Abstract
Objective: To integrate gene expression profiling into the management of high-risk cutaneous squamous cell carcinoma (cSCC) within the National Comprehensive Cancer Network (NCCN) guidelines to improve risk-aligned management recommendations.
Methods: A cohort of 300 NCCN-defined high-risk cSCC patients, along with the American Joint Committee on Cancer (AJCC) T stage, Brigham and Women’s Hospital (BWH) T stage, and known patient outcomes were analyzed. Risk classifications using a validated 40-gene expression profile (40-GEP) test and T stage were applied to NCCN patient management guidelines. Risk-directed patient management recommendations within the NCCN guidelines framework were aligned based on risk for metastasis.
Results: Of the 300 NCCN high-risk cSCC patients, 159 (53.0%) were 40-GEP Class 1 and AJCC T1-T2, and 173 (57.7%) were Class 1 and BWH T1-2a, indicating low risk for metastasis and, thereby, suggesting low management intensity. The 40-GEP integration suggested high intensity management for only 24 (8.0%) patients (all Class 2B), and moderate intensity management for the remainder of the cohort.
Conclusions: The 40-GEP test can be integrated within existing NCCN guideline recommendations for managing cSCC patients to help refine risk-directed management decisions. Integration of the 40-GEP test would allow >50% of this NCCN-defined high-risk cohort to be managed with the lowest intensity recommendations within the broad NCCN guidelines. High intensity management was deemed risk-appropriate for a small subpopulation (8.0%). This study demonstrates that the 40-GEP test, in combination with T stage, has clinical utility to impact patient management decisions in NCCN high-risk cSCC for improving risk-aligned management within the NCCN guidelines framework.
Introduction
Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer after basal cell carcinomaCitation1–4. It occurs in approximately one million people in the U.S. and the incidence is rising, partly due to enhanced detection methods and an aging populationCitation1,Citation4–8. Overall, approximately 6% of cSCC patients develop regional or distant metastatic lesions and survival rates are low for those who do develop metastasisCitation5,Citation7,Citation9–13. The number of deaths from cSCC, a large proportion of which are preceded by metastasis, has been estimated to rival that from melanomaCitation4,Citation14. Therefore, accurate prediction of risk for metastasis is essential for optimal patient management and, ultimately, to potentially improve outcomes.
National Comprehensive Cancer Network (NCCN) guidelines outline management pathways for low- and high-risk cSCC patients. Within this framework, the NCCN management recommendations are broad for patients who have a low likelihood of recurrence and/or metastasis, as are recommendations for cSCC patients considered high risk for developing recurrence and/or metastasisCitation5. Risk stratification and staging systems for cSCC include NCCN guidelines criteria, the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (8th Edition, AJCC8), and the Brigham and Women’s Hospital (BWH) tumor classification systemCitation5,Citation15–19. These systems are based on clinical and pathological features; however, they are specifically limited in their ability to predict adverse events such as metastasis (i.e. have low positive predictive value [PPV] for metastasis)Citation17–21 and pose a challenge to implementing risk-directed patient management. Patients with cSCC would benefit from improved prognostic tools for determining which patients currently considered clinicopathologically “high risk” are truly at low risk for metastasis, which patients should consider procedures to detect nodal or distant disease (e.g. nodal biopsy versus imaging versus clinical examination only), and which should consider or be recommended adjuvant therapeutic intervention to reduce risk for recurrence and metastasis (e.g. adjuvant radiation, systemic therapy, additional surgery, and/or clinical trial enrollment). Given that risk classifications guide treatment plans, improved prognostic tools would enhance shared decision-making between physicians and their patients. Ultimately, the goal is early intervention for individuals who are likely to develop metastasis, and avoidance of unnecessary invasive or costly procedures for those who are at lower risk for developing metastasis.
