Abstract
Objective: Endometriosis affects up to 10% of women of reproductive age, and the main goal of treatment is to relieve symptoms. Progestins have been the mainstay of endometriosis suppression, of which dienogest has become an important option in many parts of the world. This is an expert literature review, with recommendations on the use of dienogest in the context of various clinical considerations when treating endometriosis.
Methods: A search of PubMed was conducted for papers published between 2007 and 2019 on the use of dienogest in endometriosis. Experts reviewed these and included those they considered most relevant in clinical practice, according to their own clinical experience.
Results: Evidence regarding the long-term use (>15 months) of dienogest for the management of endometriosis is presented, with experts concluding that the efficacy of dienogest should be assessed primarily on its impact on pain and quality of life. Fertility preservation, the option to avoid or delay surgery, and managing bleeding irregularities that can occur with this treatment are also considered. Counseling women on potential bleeding risks before starting treatment may be helpful, and evidence suggests that few women discontinue treatment for this reason, with the benefits of treatment outweighing any impact of bleeding irregularities.
Conclusions: Overall, the evidence demonstrates that dienogest offers an effective and tolerable alternative or adjunct to surgery and provides many advantages over combined hormonal contraceptives for the treatment of endometriosis. It is important that treatment guidelines are followed and care is tailored to the woman’s individual needs and desires.
Introduction
Endometriosis is defined as the presence of endometrial-like tissue outside the uterusCitation1–3, causing inflammation and pain, and resulting in scar tissue and adhesionsCitation3. However, the exact prevalence is unknown owing to misdiagnosis, diagnostic delay, and the asymptomatic nature of disease in some casesCitation2,Citation4,Citation5. Endometriosis is estimated to affect up to 10% of women of reproductive ageCitation5 and up to 50% of women with subfertilityCitation6. Pain is considered to be the defining symptom of endometriosis and can include dysmenorrhea, noncyclic pelvic pain, and/or dyspareuniaCitation2,Citation7. Endometriosis can be both physically and emotionally debilitating, thereby significantly reducing a woman’s quality of lifeCitation8–11.
Although there is substantial variation in the recommendations and methodologic quality of the guidelines for endometriosis, many guidelines agree that this chronic condition requires long-term medical treatment despite the availability of surgical management optionsCitation1,Citation2,Citation12–14. The management of symptoms is the primary goal of treatment for endometriosisCitation1,Citation15. Thus, diagnosis should be based on clinical symptoms, and subsequent empirical treatment with hormonal treatments is recommended for women with symptoms suspected to be caused by endometriosisCitation2,Citation12. Progestins are recommended as a first-line hormonal therapy for the treatment of endometriosis-related pain and may compare favorably with other treatment optionsCitation16. Dienogest 2 mg daily is a fourth-generation progestin that first received approval for the treatment of endometriosis in the European Union in 2009Citation17–20. Dienogest binds to the progesterone receptor and, when taken continuously, inhibits systemic gonadotropin secretion and has local antiproliferative and anti-inflammatory effects on endometriotic lesionsCitation17,Citation21–23. These antiproliferative and antiangiogenic properties of the compound differentiate dienogest from other progestins in the same classCitation20,Citation23.
Given the chronic nature of the condition, medical treatments for endometriosis need to balance clinical efficacy and symptom relief with an acceptable long-term safety profileCitation15. This paper provides an expert review of the evidence for the use of dienogest in the long-term management of endometriosis, including its efficacy, safety profile, and use in the treatment of special patient populations, with recommendations for consideration by those in clinical practice.
Methods
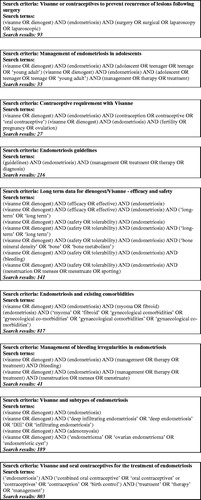
This is a narrative review, for which separate PubMed searches were conducted to retrieve publications on the use of dienogest in endometriosis published between 2007 to 2019. Experts selected publications for inclusion based on a) factors they considered relevant to the clinical management of endometriosis, such as the efficacy and safety of dienogest, and b) their own experiences in clinical practice. presents the search terms used in each search strategy and the number of citations resulting from these (with removal of duplicate results).
Efficacy with long-term dienogest therapy for endometriosis
There is no standard definition of “long-term” treatment for endometriosis, and dienogest 2 mg was approved on the basis of the clinical development program, which included studies lasting up to 15 monthsCitation24–28. Although there have been few interventional studies since to investigate treatment durations beyond 15 months, supportive evidence for long-term dienogest treatment is available from a number of studies ()Citation1–13. These studies show that administration of dienogest for up to 5 years is effective in preventing recurrence of disease and/or symptoms following surgery, and reducing endometriosis-associated painCitation19,Citation29–37.
Table 1. Dienogest efficacy and safety studies with a duration ≥1 year.
A number of prospective, observational real-world studies of long-term dienogest 2 mg treatment in women with endometriosis have been conducted, including VIPOS and ENVISIOeNCitation38,Citation39. VIPOS was a post-approval study conducted in six European countries, to evaluate the safety of dienogest and other hormonal treatments for endometriosis. A total of 27,840 women were followed for up to 7 years (NCT01266421). ENVISIOeN was a multicenter study assessing the effectiveness of dienogest in improving quality of life in more than 800 women over 2 years (NCT02425462). Together, findings from these large studies are expected to provide further insights into the use of long-term treatment with dienogest 2 mg.
Additionally, treatment with dienogest 2 mg/day in 54 women for up to 24 months was associated with significant improvements in physical, mental, social, emotional, and general health parameters, compared with baseline valuesCitation37. Similarly, significant improvements in quality-of-life categories were reported in women receiving a continuous regimen of a combined oral contraceptive (COC) containing dienogest 2 mg/30 μg ethinyl estradiol (n = 63), for up to 6 monthsCitation40. Significant improvements in sexual functioning and monthly frequency of sexual intercourse were also seen after treatment with dienogest 2 mg/day, with improvements in functioning seen as early as 6 months after beginning treatmentCitation37.
