Abstract
Objective: To determine how results from a prognostic 40-gene expression profiling (40-GEP) test would impact clinician management decisions and how their choices would align with a National Comprehensive Cancer Network (NCCN) compliant, risk-directed management plan for high-risk cutaneous squamous cell carcinoma (cSCC).
Methods: Clinicians attending a national dermatology conference were presented with 40-GEP test validation data. They were asked to rate clinicopathological features and molecular test results to assess their opinion of how concerning each is to cSCC prognosis. When presented with vignettes describing patients with NCCN-defined high-risk features, clinicians were asked to select a treatment plan using pre-test (no 40-GEP results), then, post-test (40-GEP Class 1, 2A, or 2B results) methodology along with corresponding metastasis rates for each test group.
Results: Risk factors deemed of highest concern for metastatic outcomes were a Class 2B 40-GEP result, perineural invasion, immunosuppression, invasion beyond subcutaneous fat, and tumor diameter >1 cm on the scalp. When presented with a 40-GEP result that indicated reduced risk of metastasis (Class 1), clinicians altered their treatment management plan accordingly. Specifically, there was significant reduction in the recommendations for sentinel lymph node biopsy, adjuvant radiation or chemotherapy, follow-up time, and nodal imaging. By comparison, when a 40-GEP result indicated an increased risk of metastasis (Class 2B), significant risk-appropriate increases in management intensity was observed for the aforementioned clinical decisions.
Conclusion: Integration of 40-GEP results impacted management decisions in a significant and risk-appropriate manner for high-risk cSCC patient scenarios, while remaining aligned with national guidelines for patient management.
Introduction
With more than 1 million cases being diagnosed in the U.S. annuallyCitation1, cutaneous squamous cell carcinoma (cSCC) is the second most common skin cancer with a rapidly rising incidence rateCitation2. An estimated 1.5–2% of cSCC patients die annually, and it is projected that more patients will die from cSCC than melanomaCitation1. National Comprehensive Cancer Network (NCCN) guidelines are broad in their criteria for identifying high-risk cSCC and in their approaches for management of cSCC patients considered high risk for developing recurrence and/or metastasisCitation3. Unfortunately, this broad definition of high-risk cSCC results in a low positive predictive value (PPV)Citation4, meaning many patients deemed high risk are unlikely to develop recurrences or metastasis. Since American Joint Committee on Cancer (AJCC) and Brigham and Women’s Hospital (BWH) staging systems are also limited in their accuracy for identifying high-risk patientsCitation5–9, establishing risk-appropriate patient management plans remains an unmet clinical need. Therefore, improved prognostic tools and a better-defined risk stratification system would positively enhance patient management.
A 40-gene expression profile (40-GEP) test that assesses the biology of a primary cSCC tumor was recently validated for determining metastatic potentialCitation10. The 40-GEP is a prognostic test that classifies patients into three risk groups: low (Class 1), high (Class 2A), and highest (Class 2B) risk for developing regional or distant metastasis within 3 years post-diagnosis. Accurately aligning a patients’ risk for metastasis with recommended treatment pathways would reduce unnecessary interventions for patients at low risk for metastasis and, conversely, would more precisely identify patients who would most benefit from more intense interventions/therapies. Based on the potential utility of the 40-GEP test for guiding cSCC patient management decisions, a clinical impact study was undertaken to assess if, and to what extent, more precise risk assessment through 40-GEP testing would alter physicians’ management decisions for different patient scenarios.
Methods
Data collection
Clinician attendees at a national dermatology conference were shown a presentation describing cSCC risk factors, along with data detailing the development and validation of the 40-GEP prognostic test. Demographic information from each clinician was obtained, including years of clinical experience, specialty, percentage of high-risk cSCC patients seen annually, and staging methods used in their clinical settings. Clinicians then rated, on a scale of 1–10 (1, lowest; 10, highest), the level of risk for metastasis associated with each of the following: 40-GEP Class 1, Class 2A, Class 2B; tumor on the mask area; tumor on the scalp ≥1 cm; tumor below the neck ≥2 cm; invasion beyond the subcutaneous fat; perineural invasion ≥0.1 mm; and immunosuppressed patient. Clinicians were then presented with two patient vignettes depicting the following clinical characteristics: age, sex, tumor location, size and depth of lesion, margin status, histological differentiation, and AJCC T stage. They were asked to select management decisions in the absence and then the presence of 40-GEP test results. This study protocol was submitted for Institutional Review Board (IRB) approval and determined to be IRB exempt.