A 40-gene expression profile (40-GEP) test using archival, formalin-fixed paraffin-embedded (FFPE), primary cSCC tissue was recently developed and validatedCitation22. This test stratifies clinicopathologically-confirmed cSCC tumors into three risk groups based on low (Class 1), high (Class 2A), and highest (Class 2B) risk for regional or distant metastasis at 3 years after diagnosisCitation22. A substantially higher PPV (60.0%) was found for the 40-GEP test for Class 2B relative to that found for the AJCC8 (32.8%) and BWH (35.1%) staging systems, while maintaining a negative predictive value (NPV) of approximately 90.0% (which is similar to that of the AJCC8 and BWH systems)Citation22. The primary goal of developing and validating the 40-GEP test was to improve metastasis risk prediction. Similar prognostic tests for cutaneous melanomaCitation23–26, uveal melanomaCitation27, breast cancerCitation28–30, prostate cancerCitation31,Citation32, and thyroid cancerCitation33,Citation34 have demonstrated clinical utility for guiding patient management decisionsCitation35–39.
The current study aimed to refine risk-directed patient management recommendations by integrating 40-GEP test results from NCCN-defined high-risk patients within the broad framework of the NCCN guidelinesCitation22. Herein, we demonstrate that integration of this molecular prognostic tool, which has higher PPV (and similar NPV) relative to current staging systems, identified >50% of the NCCN-defined high-risk cohort as low risk for metastasis (40-GEP Class 1 and low-risk T stage), with metastasis rates similar to rates in clinicopathologic low-risk cases. This suggests that this subgroup could be managed at the lower end of intensity within the NCCN guidelines for high-risk patients. By comparison, integration of the 40-GEP test also identified a smaller subgroup of patients (8% of the high-risk cohort) as the highest risk for metastasis (Class 2B tumor). This would warrant more aggressive intervention aligned with management at the higher end of intensity within the NCCN guidelines for high-risk patients, thereby achieving risk-appropriate allocation of surgical, imaging, and therapeutic resources. In all, integrating the 40-GEP test within risk-directed NCCN guidelines for patient management resulted in more personalized, risk-aligned treatment recommendations and potential improvement in net health outcomes.
Materials and methods
The 40-GEP test and data from the clinical validation cohort
Recently validated and reported by Wysong et al.Citation22, the 40-GEP test is an RT-PCR-based, gene expression profile (GEP), prognostic test that stratifies patients with clinicopathologically-confirmed cSCC tumors into three groups based on risk for metastasis (regional or distant) within 3 years of diagnosis: low (Class 1), high (Class 2A), and highest (Class 2B) risk. The current manuscript includes analysis of data from only those patients (n = 300) in the clinical validation cohort (described in Wysong et al.) who had high-risk cSCC per the NCCN guidelines definition. This high-risk cSCC cohort was analyzed to determine the impact of integrating the 40-GEP test into management of high-risk cSCC patients within the framework established by NCCN patient management guidelines. The following data for these high-risk cSCC patients were analyzed: metastasis risk class (40-GEP results), T stage (AJCC8 and BWH), and known patient outcomes (i.e. metastasis versus no metastasis). This high-risk cohort included only cases meeting study criteria and having one or more NCCN-defined high-risk features, as noted in . Briefly, criteria for study inclusion were pathologically-confirmed cSCC diagnosed after 1 January 2006; available FFPE primary cSCC tumor tissue; complete case report forms; and documented metastasis or minimum follow-up period of 3 years without metastasis. Study cohort demographics and clinical characteristics were monitored and underwent centralized pathology review ().
Table 1. Characteristics of the NCCN high-risk cSCC cohort (n = 300).