General safety profile with long-term dienogest therapy for endometriosis
The general safety profile of dienogest indicates that it is well tolerated in patients with endometriosis. In a pooled analysis of four clinical trials, dienogest 2 mg was well tolerated, with a favorable safety profile extending for up to 65 weeks. The most commonly reported adverse events were headache, breast discomfort, depressed mood, and acne, each occurring in <10% of patients, which were generally mild to moderate in intensity and associated with low discontinuation ratesCitation41. Administration of dienogest for up to 5 years has also demonstrated a favorable safety and tolerability profileCitation29–34.
Bone health
Maintenance of bone health is an important consideration for the long-term management of endometriosis, particularly in adolescents, given that treatments can involve suppression of estrogen levels outside of the suggested therapeutic window (20–60 pg/mL)Citation34,Citation42,Citation43. Gonadotropin-releasing hormone agonists, which are effective in the relief of endometriosis-associated painCitation2, induce a hypoestrogenic state, resulting in a 4−6% decrease in bone mineral density (BMD) after 24 weeks of treatment, without add-back therapyCitation44,Citation45. Similarly, the use of depot medroxyprogesterone acetate is associated with loss of BMD, although evidence suggests that BMD recovers following its cessationCitation46.
Treatment with dienogest 2 mg has been associated with moderate suppression of estrogen but with levels remaining within the therapeutic windowCitation34,Citation47. There is limited evidence of the effect of dienogest 2 mg on BMD in patients with endometriosis, and small decreases of 0.5% to 2.7% BMD in the lumbar spine after 1 year have been observed in 20−75% of study populationsCitation24,Citation30,Citation48,Citation49. Several studies have reported changes in BMD ()Citation24,Citation30,Citation48,Citation49; however, it is not well understood why some women experience BMD loss and others do not. One study reported that loss in BMD is largely thought to occur within the first 6 months of dienogest treatment, with some further decline up to 1 year, followed by stabilizationCitation49. Following treatment cessation, BMD levels have been shown to partially recover by 6 months after treatmentCitation24.
Table 2. Summary of impact of dienogest treatment on lumbar BMD across studies.
The clinical significance of the observed decreases in BMD with dienogest 2 mg in women with endometriosis remains unclear, and may be due, at least in part, to the limitations of BMD as a surrogate marker for osteoporosis, as it addresses bone quantity rather than qualityCitation50. The National Osteoporosis Foundation states that changes in bone density are often smaller than the measurement error of most dual-energy x-ray absorptiometry (DEXA) scannersCitation51. The errors in BMD measurement are well recognized and include differences in reference populations and variations in calculation methods that can all affect estimated T-score and Z-score values. Despite attempts to standardize these values, inconsistencies remain within and across BMD assessment technologies that provide challenges in interpreting results. As a consequence, many possible T-score and Z-score values exist for any given BMD value, with significantly greater variation in Z-scores, which are usually adjusted for characteristics such as age, ethnicity, and occasionally size or bone ageCitation52. In addition, according to the US Preventive Services Task Force statement, 2 years may be the minimum BMD screening interval necessary to detect possible bone density loss due to the precision error of the methodologyCitation53. Even longer intervals may be necessary to improve fracture risk predictionCitation53,Citation54, as confirmed in a Canadian studyCitation55. Similarly, the American College of Preventive Medicine recommends that screening for osteoporosis should not occur more often than every 2 yearsCitation50. Thus, the BMD screening intervals used in clinical trials may be too short to detect the possible bone density loss that can result due to treatment.
Data suggest that changes in BMD should not prevent long-term use of dienogest in women with endometriosis. However, further research is required in this area to address outstanding questions, including whether there is an effect of dienogest on BMD beyond the first year of treatment, and the clinical significance of BMD reductions in premenopausal patients, especially adolescent girls. International guidelinesCitation56 and recommendations given in the product labelCitation17 should be consulted in the context of a woman’s overall bone health. Osteoporosis risk factors should be considered, and health factors, such as intake of calcium or vitamin D for general bone health, should be discussed with patients (although currently there is no evidence to suggest additional monitoring or supplements are required with dienogest treatment)Citation17. In addition, the clinical significance of BMD findings must be considered in the context of the well-known limitations of BMD assessment.
Breast health
Women with endometriosis may be concerned about a potential increased cancer risk with hormonal treatmentsCitation57. Although evidence for the clinical effects of dienogest on breast tissue is limited, no serious adverse events related to breast disorders were reported in the clinical development programCitation41. Breast discomfort was the most frequently reported breast-related adverse event considered to be possibly treatment related in clinical trialsCitation17,Citation41. A pilot study investigating the impact of high-dose dienogest (20 mg daily for 24 weeks) on breast tissue in women with endometriosis, identified no adverse effects on the breast gland, fat layer thickness at the areola edge, or duct diameterCitation58.
The relationship between COCs and progestins with breast cancer has been controversial and mainly based on population-based epidemiologic studiesCitation59–62. The risk of breast cancer was shown to be similar between different COCs, and findings for different progestin-only formulations were inconsistentCitation57. Furthermore, in a mouse model, dienogest demonstrated potent anticancer activity against hormone-dependent cancers in two cell lines derived from human endometrial carcinoma, and one cell line derived from human breast carcinoma, where other progestins showed either no response or were only effective at a higher dose than that of dienogestCitation63. It has also been demonstrated that dienogest exhibits antiangiogenic activity, which suggests that, in animal models, it may have antitumor effects on human hormone-dependent cancer xenografts, such as endometrial and breast cancersCitation64. In summary, there is no direct evidence indicating an additional risk of breast cancer with dienogest treatment in humans, but the risk is likely to be similar to that of other progestins.