Data analysis
The primary objective was to determine how clinicians would alter management decisions with the incorporation of 40-GEP test results. Statistical analysis was performed using Friedman tests with a Dunn’s correction for multiple comparisons and Freeman-Halton extension of Fisher’s exact tests. Rating of risk features was compared in all combinations (). For analysis of clinician management decisions for specific patient vignettes, all management decisions made post-40-GEP test results were compared back to the corresponding management decisions pre-40-GEP test result. p-Values < .05 were considered statistically significant. Baseline metastatic rates for each vignette were calculated using a weighted average of AJCC T stage metastasis incidence and number of patients found in from Farberg et al.Citation11
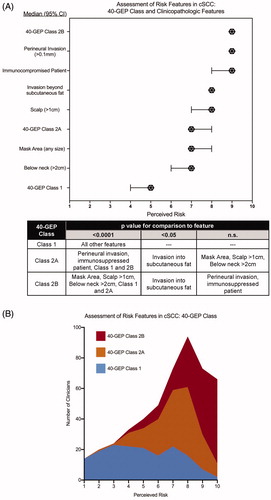
Figure 1. Clinician assessment of perceived risk of metastasis with molecular 40-GEP Class and clinicopathologic features in cutaneous squamous cell carcinoma. (A) Clinicians (n = 162) reported how risky they perceived a feature with 1 and 10 being the lowest and highest risk, respectively. Median values are plotted with error bars denoting 95% confidence intervals. p Values for comparisons of risk between two features are shown in the table and reflect Friedman tests with a Dunn’s correction for multiple comparisons. (B) Stacked histogram for clinician rating of risk of 40-GEP Class call: Class 1 (blue); Class 2A (orange); Class 2B (red).
Results
Clinician demographics and characteristics
Overall, 162 clinicians completed the study () with the vast majority identifying as dermatology clinicians (98.1%) of which 11.1% were Mohs surgeons. The greatest number of clinicians, 40.7%, had been in practice for 1–10 years, followed by 19.8% for 21–30 years, 14.2% for 11–20 years, 13.6% for greater than 30 years, and 11.7% who were current residents. Diversity among clinicians was observed regarding whether they use current staging systems and, if so, which systems are implemented in their clinical practice. Overall, 43.9% of clinicians stated that they did not use or were not aware of current staging methods. Of the 91 participants who do utilize staging systems for cSCC tumors, 58.2% reported using AJCC 8th Edition, with the remaining clinicians using AJCC 7th edition (17.6%) and BWH staging criteria (24.2%).
Table 1. Clinician Demographics (n = 162).
Perception of metastasis risk associated with molecular-based prognostic risk assessment and NCCN-defined high-risk features
Results of the 40-GEP were ranked in a risk-appropriate manner, with a Class 1 result considered to be of the lowest perceived risk (median risk score: 5), a Class 2A result associated with a moderate risk of metastasis (median risk score: 7), and a Class 2B result considered a high-risk factor (median risk score: 9) (). A greater degree of uniformity in responses from clinician regarding Class 2A and Class 2B as a perceived higher risk for metastasis was observed; whereas, a greater diversity of responses was observed for assessment of metastatic risk regarding a Class 1 result (). When assessing clinicopathological features, clinicians rated immunosuppression, perineural invasion ≥0.1 mm (PNI), invasion beyond subcutaneous fat, and tumor diameter >1 cm on the scalp as the clinicopathologic features associated with the highest risk for metastasis with median risk scores of 9, 9, 8, and 8, respectively. By comparison, clinicians found tumor diameter ≥2 cm below the neck and tumor in mask area (any size), to be of moderate risk with median risk scores of 7 and 7, respectively. Multiple comparison testing to compare the perceived risks associated with all considered features ( table) determined that a Class 1 result was perceived to be of statistically significant lower risk than all other features (p < .001), while Class 2B, along with PNI and immunosuppression, outranked all features as highest risk (p < .05).