Statistical analysis and integration of 40-GEP results within risk-aligned management recommendations
For the NCCN-defined high-risk cohort (n = 300), the numbers of patients stratified in each 40-GEP Class and per AJCC8 and BWH T stage were analyzed. Corresponding metastasis rates for groups defined by 40-GEP Class 1, 2A, and 2B results, and by AJCC8 (T1-T2 and T3-T4) and BWH (T1-T2a and T2b-T3) T stage were categorized into risk bins of <10%, 10–49%, or ≥50% risk for metastasis, which were aligned with risk-appropriate recommendations for management intensity (low, moderate, or high, respectively). Kaplan–Meier analysis with log-rank testing was performed, as previously describedCitation22, to compare metastasis-free survival (MFS) rates of the patients across the three management intensity categories per AJCC8 or BWH T stage. A p-value of ≤ .05 was considered statistically significant.
Within the broad range of existing recommendations for high-risk cSCC patients with localized disease, NCCN management recommendations for follow-up frequency, nodal assessment (with or without imaging, biopsy, and/or neck dissection), adjuvant therapy, and clinical trials were aligned with low, moderate, and high intensity management strategies. Corresponding risk-aligned management plans within the lower, mid (moderate), and higher intensity ranges of the NCCN guidelines framework were recommended for high-risk cSCC patients.
Results
Cohort characteristics, 40-GEP risk classification, and outcomes
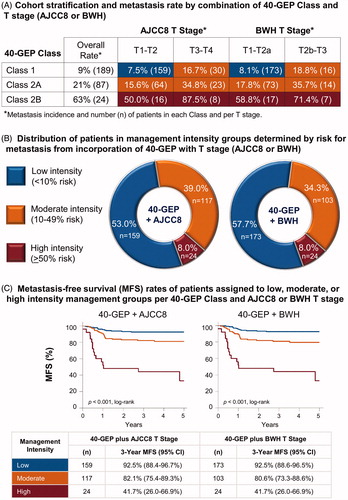
A 300-case cohort of NCCN-defined high-risk cSCC patients () was analyzed to integrate the recently validated 40-GEP test into NCCN guidelines and T stage criteria for patient management to improve risk-aligned management recommendations. The 40-GEP test classifies patients into three risk groups: Class 1, Class 2A, and Class 2B, having low, high, and highest risk for metastasis at 3 years post-diagnosis, respectively. Of the 300 cases, 189 (63.0%) were Class 1, 87 (29.0%) were Class 2A, and 24 (8.0%) were Class 2B with overall metastasis rates of 9%, 21%, and 63%, respectively (). More than 50% of the cases were Class 1 and AJCC8 T1-T2 (n = 159, 53.0%) or BWH T1-T2a (n = 173, 57.7%) with metastasis rates below 10% (AJCC8, 7.5%; BWH, 8.1%) (). Whereas, Class 1 cases that were also AJCC8 T3-T4 or BWH T2b-T3, as well as all Class 2A cases, had metastasis rates above 10%, but lower than 50%. All Class 2B cases (8.0% of the cohort) had metastasis rates that were greater than 50%.
Figure 1. Application of 40-GEP test results to NCCN-defined high-risk status and T stage for improving risk-appropriate management of cSCC. (A) Using a cohort (n = 300) of clinicopathologically-defined cSCC patients meeting study criteria and who were NCCN-defined high risk, the 40-GEP test stratified the patients into three groups depending on risk for metastasis at 3 years post-diagnosis: low (Class 1, n = 189), high (Class 2A, n = 87), or highest (Class 2B, n = 24). Patients stratified as Class 1, 2A, and 2B had a 9%, 21%, and 63% risk for metastasis, respectively, per the 40-GEP test alone. Corresponding AJCC8 and BWH T stages and metastasis rates were analyzed. (B) Incorporation of 40-GEP Class plus AJCC8 and BWH T stages into three metastasis risk bins (<10%, 10–49%, and ≥50% risk) resulted in low, moderate, and high intensity management strategies. The 40-GEP integration demonstrates low management intensity for 53.0% (AJCC8) or 57.7% (BWH), high intensity management for 8.0%, and moderate intensity management for the remainder (39.0%, AJCC8; 34.3%, BWH) of the 300-patient cohort. (C) Metastasis-free survival (MFS) rates of patients from the cohort risk-aligned with low, moderate, or high intensity management per 40-GEP Class and AJCC8 or BWH T stage. Kaplan–Meier analysis revealed 3-year MFS rates of 92.5% for patients in the low intensity management category (both AJCC8 and BWH), 82.1% and 80.6% for those in the moderate intensity category (AJCC8 and BWH, respectively), and 41.7% for patients in the high intensity category (both AJCC8 and BWH). Statistically significant differences were found for these MFS curves for both 40-GEP Class plus AJCC8 and 40-GEP Class plus BWH T stage (p ≤ .001, log-rank).