Expert recommendations
Long-term treatment of endometriosis with dienogest 2 mg (>15 months) should continue for as long as needed by the individual woman (e.g. until pregnancy is desired, disease recurs, or side effects occur), based on local treatment labels
The efficacy of dienogest 2 mg should be measured primarily by its impact on pain and quality of life for the woman
^ Other important considerations are effects of medical management on menstrual bleeding, reduction of lesion size, preservation of fertility, and the ability to avoid or delay surgery
Small decreases in BMD have been seen with dienogest treatment of up to 52 weeks; however, there does not appear to be a cumulative decrease in BMD, and there is evidence of partial recovery in BMD following treatment cessationCitation24,Citation48
^ The clinical significance of the decreases in BMD observed with dienogest treatment are currently unknown
^ There is no evidence suggesting the need for additional monitoring or supplements for bone health with dienogest treatment
^ There is no evidence to suggest an increased risk of fracture in later life
^ Changes in BMD should not prevent long-term treatment with dienogest, but patients should be advised about the risks of decreased BMD, particularly if already predisposed to osteoporosis due to factors including chronic steroid use, previous fragility fractures, smoking, and malabsorption conditions, such as inflammatory bowel disease
^ Lifestyle modifications should be discussed with patients, with suggestions including (a) calcium and vitamin D supplementation, (b) weight-bearing exercise, (c) smoking cessation, and (d) avoidance of excessive alcohol use
In terms of cancer risk, women receiving dienogest do not need to be treated or monitored differently from other women. Women should be offered appropriate counseling and time to ask questions. Ovarian masses should be monitored and treated according to published clinical practice guidelines, which aim to minimize morbidity with conservative management, laparoscopic techniques, and appropriate referralCitation65.
Comparison of combined oral contraceptives and dienogest
Combined hormonal contraceptives, including COCs, are relatively inexpensive, with a well-established safety profile. Although there is some evidence that COCs can be effective in relieving dysmenorrhea, the off-label use of COCs in the treatment of endometriosis has been largely based on data from uncontrolled trialsCitation16,Citation66,Citation67. Therefore, there remains a lack of solid clinical trial evidence supporting the efficacy of COCs in alleviating the symptoms of endometriosis, such as painCitation16,Citation68. A recent Cochrane review concluded that, based on the limited evidence and high risk of bias, there is insufficient support to judge the effectiveness of COCs alone compared with placebo, or combined with other medical treatmentsCitation68. In addition, the rationale for the use of COCs has been questioned, in that they provide a higher than physiologic dose of estrogen that maintains the existing estrogen–progesterone disequilibrium, and may stimulate the diseaseCitation69. Furthermore, medical contraindications limit the use of COCs in clinical practiceCitation16. The use of oral contraceptives (OCs) has also been studied, indicating the association between past use of OCs for severe primary dysmenorrhea and surgical confirmation of endometriosis, particularly of deep-infiltrating endometriosis (DIE)Citation70. Conversely, as described in a meta-analysis, no such association is apparent between current OC use and endometriosis, a result found in agreement with a meta-analysisCitation71.
As a consequence, some experts have suggested that progestins be used as first-line medical treatment for endometriosisCitation16,Citation69. Some clinical practice guidelines also recommend progestins as first-line therapyCitation2,Citation13.
Unlike COCs, dienogest 2 mg has not been developed as a contraceptive, and barrier contraception is recommended during treatment to prevent pregnancy, although ovulation inhibition was demonstrated in early clinical trialsCitation17,Citation72. Numerous studies have shown that progestin-releasing intrauterine systems or implants improve pelvic pain, dysmenorrhea, and quality of life in women with surgically confirmed endometriosisCitation66,Citation73.
Expert recommendations
• Data support the efficacy of COCs in reducing dysmenorrhea in women with endometriosis but not the relief of other typical pain symptoms (e.g. dyspareunia, non-cyclic pelvic pain)
^ COCs containing estrogen (ethinylestradiol or estradiol) and progestin components have additional contraindications and side effects compared with progestin-only products, such as dienogestCitation16. A history of OC use for dysmenorrhea is associated with diagnosis of endometriosis, particularly of DIE, later in lifeCitation70
^ The rationale and evidence for OCs in the treatment of endometriosis are limited. Progestins (including dienogest) may be a better first-line treatment option with fewer contraindications compared with COCsCitation16
• Evidence supports the efficacy of dienogest 2 mg in reducing multiple types of endometriosis-associated pain, including pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia
• The role of dienogest 2 mg in the management of fertility requires further investigation
Management of bleeding irregularities
Treatment with dienogest 2 mg, as with other progestins, leads to endometrial regression and bleeding irregularitiesCitation22. Initial bleeding during the first few months can be consistent, typically lasting for 8–10 days, with decreases in intensity and frequency over timeCitation28,Citation41,Citation47,Citation74. In addition, spotting can occur with long-term dienogest treatmentCitation17. Although the potential impact of bleeding irregularities on patient acceptance and compliance with dienogest 2 mg is a recognized challenge, <1% of patients in clinical trials discontinued treatment for this reasonCitation41,Citation74. However, given that irregular bleeding patterns in the first 3 months of treatment are thought to occur in ∼20% of patients, it is recommended that patients be counseled to prepare and reassure them of possible bleedingCitation41. Regular follow-up and support at this time are also advised.
Abnormal uterine bleeding may require further investigation via transvaginal ultrasound examination and physical and laboratory assessment, including recording sexual history and screening for sexually transmitted infections, such as chlamydia, when applicableCitation75,Citation76.
Expert recommendations
Before treatment initiation, patients should be counseled on what to expect regarding bleeding pattern changes
^ Women should be reassured that bleeding with dienogest 2 mg is not a sign of a lack of efficacy or recurrence of disease
^ Two distinct types of bleeding irregularities may occur during dienogest 2 mg treatment: initial bleeding during the first few months, and bleeding/spotting with longer-term use
^ Initial bleeding can be consistent and typically lasts for 8–10 days. A regimen involving treatment with a gonadotropin-releasing hormone followed by long-term dienogest therapy may reduce initial irregular bleedingCitation77. Furthermore, initiation of dienogest 2 mg at the onset of menses may also decrease initial bleeding
^ Bleeding that occurs during long-term treatment is typically spotting. If the sonographic endometrium thickness is low, management can include a treatment break of 5–7 days to allow for the recovery of the atrophy of the endometrium, or a short-term application of 1 mg oral or transdermal estradiol (5–7 days)
The occurrence of abnormal uterine bleeding may require further investigation, such as by ultrasound examination
^ Persistence of abnormal uterine bleeding should prompt further investigations for other uterine pathologies beyond endometriosis
Recording sexual history and screening for sexually transmitted infections may be advised
Preventing postsurgical recurrence of endometriosis
Endometriosis is a chronic disease requiring long-term management, with the goal of maximizing the use of medical treatment and avoiding repeated surgical proceduresCitation78. Even after successful surgery, recurrence of endometriosis-associated symptoms is frequently observedCitation79,Citation80, and prescription of medical treatment for the long-term prevention of recurrence is recommendedCitation2. Several studies support the use of long-term dienogest for the prevention of recurrenceCitation29,Citation30,Citation33,Citation81–87, and a reduction in recurrent endometrioma sizeCitation30 has been observed for up to 5 years following surgery ()Citation29,Citation30,Citation33,Citation81–85. In a retrospective cohort study of 568 women with endometrioma, cumulative disease recurrence rates 5 years postsurgery were 69% in women receiving no medical treatment, compared with 4% in women taking dienogest 2 mgCitation81. Additionally, in a retrospective review, 55.5% of women who discontinued treatment with OCs experienced postoperative recurrence of ovarian endometriomas, compared with a rate of 0% in those who received continuous treatment with dienogestCitation82. In contrast, evidence to suggest the efficacy of COC regimens in the reduction of postsurgical recurrence is limitedCitation16,Citation88.