Impact of 40-GEP test results on clinical management decisions of patient vignettes
Based upon clinical outcomes data from Farberg et al.Citation11, the pre-test baseline risk of metastasis was 17% and 32% (as described in Methods) for vignette 1 and vignette 2, respectively. The post-test Class 1 result for vignette 1 and 2 (reducing the risk for metastasis to 8% and 17%, respectively) caused clinicians to substantially increase their avoidance of sentinel lymph node biopsy (SLNB), adjuvant radiation, adjuvant chemotherapy, and nodal imaging, as well as shift toward longer follow-up intervals (), all of which aligns with a management plan with lower intensity interventions. When a Class 2A result was given (slightly altering the risk of metastasis of vignette 1 and vignette 2 to 16% and 35%, respectively), the clinicians made only minor changes to their management. A Class 2B result (increasing the risk for metastasis for vignette 1 and vignette 2 to 50% and 88%, respectively) led clinicians to choose a higher intensity management plan for both patient vignettes with significant increases in recommendations for SLNB, adjuvant radiation, adjuvant chemotherapy, nodal imaging, as well as shorter follow-up intervals. Across all decision points for both vignettes, ≥90% of changes in management were aligned with the appropriate intensity change for Class 1 (reduced) or for Class 2B (increased) ().
Figure 2. Effect of 40-GEP test results on clinicians’ management decisions regarding: follow-up intervals, SLNB, nodal imaging, adjuvant radiation, and adjuvant chemotherapy. Percentage of clinicians who would develop either a low (blue bar), moderate (orange bar), or high (red bar) intensity management plan based on pre-test (no 40-GEP data), then, post-test (Class 1, 2A, or 2B) results. Using a Friedman’s test with Dunn’s multiple comparisons correction, statistical significance was determined for each vignette when all post-test 40-GEP results (Class 1, 2A, or 2B) were compared to pre-test 40-GEP (no 40-GEP). F/U: follow-up; SLNB: sentinel lymph node biopsy; imaging: nodal imaging; chemo: adjuvant chemotherapy; RT: adjuvant radiation.
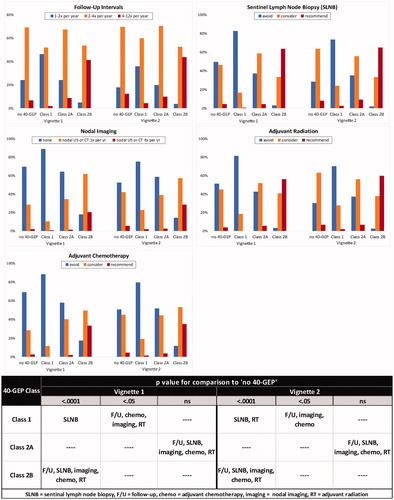
Table 3. Comparison of changes by management modality.
Discussion
In cSCC, the risk of metastasis determines treatment plans and risk estimation from current clinicopathologic paradigms (i.e. AJCC and BWH) alone is limited. Thus, there is a need for improved prognostic risk assessment methods to help clinicians make informed decisions about cSCC patient management.
NCCN guidelines encompass the most comprehensive list of features that define high-risk cSCC and also provide broad recommendations for management plans of both low- and high-risk patients. This study identified diversity among clinicians as to which clinical and pathological features, including 40-GEP Class results, they associate with a patient’s risk of metastasis (). Distinguishing which cSCC patients are at greatest risk for metastasis can be a significant challenge during patient management.