Clinical utility of integrating the 40-GEP test
By combining the low-risk Class 1 result with AJCC8 T1-T2 stage or BWH T1-T2a stage, a 3-year metastasis rate of 7.5% (NPV, 92.5%) or 8.1% (NPV, 91.9%), respectively, was identified for this subgroup within the NCCN-defined high-risk cohort (). This metastasis rate approaches the rate reported for the general cSCC patient population (≤6% metastasis)Citation7,Citation9–11,Citation13,Citation17. Of the Class 1 cases, 159 and 173 were AJCC8 T1-T2 and BWH T1-T2a, respectively, and were risk-aligned (<10% risk for metastasis) for receiving low intensity management (). The 40-GEP test identified a highest-risk (Class 2B) subpopulation (n = 24, 8.0%), which was risk-aligned (≥50% risk for metastasis) for receiving high intensity management (16 and 8 of the patients were AJCC8 T1-T2 and T3-T4, respectively; 17 and 7 were BWH T1-2a and T2b-T3, respectively). Of the remainder of the cohort, 64 were Class 2A/AJCC8 T1-T2 and 73 were Class 2A/BWH T1-T2a, with a risk for metastasis of 15.6% and 17.8%, respectively (). These rates are lower than that for the overall Class 2A rate for the cohort, but still more than twice that of the general cSCC patient population. Moderate intensity management was suggested for this group, as well as those patients who were Class 1 or 2A and AJCC8 T3-T4 or BWH T2b-T3 (10–49% risk for metastasis, ).
The 40-GEP test results, when adjusted for AJCC8 or BWH T stage in this study, suggest low management intensity for 53.0% or 57.7% of the 300-patient cohort, respectively (). Integration of the 40-GEP test also suggests maintaining moderate intensity management for 39.0% (40-GEP + AJCC8) or 34.3% (40-GEP + BWH), and high intensity patient management for 8.0% of the cohort (). Kaplan–Meier analysis determined a statistically significant difference in 3-year MFS rates for the cases aligned with the low, moderate, and high-intensity management strategies (p ≤ .001, log-rank). The low management intensity group had an MFS rate of 92.5% (95% CI, 88.4–96.7% or 88.6–96.5% for 40-GEP Class plus AJCC8 or BWH staging, respectively) (). The moderate management intensity group had an MFS rate of 82.1% (95% CI, 75.4–89.3%) or 80.6% (95% CI, 73.3–88.6%) for 40-GEP Class plus AJCC8 or BWH, respectively, and the high management intensity group had an MFS rate of 41.7% (95% CI, 26.0–66.9%) for 40-GEP Class plus AJCC8 or BWH ().
Discussion
The 40-GEP test was recently reported to be an independent predictor of 3-year metastasis risk in cSCC with improved metastasis risk prediction compared to AJCC8 and BWHCitation22. Herein, the 40-GEP test results for a cohort of 300 NCCN-defined high-risk cSCC patients were combined with T stage, and corresponding metastasis risk bins (<10%, 10–49%, and ≥50% risk) were aligned with risk-directed management intensities (low, moderate, and high intensity, respectively). Statistical analysis demonstrated significant differences in MFS rates at three years post-diagnosis (the period during which most cSCC metastases occur) for patients across the three management intensity categories when 40-GEP Class was combined with either AJCC8 or BWH T stage. Recommendations for improving risk-aligned patient management via low, moderate, and high intensity management plans were developed within the NCCN guidelines framework.