Table 3. Data supporting the efficacy of dienogest for the prevention of postsurgical recurrence.
Expert recommendations
Asymptomatic ovarian endometriomas should be monitored but do not require medical or surgical treatment; if the endometrioma is large and there is a risk of rupture, then surgery should be considered
Surgery should be considered in cases of atypical findings via ultrasound examination
Painful ovarian cysts >3–4 cm in diameter should be treated surgically, in line with treatment guidelinesCitation2
Medical treatments can be prescribed for symptomatic relief when awaiting surgery
Medical treatments, including dienogest 2 mg, should be prescribed postsurgery to prevent the recurrence of endometriosis, unless there is an immediate desire for pregnancy. Postoperative dienogest 2 mg treatment has been effective in the prevention of endometriosis symptom recurrence and endometriomaCitation29,Citation30,Citation33,Citation81–86
Long-term treatment with dienogest 2 mg has been shown to decrease recurrent endometrioma size, which may indicate an additional benefit of its use in medical treatmentCitation30
Treating complex patients
The available evidence indicates a role for dienogest 2 mg in the management of adenomyosis, DIE, and endometrioma.
Adenomyosis
Adenomyosis is an estrogen-dependent disease that is characterized by the growth of ectopic endometrial-like tissue within the myometrium of the uterus, and studies have reported a prevalence of approximately 22−43% in women with endometriosisCitation89–91. Conservative surgical procedures in the treatment of adenomyosis are associated with a high rate of recurrence in the long-term (38−49% 2–3 years after the procedure); however, evidence indicates that concurrent medical treatment can reduce this recurrence rateCitation92–95. In addition, the risk of bleeding with surgical procedures is high, owing to the proximity of the myometrial surface to significant arteriesCitation94.
Guidelines recommend that a levonorgestrel-releasing intrauterine device (LNG-IUD) should be considered initially in women with fibroids <3 cm in diameterCitation96. Where an alternative treatment is preferred by the woman or is necessary, evidence suggests that dienogest is effective and well tolerated in the treatment of painful symptoms in patients with this conditionCitation89. In a phase III randomized trial of women with adenomyosis (n = 67), treatment with dienogest was associated with a significant decrease in visual analog scale scores versus placebo (−58.4 ± 23.6 mm vs. −20.6 ± 23.6 mm, p < .001)Citation89. It is recommended that physicians monitor patients, particularly those of a younger age, for anemiaCitation17,Citation97. In the long term, the use of medical management is preferred for the treatment of adenomyosis, thus avoiding surgery and allowing patients to conceive if desiredCitation98.
Deep-infiltrating endometriosis
DIE is a severe form of endometriosis that occurs when the peritoneal surface is invaded and areas surrounding the uterus are affected. DIE lesions often extend >5 mm into the peritoneum and the depth of infiltration is often related to the type and severity of symptomsCitation99–101. DIE is associated with high levels of pelvic pain, as well as infertility, dysmenorrhea, and dyspareuniaCitation102. Evidence suggests that dienogest is effective in the management of DIECitation84,Citation103. Treatment with dienogest 2 mg for up to 12 months in women with DIE was associated with reductions in a number of endometriosis-associated pain symptoms, including dysmenorrhea, pelvic pain, dyspareunia, and bowel/intestinal painCitation103,Citation104. In a single small study, postsurgical dienogest 2 mg treatment was associated with a reduced occurrence of endometriosis-associated pain compared with no medical treatmentCitation84. Surgery should be considered in cases that are complicated by urethra, bowel, or kidney obstruction, or fistulae in rectovaginal endometriosis because the risk of complications is highCitation2.
Comorbidities in patients with endometriosis
The symptoms of endometriosis frequently affect psychologic and social functioning, significantly compromising patients’ relationships, sexuality, and mental healthCitation105,Citation106. Furthermore, women who suffer from endometriosis report high levels of anxiety, depression, and other psychiatric disorders, which may amplify the severity of painCitation105. Psychologic issues should be considered by healthcare professionals and openly discussed with patients before initiation of hormonal treatments for endometriosisCitation105. Depressed mood can be observed with dienogest 2 mg as with other hormonal treatments, occurring in <10% of women in a pooled analysis of safety data from pivotal dienogest studiesCitation41. Patients should be advised that mood changes or depression are a possible result of all progestins, not specifically dienogest, and they should be watchful for any changes that may occur.
Studies have also suggested that endometriosis may be associated with irritable bowel syndrome (IBS), with a five times greater prevalence of IBS observed in women with endometriosis compared with those without endometriosisCitation107. Additionally, adolescent patients with endometriosis may be more likely to report migraines than those without, with nearly five-fold greater odds of migraines reported among those with endometriosis, compared with those withoutCitation108.
