, Laurberg S, et al. Chronic diarrhoea following surgery for Colon cancer-frequency, causes and treatment options. Int J Colorectal Dis. 2018;33(6):683–694. doi: 10.1007/s00384-018-2993-y.
- Haas S, Mikkelsen AH, Kronborg CJS, et al. Management of treatment-related sequelae following colorectal cancer. Colorectal Dis. 2023;25(3):458–488. doi: 10.1111/codi.16299.
Appendix A
Table A1. PROMs included in the survey.