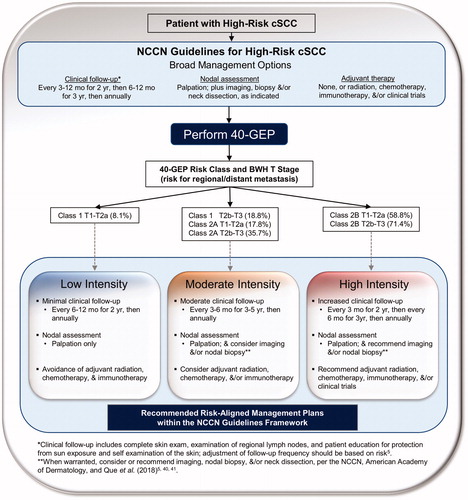
As shown in , within the NCCN guidelines framework, low intensity management for these types of Class 1 patients would involve low frequency follow-up visits (1–2 visits/year), nodal assessments via palpation only, and avoidance of adjuvant radiation therapy (RT) or systemic therapy. Moderate intensity management based on NCCN recommendations would allow for fewer follow-up visits relative to high intensity management (). Follow-up would include nodal palpation and consideration of imaging and/or nodal biopsy or dissection (when warranted)Citation5,Citation40,Citation41 as well as adjuvant radiation, chemotherapy, and/or immunotherapy during moderate intensity management. This would potentially result in fewer invasive procedures (less imaging, fewer nodal biopsies and dissections) and more sparing use of adjuvant therapy relative to high intensity management during which many of these procedures, including clinical trials, would be recommended ().
Figure 2. Recommended risk-aligned cSCC patient management plans within the NCCN guidelines framework for prognostic groups based on 40-GEP Class and T stage. Risk for regional/distant metastasis is reported for 40-GEP Class and BWH T stage. *Adjustment of clinical follow-up should be based on risk per the NCCN guidelines. **Imaging, nodal biopsy, and/or neck dissection should be considered (moderate intensity management) or recommended (high intensity management) when warranted per the NCCN, American Academy of Dermatology, and Que et al.Citation41.
This integration demonstrates the validated 40-GEP prognostic test has clinical utility for complementing current staging systemsCitation15–17 and refining risk-appropriate pathways recommended by national guidelinesCitation5 for improved management of cSCC patients deemed high risk by clinicopathologic methods. The 40-GEP test provides more accurate prediction of risk for metastasis in NCCN-defined high-risk cSCC patients within three years post-diagnosis, enabling improved risk-directed management decisions for therapy and surveillance. The current study reports the value of the test to identify within an NCCN high-risk cSCC patient population: (1) low-risk patients, having metastasis rates similar to rates of the general cSCC patient population, and who could benefit from management within the lower intensity end of the spectrum of guideline recommendations, including avoiding adjuvant therapy; and (2) truly high-risk patients who may benefit from management aligned within the highest intensity end of the spectrum of existing management recommendations. The value of more accurate prognosis is the ability to have better-informed decision making for aligning low and high risk of poor outcomes with appropriate management strategies. Collectively, the 40-GEP test may, in combination with AJCC8 or BWH T stage, help refine risk-directed management of high-risk cSCC patients within the current framework of NCCN guidelines for these patients. In summary, integration of the 40-GEP test into management of high-risk cSCC could enable improved risk-aligned management for the majority of patients tested and potentially improve net health outcomes.
Conclusions
The 40-GEP test can be integrated within NCCN guideline recommendations and, in combination with T stage, may have clinical utility for impacting and improving risk-aligned cSCC patient management.
Transparency
Declaration of funding
Research for this study and writing of this manuscript were supported by Castle Biosciences, Inc.