Expert recommendations
The use of medical treatments should be maximized for patients with adenomyosis
^ Dienogest 2 mg treatment effectively reduces pain for patients with adenomyosis and may be an alternative treatment to LNG-IUD
Patients with symptomatic DIE can be managed with dienogest 2 mg
Where extragenital endometriosis results in urethra, bowel, or kidney obstruction, or fistulae in rectovaginal endometriosis, surgical treatment options should be considered
Management of mood disturbances and depression with dienogest 2 mg should include regular monitoring during routine follow-up appointments and may require additional steps for women with a history of depression
^ Patients should be made aware that depression and mood disturbances can occur with dienogest 2 mg treatment, as with all hormonal therapies
^ As part of routine follow-up during treatment, patients should be monitored for signs of depression or mood disturbances
^ Possible introduction of a “treatment break” might be warranted in the context of long-term treatment for patients with mood symptoms
^ If women have a history or current diagnosis of clinical depression, care should be taken in the prescription of dienogest 2 mg or other hormonal treatments. In addition, the involvement of a mental-health specialist may be warranted
Diagnosis and management of endometriosis in adolescent patients
Endometriosis affects a significant proportion of adolescent girls, but diagnostic delays are common in this populationCitation109,Citation110, partially due to the differences in clinical presentation compared with adults. These delays can partly explain the severity of disease affecting these younger patients and support the need for a simple, noninvasive tool for the screening of endometriosis in this populationCitation110. It is important that physicians recognize endometriosis in younger patients so timely treatment can be givenCitation109. As such, patients presenting with dysmenorrhea and chronic pelvic pain should not be underestimated, and a detailed and accurate history should be obtained before performing clinical evaluation and pelvic sonographyCitation111. Furthermore, in adolescent patients, the focus should be on clinical rather than surgical/laparoscopic diagnosis. Receiving a diagnosis of endometriosis can provide some reassurance to adolescent patients regarding the cause of their pain, but it can also cause anxiety upon learning that it may require long-term treatment and surgery, and may cause infertility; therefore, scheduling frequent follow-up visits is important.
Current surgical treatment options have potential deleterious effects on the ovarian follicle reserve, and some studies have suggested a recurrence rate of up to 56% in adolescent patients with endometriosisCitation110. As it stands, a conservative treatment approach, avoiding surgery, is desirable, and hormonal therapies and analgesics are recommended for relief of endometriosis-related painCitation2. In a 52-week, multicenter study of adolescent patients, dienogest 2 mg was effective in relieving the symptoms (pelvic pain, dysmenorrhea, and dyspareunia) and signs (pelvic tenderness and induration) of endometriosis. In addition, dienogest 2 mg was generally well tolerated, consistent with findings previously reported in adults with endometriosisCitation24. Given that adolescence is a crucial time for accumulation of BMD, data on the impact of endometriosis treatment at this age are of particular interestCitation24. In adolescents treated with dienogest 2 mg for 1 year, a 1.2% reduction in BMD from baseline to the end of treatment was observedCitation24. This slight reduction should be balanced against recovery of BMD once treatment is stopped, and the significant reductions in endometriosis-associated pain observed with treatmentCitation24. The impact of these observations in adolescent patients must also be considered in context with the fact that BMD is only a surrogate marker for bone healthCitation50,Citation112. Important confounding factors should be considered when performing DEXA, especially in children and adolescent patients, including variations in age, race, gender, pubertal status, and height. Thus, errors in interpretation of bone densitometry in children and adolescents can lead to significant overdiagnosis of osteopenia, or even osteoporosis, on the basis of low BMD scores inferred from DEXACitation112.
In conclusion, although challenges with the interpretation of BMD in the adolescent population must be recognized, dienogest 2 mg may be favorable owing to the lack of alternative treatments when the benefit–risk profile is considered.
Expert recommendations
The occurrence of endometriosis and the impact of symptoms should not be underestimated in adolescent patients, to ensure timely diagnosis and treatment initiation
In adolescent patients, surgical diagnosis of endometriosis should be avoided in favor of clinical diagnosis based on symptoms
For adolescent patients with endometriosis, multiple treatment options are available and the use of dienogest 2 mg has been investigated in this patient population. However, treatment decisions should be made on an individual basis, using a risk–benefit approach that considers efficacy and safety
Conclusions
Endometriosis is a chronic disease and, as such, medical treatment should be maximized and surgical interventions avoided and minimized, unless necessary. Dienogest 2 mg offers an effective and tolerable alternative to surgical intervention for the long-term management of endometriosis, providing several important advantages over COCs. Furthermore, the evidence highlights that patients are willing to accept the bleeding irregularities that often occur with dienogest 2 mg, given the pain relief experienced. In clinical practice, counseling patients regarding the expected side effects, weighing up the efficacy and safety of each treatment approach, and following treatment guidelines to provide tailored care according to each woman’s needs and desires, are all important components of management.
Transparency
Declaration of funding
All authors attended a meeting to discuss the topics and to develop the expert recommendations included in this manuscript. This meeting was financially supported Bayer AG. Medical writing support provided by Huntsworth Health Ltd was also funded by Bayer AG.
Declaration of financial/other relationships
AM has received grants/research funding from AbbVie, Allergan, and Bayer; has acted as a consultant/advisor for AbbVie, Allergan, Bayer, and Hologic; and has participated in speaker bureaus for AbbVie, Allergan, Bayer, and Hologic. KB has acted as a consultant/advisor for Bayer. JL has acted as a consultant/advisor for Bayer. MDM has acted as a consultant/advisor for Bayer, Roche, Pfizer, Terumo, and MSD; and has participated in speaker bureaus for Bayer, Storz, and MSD. TR has acted as a consultant/advisor for Bayer, Exeltis, Aristo, Gedeon Richter, and DR. KADE. MV has acted as a consultant/advisor for Bayer. MY has acted as a consultant/advisor and has participated in speaker bureaus for Bayer. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in the analysis and interpretation of the data, the drafting of the paper, and critical revision of the content for intellectual integrity. All authors provided final approval of the manuscript version to be published and agree to be accountable for all aspects of the work.
Acknowledgements
Medical writing support was provided by Huntsworth Health Ltd, with funding from Bayer AG.
References
- Johnson NP, Hummelshoj L. World Endometriosis Society Montpellier C. Consensus on current management of endometriosis. Hum Reprod. 2013;28(6):1552–1568.
- Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Klemmt PAB, Starzinski-Powitz A. Molecular and cellular pathogenesis of endometriosis. Curr Womens Health Rev. 2018;14(2):106–116.
- Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34–41.
- Eisenberg VH, Weil C, Chodick G, et al. Epidemiology of endometriosis: a large population-based database study from a healthcare provider with 2 million members. BJOG. 2018;125(1):55–62.
- Meuleman C, Vandenabeele B, Fieuws S, et al. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril. 2009;92(1):68–74.
- Sinaii N, Plumb K, Cotton L, et al. Differences in characteristics among 1,000 women with endometriosis based on extent of disease. Fertil Steril. 2008;89(3):538–545.