Multiple studies have used a variety of methods to rank individual clinicopathologic features according to metastatic risk (independent of other patient clinical characteristics), resulting in a diversity of outcomesCitation12–15. These studies reflect the inconsistent risk that is reported from study to study for features generally considered to determine the likelihood of metastasis and advocates the need for improvement upon risk-alignment during clinical management decisions. Our study demonstrates how the addition of molecular-based prognostic information could be integrated into current clinical practice to help guide management choices. shows that there was some variance in the perceived metastatic risk when considering only a 40-GEP Class 1 result. Then, when given the clinical characteristics of the patient vignettes () to create a more comprehensive representation of an actual patient, clinicians were able to apply a 40-GEP Class 1 result in a more consistent risk-appropriate manner.
Table 2. Clinical characteristics of patient vignettes.
In this study, a further effect of using molecular-based information as a method to guide clinical decisions can be seen when comparing management decisions between vignettes 1 and 2. In vignette 2, the introduction of “tumor invasion beyond subcutaneous fat,” a clinicopathological feature deemed to be of highest concern for metastasis (), led clinicians to considerably elevate the intensity of management at the pre-test “no 40-GEP” baseline when compared to the pre-test “no 40-GEP” of vignette 1 for SLNB, adjuvant radiation, adjuvant chemotherapy, and imaging. Interestingly, regardless of their response in the pre-test, the presence of a Class 1 40-GEP result drove the clinicians to select similar management strategies for vignettes 1 and 2 (). This same trend of making risk-aligned management decisions based on the risk of metastasis as determined by the 40-GEP test was seen when either a Class 2A or Class 2B result was given. These responses demonstrate that when given an objective, molecular prognostic factor, such as a 40-GEP test result, a standardized risk-aligned management plan could be applied appropriately.
A limitation of the study was the capture of data during a national dermatology conference, which does not allow for the follow-up studies that would benefit from the use of the same population of respondents. Also, it is important to note that this study does not emphasize the prognostic capabilities of the 40-GEP test itself, yet only how clinicians may alter management decisions for their cSCC patients.
The findings of this study suggest that the independent, additional information provided by the 40-GEP test can appropriately augment management of cSCC patients while remaining within established guidelines. Clinicians most commonly chose a moderate level (considering adjuvant therapies) of management as a baseline for vignette 1 and 2. With the introduction of a 40-GEP Class 1 result, they made a more definitive, risk-appropriate decision toward a NCCN low management approach. According to Farberg et al., the scenario in which a patient is deemed high-risk by T stage and/or NCCN guidelines, but is biologically low risk, as evidenced by a Class 1 result, may be quite common. This study shows that of 300 NCCN-defined high-risk patients, more than 50% were defined as Class 1, meaning their treatment could be managed more conservatively. When clinicians were given a Class 2A result, which only slightly changed the risk of metastasis, a moderate management plan was typically chosen. This treatment approach also aligns with Farberg’s study in which metastasis risk bins were stratified, based on 40-GEP results and T stage, into low, moderate, and high intensity management to correspond with metastasis risk of <10%, 10–50%, and >50%, respectively. When presented with a Class 2B result, clinicians elevated management to align with risk, more frequently recommending adjuvant therapies, nodal imaging, and follow-ups. Interestingly, of the 300 NCCN high-risk patients from Farberg’s study, only 8% were defined as Class 2B, warranting the high intensity management plan. This implies that a significant proportion of patients defined by NCCN as high risk, may be receiving a more aggressive management regimen than might be necessary.
Conclusions
The data presented in this study support that clinicians can appropriately incorporate 40-GEP test results to assist in management decisions for high-risk cSCC patients. Management plans were altered in a risk-appropriate manner to align with 40-GEP Class results and clinicopathologic features. The findings of this study suggest the possibility of more appropriate management and efficient resource allocation for cSCC patients when 40-GEP information is included in prognostic risk assessment.
Transparency
Declaration of funding
Research for this study was supported by Castle Biosciences, Inc.
Declaration of financial/other relationships
GHL participated in a research fellowship, which was partially funded by Castle Biosciences, Inc. DSR is a consultant and a member of the Speaker Bureau for Castle Biosciences, Inc. ALF, SJK, and RWC are employees and also hold stock options at Castle Biosciences, Inc. A peer reviewer on this manuscript discloses participation in research projects with Castle Biosciences, but they have not been implicated in this manuscript nor in this study. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no other relevant financial relationships to disclose.