Declaration of financial/other relationships
ASF and SMD serve as members on the advisory board for Castle Biosciences, Inc. MAH, KRC, SJK, and RWC are employees and also hold stock options at Castle Biosciences, Inc. A peer reviewer on this manuscript discloses cooperation with Castle Biosciences in research works on cutaneous squamous cell carcinoma, but they have not been implicated in this particular paper. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no other relevant financial relationships to disclose.
Author contributions
ASF, RWC, SJK, KRC, and SMD were involved in the conception and design, as well as analysis and interpretation of data. MAH, SJK, and RWC drafted the manuscript. ASF, MAH, LD, SJK, RWC, and SMD were involved with revising it critically for intellectual content. All authors approved the final version of the manuscript submitted and agree to be accountable for all aspects of the work.
Previous presentations
Data from this study were submitted for poster presentation at the 2020 Winter Clinical Dermatology Conference, January 17–22, 2020, and the Maui Derm for Dermatologists 2020 meeting, January 25–29, 2020.
Acknowledgements
The authors thank Federico Monzon, MD for his expert advice and intellectual guidance while writing and revising this manuscript, as well as Alison Fitzgerald, PhD for her intellectual contributions (both employees at Castle Biosciences, Inc.).
References
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33(1):1–12.
- Genders RE, Weijns ME, Dekkers OM, et al. Metastasis of cutaneous squamous cell carcinoma in organ transplant recipients and the immunocompetent population: is there a difference? A systematic review and meta‐analysis. J Eur Acad Dermatol Venereol. 2019;33(5):828–841.
- Keyal U, Bhatta AK, Zhang G, et al. Present and future perspectives of photodynamic therapy for cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2019;80(3):765–773.
- Skin Cancer Foundation: Skin Cancer Facts & Statistics [Internet]. The Skin Cancer Foundation, 2019. [cited 2019 Oct 21]. Available from: https://www.skincancer.org/skin-cancer-information/skin-cancer-facts/
- National Comprehensive Cancer Network: Squamous Cell Skin Cancer, NCCN Guidelines Version 1.2020, in NCCN Clinical Practice Guidelines in Oncology [Internet]. 2019. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx#site
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151(10):1081–1086.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: Estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957–966.
- Muzic JG, Schmitt AR, Wright AC, et al. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2017;92(6):890–898.
- Feinstein S, Higgins S, Ahadiat O, et al. A retrospective cohort study of cutaneous squamous cell carcinoma with lymph node metastasis: risk factors and clinical course. Dermatol Surg. 2019;45(6):772–781.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma outcomes: a systematic review and meta-analysis. JAMA Dermatol. 2016;152(4):419–428.
- Belkin D, Carucci JA. Mohs surgery for squamous cell carcinoma. Dermatol Clin. 2011;29(2):161–174.
- Yom SS. Integrating the management of nodal metastasis into the treatment of nonmelanoma skin cancer. Semin Radiat Oncol. 2019;29(2):171–179.
- Karia PS, Morgan FC, Ruiz ES, et al. Clinical and incidental perineural invasion of cutaneous squamous cell carcinoma: a systematic review and pooled analysis of outcomes data. JAMA Dermatol. 2017;153(8):781.
- National Cancer Institute, National Institutes of Health: Melanoma of the Skin - Cancer Stat Facts [Internet]. SEER [cited 2019 Oct 24]. Available from: https://seer.cancer.gov/statfacts/html/melan.html.
- Amin MB, Edge S, Greene F, et al., editors. AJCC cancer staging manual. 8th ed. New York: Springer International Publishing, 2017.
- Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99.
- Ruiz ES, Karia PS, Besaw R, et al. Performance of the American Joint Committee on cancer staging manual, 8th edition vs the Brigham and Women’s Hospital Tumor Classification System for Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2019;155(7):819.
- Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149(4):402.
- Karia PS, Jambusaria-Pahlajani A, Harrington DP, et al. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital Tumor Staging for Cutaneous Squamous Cell Carcinoma. JCO. 2014;32(4):327–334.