- Ramin-Wright A, Schwartz ASK, Geraedts K, et al. Fatigue – a symptom in endometriosis. Hum Reprod. 2018;33(8):1459–1465.
- Chauvet P, Guiguet-Auclair C, Comptour A, et al. Feelings and expectations in endometriosis: Analysis of open comments from a cohort of endometriosis patients. J Gynecol Obstet Hum Reprod. 2018;47(7):281–287.
- Lagana AS, Condemi I, Retto G, et al. Analysis of psychopathological comorbidity behind the common symptoms and signs of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2015;194:30–33.
- Soliman AM, Coyne KS, Zaiser E, et al. The burden of endometriosis symptoms on health-related quality of life in women in the United States: a cross-sectional study. J Psychosom Obstet Gynaecol. 2017;38(4):238–248.
- National Institute for Health and Care Excellence (NICE). Endometriosis: diagnosis and management. Available from: https://www.nice.org.uk/guidance/ng73.
- Leyland N, Casper R, Laberge P, et al. Sogc. Endometriosis: diagnosis and management. J Obstet Gynaecol Can. 2010;32(7 Suppl 2):S1–S32.
- Hirsch M, Begum MR, Paniz E, et al. Diagnosis and management of endometriosis: a systematic review of international and national guidelines. BJOG. 2018;125(5):556–564.
- Ferrero S, Evangelisti G, Barra F. Current and emerging treatment options for endometriosis. Expert Opin Pharmacother. 2018;19(10):1109–1125.
- Casper RF. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil Steril. 2017;107(3):533–536.
- Visanne. Summary of product characteristics. Pymble (NSW): Bayer Australia. 2016.
- Angioni S, Cofelice V, Pontis A, et al. New trends of progestins treatment of endometriosis. Gynecol Endocrinol. 2014;30(11):769–773.
- Paulo Leonardo-Pinto J, Laguna Benetti-Pinto C, Angerame Yela D. When solving dyspareunia is not enough to restore sexual function in women with deep infiltrating endometriosis treated with dienogest. J Sex Marital Ther. 2019;45(1):44–49.
- Barra F, Scala C, Ferrero S. Current understanding on pharmacokinetics, clinical efficacy and safety of progestins for treating pain associated to endometriosis. Expert Opin Drug Metab Toxicol. 2018;14(4):399–415.
- Foster RH, Wilde MI. Dienogest. Drugs. 1998;56(5):825–833. discussion 34-(5).
- McCormack PL. Dienogest: a review of its use in the treatment of endometriosis. Drugs. 2010;70(16):2073–2088.
- Schindler AE. Dienogest in long-term treatment of endometriosis. Int J Womens Health. 2011;3:175–184.
- Ebert AD, Dong L, Merz M, et al. Dienogest 2 mg daily in the treatment of adolescents with clinically suspected endometriosis: The VISanne Study to Assess Safety in ADOlescents. J Pediatr Adolesc Gynecol. 2017;30(5):560–567.
- Petraglia F, Hornung D, Seitz C, et al. Reduced pelvic pain in women with endometriosis: efficacy of long-term dienogest treatment. Arch Gynecol Obstet. 2012;285(1):167–173.
- Strowitzki T, Faustmann T, Gerlinger C, et al. Dienogest in the treatment of endometriosis-associated pelvic pain: a 12-week, randomized, double-blind, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2010;151(2):193–198.
- Strowitzki T, Marr J, Gerlinger C, et al. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial. Hum Reprod. 2010;25(3):633–641.
- Kohler G, Faustmann TA, Gerlinger C, et al. A dose-ranging study to determine the efficacy and safety of 1, 2, and 4 mg of dienogest daily for endometriosis. Int J Gynaecol Obstet. 2010;108(1):21–25.
- Lee SR, Yi KW, Song JY, et al. Efficacy and safety of long-term use of dienogest in women with ovarian endometrioma. Reprod Sci. 2018;25(3):341–346.
- Park SY, Kim SH, Chae HD, et al. Efficacy and safety of dienogest in patients with endometriosis: a single-center observational study over 12 months. Clin Exp Reprod Med. 2016;43(4):215–220.
- Sugimoto K, Nagata C, Hayashi H, et al. Use of dienogest over 53 weeks for the treatment of endometriosis. J Obstet Gynaecol Res. 2015;41(12):1921–1926.
- Morelli M, Sacchinelli A, Venturella R, et al. Postoperative administration of dienogest plus estradiol valerate versus levonorgestrel-releasing intrauterine device for prevention of pain relapse and disease recurrence in endometriosis patients. J Obstet Gynaecol Res. 2013;39(5):985–990.
- Chandra A, Rho AM, Jeong K, et al. Clinical experience of long-term use of dienogest after surgery for ovarian endometrioma. Obstet Gynecol Sci. 2018;61(1):111–117.
- Römer T. Long-term treatment of endometriosis with dienogest: retrospective analysis of efficacy and safety in clinical practice. Arch Gynecol Obstet. 2018;298(4):747–753.
- Lee JH, Song JY, Yi KW, et al. Effectiveness of dienogest for treatment of recurrent endometriosis: multicenter data. Reprod Sci. 2018;25(10):1515–1522.
- Del Forno S, Mabrouk M, Arena A, et al. Dienogest or norethindrone acetate for the treatment of ovarian endometriomas: can we avoid surgery? Eur J Obstet Gynecol Reprod Biol. 2019;238:120–124.
- Caruso S, Iraci M, Cianci S, et al. Effects of long-term treatment with dienogest on the quality of life and sexual function of women affected by endometriosis-associated pelvic pain. JPR. 2019;12:2371–2378.
- Bayer. Visanne Post-approval Observational Study (VIPOS). [cited 2019 Nov]; Available from: https://clinicaltrials.bayer.com/study/20018
- Bayer. To evaluate effectiveness of Visanne in improving quality of life in Asian women with Endometriosis. [cited 2019 Nov]; Available from: https://clinicaltrials.bayer.com/study/2084
- Caruso S, Iraci M, Cianci S, et al. Comparative, open-label prospective study on the quality of life and sexual function of women affected by endometriosis-associated pelvic pain on 2 mg dienogest/30 microg ethinyl estradiol continuous or 21/7 regimen oral contraceptive. J Endocrinol Invest. 2016;39(8):923–931.