Author contributions
GHL, ALF, and DSR were involved in the design of the study and prepared all documents necessary for IRB exempt status. GHL, ALF, and SJK analyzed and interpreted the data. ALF drafted the manuscript. GHL, SJK, RWC, and DSR were involved with revising the manuscript critically for intellectual content. DSR presented material necessary for clinicians to participate in the study. All authors approved the final version of the manuscript submitted and agree to be accountable for all aspects of the work.
Acknowledgements
The authors thank Derek Maetzold and Federico Monzon, MD for their expert advice and intellectual guidance while writing this manuscript, as well as Mary A. Hall, PhD for her intellectual contributions (all are employees at Castle Biosciences, Inc.).
References
- Skin Cancer Foundation: Skin Cancer Facts & Statistics [Internet]. The Skin Cancer Foundation, 2019. [cited 2019 Oct 21]. Available from: https://www.skincancer.org/skin-cancer-information/skin-cancer-facts/#nonmelanoma
- Muzic JG, Schmitt AR, Wright AC, et al. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: a population-based study in Olmsted County, Minnesota, 2000 to 2010. Mayo Clin Proc. 2017;92(6):890–898.
- National Comprehensive Cancer Network: Squamous Cell Skin Cancer, NCCN Guidelines Version 1.2020, in NCCN Clinical Practice Guidelines in Oncology [Internet]. 2019. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx#site
- Wysong A, Newman J, Covington K, et al. Development and validation of a prognostic gene expression profile (GEP) for stratification of cutaneous squamous cell carcinoma (SCC) patients by 3-year risk of regional or distant metastases. Presented at American Society for Dermatologic Surgery Annual Meeting, October 2019, Chicago, IL.
- Ruiz ES, Karia PS, Besaw R, et al. Performance of the American Joint Committee on Cancer Staging Manual, 8th Edition vs the Brigham and Women’s Hospital Tumor Classification System for cutaneous squamous cell carcinoma. JAMA Dermatol. 2019;155(7):819.
- Karia PS, Morgan FC, Califano JA, et al. Comparison of tumor classifications for cutaneous squamous cell carcinoma of the head and neck in the 7th vs 8th edition of the AJCC Cancer Staging Manual. JAMA Dermatol. 2018;154(2):175–181.
- Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149(4):402.
- Karia PS, Jambusaria-Pahlajani A, Harrington DP, et al. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital Tumor Staging for cutaneous squamous cell carcinoma. JCO. 2014;32(4):327–334.
- Canueto J, Burguillo J, Moyano-Bueno D, et al. Comparing the eighth and the seventh editions of the American Joint Committee on Cancer Staging System and the Brigham and Women’s Hospital Alternative Staging System for cutaneous squamous cell carcinoma: implications for clinical practice. J Am Acad Dermatol. 2019;80(1):106–113.
- Wysong A, Newman JG, Covington KR, et al. Validation of a 40-gene expression profile test to predict metastatic risk in localized high-risk cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2020. DOI:10.1016/j.jaad.2020.04.088
- Farberg A, Hall M, Douglas L, et al. Integrating gene expression profiling into NCCN high-risk cutaneous squamous cell carcinoma management recommendations: impact on patient management. Curr Med Res Opin. 2020. DOI:10.1080/03007995.2020.1763284
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152(4):419–428.
- Peat B, Insull P, Ayers R. Risk stratification for metastasis from cutaneous squamous cell carcinoma of the head and neck. ANZ J Surg. 2012;82(4):230–233.
- Haisma M, Plaat B, Bijl H, et al. Multivariate analysis of potential risk factors for lymph node metastasis in patients with cutaneous squamous cell carcinoma of the head and neck. J Am Acad Dermatol. 2016;75(4):722–730.
- Stewart T, Saunders A. Risk factors for positive margins after wide local excision of cutaneous squamous cell carcinoma. J Dermatolog Treat. 2018;29(7):706–708.