- Roscher I, Falk RS, Vos L, et al. Notice of retraction and replacement: Roscher et al. validating 4 staging systems for cutaneous squamous cell carcinoma using population-based data: a nested case-control study. JAMA Dermatol. 2018;154(4)428–434.
- Karia PS, Morgan FC, Califano JA, et al. Comparison of tumor classifications for cutaneous squamous cell carcinoma of the head and neck in the 7th vs 8th edition of the AJCC cancer staging manual. JAMA Dermatol. 2018;154(2):175.
- Wysong A, Newman JG, Covington KR, et al. Validation of a 40-gene expression profile test to predict metastatic risk in localized high-risk cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2020. DOI:10.1016/j.jaad.2020.04.088
- Gerami P, Cook RW, Wilkinson J, et al. Development of a prognostic genetic signature to predict the metastatic risk associated with cutaneous melanoma. Clin Cancer Res. 2015;21(1):175–183.
- Cook RW, Middlebrook B, Wilkinson J, et al. Analytic validity of DecisionDx-Melanoma, a gene expression profile test for determining metastatic risk in melanoma patients. Diagn Pathol. 2018;13(1):13.
- Keller J, Schwartz TL, Lizalek JM, et al. Prospective validation of the prognostic 31-gene expression profiling test in primary cutaneous melanoma. Cancer Med. 2019;8(5):2205–2212.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1-T2 melanoma using gene expression profiling. Future Oncol. 2019;15(11):1207–1217.
- Onken MD, Worley LA, Char DH, et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012;119(8):1596–1603.
- Scope A, Essat M, Pandor A, et al. Gene expression profiling and expanded immunohistochemistry tests to guide selection of chemotherapy regimens in breast cancer management: a systematic review. Int J Technol Assess Health Care. 2017;33(1):32–45.
- Ward S, Scope A, Rafia R, et al. Gene expression profiling and expanded immunohistochemistry tests to guide the use of adjuvant chemotherapy in breast cancer management: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2013;17(44):1–302.
- McVeigh TP, Kerin MJ. Clinical use of the Oncotype DX genomic test to guide treatment decisions for patients with invasive breast cancer. BCTT. 2017;9:393–400.
- Alford AV, Brito JM, Yadav KK, et al. The use of biomarkers in prostate cancer screening and treatment. Rev Urol. 2017;19:221–234.
- Kristiansen G. Markers of clinical utility in the differential diagnosis and prognosis of prostate cancer. Mod Pathol. 2018;31(S1):143–155.
- Vargas-Salas S, Martínez JR, Urra S, et al. Genetic testing for indeterminate thyroid cytology: review and meta-analysis. Endocr Relat Cancer. 2018;25(3):R163–R177.
- Yip L. Molecular markers for thyroid cancer diagnosis, prognosis, and targeted therapy. J Surg Oncol. 2015;111(1):43–50.
- Berger AC, Davidson RS, Poitras JK, et al. Clinical impact of a 31-gene expression profile test for cutaneous melanoma in 156 prospectively and consecutively tested patients. Curr Med Res Opin. 2016;32(9):1599–1604.
- Farberg AS, Glazer AM, White R, et al. Impact of a 31-gene expression profiling test for cutaneous melanoma on dermatologists’ clinical management decisions. J Drugs Dermatol. 2017;16(5):428–431.
- Dillon LD, Gadzia JE, Davidson RS, et al. Prospective, multicenter clinical impact evaluation of a 31-gene expression profile test for management of melanoma patients. J Skin. 2018;2(2):111–121.
- Aaberg TM, Cook RW, Oelschlager K, et al. Current clinical practice: differential management of uveal melanoma in the era of molecular tumor analyses. Clin Ophthalmol. 2014;8:2449–2460.
- Plasseraud KM, Cook RW, Tsai T, et al. Clinical performance and management outcomes with the DecisionDx-UM gene expression profile test in a prospective multicenter study. J Oncol. 2016;2016:1–9.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78(3):560–578.
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78(2):249–261.