- Strowitzki T, Faustmann T, Gerlinger C, et al. Safety and tolerability of dienogest in endometriosis: pooled analysis from the European clinical study program. Int J Womens Health. 2015;7:393–401.
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740–745.
- Barbieri RL. Endometriosis and the estrogen threshold theory. Relation to surgical and medical treatment. J Reprod Med. 1998;43(3 Suppl):287–292.
- Moghissi KS, Schlaff WD, Olive DL, et al. Goserelin acetate (Zoladex) with or without hormone replacement therapy for the treatment of endometriosis. Fertil Steril. 1998;69(6):1056–1062.
- Franke HR, van de Weijer PH, Pennings TM, et al. Gonadotropin-releasing hormone agonist plus “add-back” hormone replacement therapy for treatment of endometriosis: a prospective, randomized, placebo-controlled, double-blind trial. Fertil Steril. 2000;74(3):534–539.
- ACOG. Committee opinion: depot medroxyprogesterone acetate and bone effects (number 602). Committee on adolescent health care and committee on gynecologic practice; 2014.
- Lang J, Yu Q, Zhang S, et al. Dienogest for treatment of endometriosis in Chinese women: a placebo-controlled, randomized, double-blind phase 3 study. J Womens Health (Larchmt). 2018;27(2):148–155.
- Momoeda M, Harada T, Terakawa N, et al. Long-term use of dienogest for the treatment of endometriosis. J Obstet Gynaecol Res. 2009;35(6):1069–1076.
- Seo JW, Lee DY, Yoon BK, et al. Effects of long-term postoperative dienogest use for treatment of endometriosis on bone mineral density. Eur J Obstet Gynecol Reprod Biol. 2017;212:9–12.
- Lim LS, Hoeksema LJ, Sherin K. Screening for osteoporosis in the adult U.S. population: ACPM position statement on preventive practice. Ame J Prevent Med. 2009;36(4):366–375.
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–2381.
- Carey JJ, Delaney MF, Love TE, et al. DXA-generated Z-scores and T-scores may differ substantially and significantly in young adults. J Clin Densitom. 2007;10(4):351–358.
- Nordin C. Screening for osteoporosis: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2011;155(4):276; author reply 76-7.
- Hillier TA, Stone KL, Bauer DC, et al. Evaluating the value of repeat bone mineral density measurement and prediction of fractures in older women: the study of osteoporotic fractures. Arch Intern Med. 2007;167(2):155–160.
- Leslie WD, Morin SN, Lix LM. Rate of bone density change does not enhance fracture prediction in routine clinical practice. J Clin Endocrinol Metab. 2012;97(4):1211–1218.
- Qaseem A, Forciea MA, McLean RM, et al. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(11):818–839.
- Morch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228–2239.
- Schindler AE, Henkel A, Christensen B, et al. Dienogest and the breast. Gynecol Endocrinol. 2009;25(7):472–474.
- Samson M, Porter N, Orekoya O, et al. Progestin and breast cancer risk: a systematic review. Breast Cancer Res Treat. 2016;155(1):3–12.
- Borges JBR, Torresan RZ. Breast cancer and hormonal contraception: Should we rethink our concepts? Rev Assoc Med Bras.2018;64(3):201–203.
- Eden J. Progestins and breast cancer. Am J Obstet Gynecol. 2003;188(5):1123–1131.
- Staffa JA, Newschaffer CJ, Jones JK, et al. Progestins and breast cancer: an epidemiologic review. Fertil Steril. 1992;57(3):473–491.
- Katsuki Y, Shibutani Y, Aoki D, et al. Dienogest, a novel synthetic steroid, overcomes hormone-dependent cancer in a different manner than progestins. Cancer. 1997;79(1):169–176.
- Nakamura M, Katsuki Y, Shibutani Y, et al. Dienogest, a synthetic steroid, suppresses both embryonic and tumor-cell-induced angiogenesis. Eur J Pharmacol. 1999;386(1):33–40.
- RCOG/BSGE Joint Guideline. Management of suspected ovarian masses in premenopausal women. Available from: https://www.rcog.org.uk/globalassets/documents/guidelines/gtg_62.pdf
- Grandi G, Barra F, Ferrero S, et al. Hormonal contraception in women with endometriosis: a systematic review. Eur J Contracept Reprod Health Care. 2019;24(1):61–70.
- Harada T, Momoeda M, Taketani Y, et al. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008;90(5):1583–1588.
- Brown J, Crawford TJ, Datta S, et al. Oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2018;5:Cd001019.
- Vercellini P, Buggio L, Frattaruolo MP, et al. Medical treatment of endometriosis-related pain. Best Pract Res Clin Obstet Gynaecol. 2018;51:68–91.
- Chapron C, Souza C, Borghese B, et al. Oral contraceptives and endometriosis: the past use of oral contraceptives for treating severe primary dysmenorrhea is associated with endometriosis, especially deep infiltrating endometriosis. Hum Reprod. 2011;26(8):2028–2035.
- Vercellini P, Eskenazi B, Consonni D, et al. Oral contraceptives and risk of endometriosis: a systematic review and meta-analysis. Hum Reprod Update. 2011;17(2):159–170.
- Klipping C, Duijkers I, Remmers A, et al. Ovulation-inhibiting effects of dienogest in a randomized, dose-controlled pharmacodynamic trial of healthy women. J Clin Pharmacol. 2012;52(11):1704–1713.
- Carvalho N, Margatho D, Cursino K, et al. Control of endometriosis-associated pain with etonogestrel-releasing contraceptive implant and 52-mg levonorgestrel-releasing intrauterine system: randomized clinical trial. Fertil Steril. 2018;110(6):1129–1136.
- Seitz C, Gerlinger C, Faustmann T, et al. Safety of dienogest in the long-term treatment of endometriosis: a one-year, open-label, follow-up study. Fertil Steril. 2009;92:S107.
- Singh S, Best C, Dunn S, et al. Abnormal uterine bleeding in pre-menopausal women. SOGC clinical practice guideline. J Obstet Gynaecol Can. 2013;35(5eSuppl):S1–S28.
- Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120(1):197–206.
- Kitawaki J, Kusuki I, Yamanaka K, et al. Maintenance therapy with dienogest following gonadotropin-releasing hormone agonist treatment for endometriosis-associated pelvic pain. Eur J Obstet Gynecol Reprod Biol. 2011;157(2):212–216.
- Practice Committee of the American Society for Reproductive M. Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927–935.
- Busacca M, Chiaffarino F, Candiani M, et al. Determinants of long-term clinically detected recurrence rates of deep, ovarian, and pelvic endometriosis. Am J Obstet Gynecol. 2006;195(2):426–432.
- Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update. 2009;15(4):441–461.
- Ota Y, Andou M, Yanai S, et al. Long-term administration of dienogest reduces recurrence after excition of endometrioma. J Endometr Pelvic Pain Disord. 2015;7(2):63–67.
- Ouchi N, Akira S, Mine K, et al. Recurrence of ovarian endometrioma after laparoscopic excision: risk factors and prevention. J Obstet Gynaecol Res. 2014;40(1):230–236.
- Adachi K, Takahashi K, Nakamura K, et al. Postoperative administration of dienogest for suppressing recurrence of disease and relieving pain in subjects with ovarian endometriomas. Gynecol Endocrinol. 2016;32(8):646–649.
- Yamanaka A, Hada T, Matsumoto T, et al. Effect of dienogest on pain and ovarian endometrioma occurrence after laparoscopic resection of uterosacral ligaments with deep infiltrating endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;216:51–55.
- Takaesu Y, Nishi H, Kojima J, et al. Dienogest compared with gonadotropin-releasing hormone agonist after conservative surgery for endometriosis. J Obstet Gynaecol Res. 2016;42(9):1152–1158.
- Koga K, Takamura M, Fujii T, et al. Prevention of the recurrence of symptom and lesions after conservative surgery for endometriosis. Fertil Steril. 2015;104(4):793–801.
- Koshiba A, Mori T, Okimura H, et al. Dienogest therapy during the early stages of recurrence of endometrioma might be an alternative therapeutic option to avoid repeat surgeries. J Obstet Gynaecol Res. 2018;44(10):1970–1976.
- Muzii L, Di Tucci C, Achilli C, et al. Continuous versus cyclic oral contraceptives after laparoscopic excision of ovarian endometriomas: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(2):203–211.
- Osuga Y, Fujimoto-Okabe H, Hagino A. Evaluation of the efficacy and safety of dienogest in the treatment of painful symptoms in patients with adenomyosis: a randomized, double-blind, multicenter, placebo-controlled study. Fertil Steril. 2017;108(4):673–678.
- Gonzales M, de Matos L, da Costa Gonçalves M, et al. Patients with adenomyosis are more likely to have deep endometriosis. Gynecol Surg. 2012;9:259–264.
- Di Donato N, Montanari G, Benfenati A, et al. Prevalence of adenomyosis in women undergoing surgery for endometriosis. Eur J Obstet Gynecol Reprod Biol. 2014;181:289–293.
- Bratby MJ, Walker WJ. Uterine artery embolisation for symptomatic adenomyosis–mid-term results. Eur J Radiol. 2009;70(1):128–132.
- Kim MD, Kim S, Kim NK, et al. Long-term results of uterine artery embolization for symptomatic adenomyosis. AJR Am J Roentgenol. 2007;188(1):176–181.
- Wood C. Surgical and medical treatment of adenomyosis. Hum Reprod Update. 1998;4(4):323–336.
- Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. 2009;92(3):876–885.
- National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management. Available from: https://www.nice.org.uk/guidance/ng88
- Nagata C, Yanagida S, Okamoto A, et al. Risk factors of treatment discontinuation due to uterine bleeding in adenomyosis patients treated with dienogest. J Obstet Gynaecol Res. 2012;38(4):639–644.
- Pontis A, D’Alterio MN, Pirarba S, et al. Adenomyosis: a systematic review of medical treatment. Gynecol Endocrinol. 2016;32(9):696–700.
- Borghese B, Santulli P, Marcellin L, et al. Definition, description, clinicopathological features, pathogenesis and natural history of endometriosis: CNGOF-HAS Endometriosis Guidelines. Gynecol Obstet Fertil Senol. 2018;46(3):156–167.
- Chapron C, Tosti C, Marcellin L, et al. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum Reprod. 2017;32(7):1393–1401.
- Agarwal N, Subramanian A. Endometriosis – morphology, clinical presentations and molecular pathology. J Lab Physicians. 2010;2(1):1–9.
- Tosti C, Pinzauti S, Santulli P, et al. Pathogenetic mechanisms of deep infiltrating endometriosis. Reprod Sci. 2015;22(9):1053–1059.
- Leonardo-Pinto JP, Benetti-Pinto CL, Cursino K, et al. Dienogest and deep infiltrating endometriosis: the remission of symptoms is not related to endometriosis nodule remission. Eur J Obstet Gynecol Reprod Biol. 2017;211:108–111.
- Yela D, Kajikawa P, Donati L, et al. Deep infiltrating endometriosis treatment with dienogest: a pilot study. J Endometr Pelvic Pain Disord. 2015;7(1):33–37.
- Lagana AS, La Rosa VL, Rapisarda AMC, et al. Anxiety and depression in patients with endometriosis: impact and management challenges. Int J Womens Health. 2017;9:323–330.
- Culley L, Law C, Hudson N, et al. The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Hum Reprod Update. 2013;19(6):625–639.
- Schomacker ML, Hansen KE, Ramlau-Hansen CH, et al. Is endometriosis associated with irritable bowel syndrome? A cross-sectional study. Eur J Obstet Gynecol Reprod Biol. 2018;231:65–69.
- Miller JA, Missmer SA, Vitonis AF, et al. Prevalence of migraines in adolescents with endometriosis. Fertil Steril. 2018;109(4):685–690.
- Arruda MS, Petta CA, Abrao MS, et al. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18(4):756–759.
- Benagiano G, Guo SW, Puttemans P, et al. Progress in the diagnosis and management of adolescent endometriosis: an opinion. Reprod Biomed Online. 2018;36(1):102–114.
- Zannoni L, Forno SD, Paradisi R, et al. Endometriosis in adolescence: practical rules for an earlier diagnosis. Pediatr Ann. 2016;45(9):e332–5.
- Gafni RI, Baron J. Overdiagnosis of osteoporosis in children due to misinterpretation of dual-energy x-ray absorptiometry (DEXA). J Pediatr. 2004;144(2):253–257.