Abstract
Objective
Evaluate clinical characteristics, comorbidity burden, major depressive disorder (MDD)-related healthcare resource utilization (HCRU), medication burden, and antidepressant treatment (ADT) patterns among older adults with MDD with and without selected comorbidities.
Methods
Using Komodo’s Healthcare Map claims data (1/1/2016-9/30/2022), patients with MDD (≥65 years) treated with ADTs were assessed 24 months preceding (baseline) and 12 months following (follow-up) first observed ADT prescription fill (index). Patients were separated into cohorts of those with ≥1 of 5 selected comorbidities and those without. Clinical characteristics, comorbidities, and MDD-related HCRU were assessed during baseline; treatment patterns were assessed during follow-up. Baseline and follow-up all-cause and comorbidity-specific medication burdens (mean prescription claims/month) were determined.
Results
Among the total cohort (N = 417,643), 97.1% had ≥1 of 5 selected comorbidities: hypertension (80.3%), hyperlipidemia (75.4%), diabetes (54.2%), anxiety disorder (39.0%), and chronic obstructive pulmonary disorder (19.5%). Baseline and follow-up all-cause medication burdens per month were 3.8 and 4.5 for patients with selected comorbidities and 1.7 and 2.3 for those without. During baseline, most patients (96.0% with selected comorbidities, 96.2% without) had ≥1 outpatient visit, and a numerically higher percentage of those with vs. without selected comorbidities had MDD-related emergency room (13.9% vs. 6.0%) and inpatient (13.5% vs. 4.1%) visits. The majority of both cohorts (61.0% with selected comorbidities, 59.5% without) underwent treatment pattern changes.
Conclusion
This study highlights the medication burden and ADT patterns in older adults with MDD, assessing these outcomes among patients with and without comorbidities. Numerically higher medication burdens among those with selected comorbidities suggests future studies could investigate the impact of comorbidities on MDD-related care.
Introduction
Major Depressive Disorder (MDD) is a common medical illness that affects millions of individuals worldwide. MDD is characterized by the presentation of five or more depressive symptoms, occurring for at least 2 weeks and representing a change from previous functioningCitation1,Citation2. Depressive symptoms may include feelings of worthlessness, fatigue, diminished interests, reduced cognitive and social functioning, and recurrent thoughts of deathCitation1. Depression can have substantial negative impacts on an individual’s quality of life, and it has been recognized as a leading cause of disability around the worldCitation3,Citation4. It is estimated that in 2021, 21 million adults experienced a major depressive episode (MDE) in the United States, of which 1.5 million were older adults (≥65 years of age)Citation5. Notably, the prevalence of MDE in older adults increased by 60% between 2010 to 2019Citation6.
MDD is a challenging condition to treat, and treatment options include, but are not limited to, medications (e.g. antidepressants), psychotherapy, and electroconvulsive therapyCitation2. Patients often require an individualized treatment plan with a “trial and error” pharmacotherapeutic approachCitation7. In addition, payers usually require “step therapy” protocols, which entail patients trying and “failing” a first-line antidepressant medication prior to receiving (and acquiring coverage for) a second-line medicationCitation8. Remission of MDD symptoms can be difficult to achieve: one study found only approximately one-third of patients achieved remission for their symptoms following first-line treatment with a selective serotonin reuptake inhibitor (SSRI), and the estimated cumulative remission rate across four lines of treatment was only 67% following 3 months of treatmentCitation9. Low remission rates and medication intolerability may lead many patients to undergo treatment changes (e.g. discontinuation of medication, switching therapies, or augmentation of current therapies)Citation8,Citation9.
The etiology of MDD is complex; MDD may develop as a result of a combination of biological, genetic, environmental, and psychological factorsCitation2. These factors are further compounded in older adults, as they often experience a number of health challenges. Diagnosing MDD in older adults can be associated with additional difficulties, as somatization is common among older adults with MDD, and depressive symptoms may manifest differently in adults with late-life depression than in younger adultsCitation10. Older adults may not recognize their depressive symptoms as stemming from a depressed mood and instead describe physical complaints, such as fatigue, weight loss, social withdrawal, problems with self-care, and other unexplained medical symptomsCitation11. Since such symptoms may be attributed to aging or to other physical conditionsCitation10, this may warrant increased scrutiny by clinicians to recognize that underlying depression may be a major contributorCitation12.
Given the potential for high-severity outcomes, such as suicide, increasing identification and treatment of MDD among older adults is crucial. Indeed, risk factors for suicide are complex and may be of particular concern among older adults. Investigations of suicidal ideation and behavior have identified several areas for future study, including relationships between MDD and neuroinflammation, specifically the dysregulation of the kynurenine pathway, which has been suggested as the potential biomarker for suicidal behavior in patients with psychiatric disordersCitation13. Additionally, the linkage between psychiatric disorders and demoralization further compounds the complexity of managing MDDCitation14. Suicide risk may be influenced by the presence of a psychiatric illness, such as MDD, as well as factors that may put older adults at particular risk, including low social connectedness and the presence of physical health conditionsCitation14,Citation15.
Older adults are frequently burdened by multiple comorbidities, which may also complicate the diagnosis, treatment, and management of MDDCitation11,Citation16. It has been well-established that older age is associated with a higher likelihood of experiencing comorbiditiesCitation17. However, the prevalence of comorbid conditions in older adults with MDD treated with ADT has not been extensively studied. This is a significant consideration, as prior studies have shown that the economic burden attributable to patients diagnosed with MDD is largely comprised of costs related to comorbid conditionsCitation18. Inadequate MDD care is also associated with a higher risk of adverse cardiovascular events, of which, both hyperlipidemia and hypertension are key risk factorsCitation19–21. Moreover, MDD has been implicated as a significant risk factor for the occurrence and worsening of a wide range of comorbid conditions (e.g. metabolic, autoimmune, and cardiovascular disorders)Citation22. Treatment of comorbid conditions can also increase the medication burden in patients which may further complicate the already difficult pharmacological management of MDDCitation23–26.
Prior evidence has indicated that older adults struggle with adherence to antidepressant treatments (ADT) due to factors including their cognitive status, comorbidities, and polypharmacy-related issuesCitation27. Polypharmacy is a well-known contributor to difficulties in medication adherence for patientsCitation28,Citation29, and it is estimated that 39% of older adults take ≥5 medications (i.e. polypharmacy)Citation30. Moreover, previous evidence has shown a positive relationship between polypharmacy and the occurrence of adverse drug events, hospitalization, and mortality in older adultsCitation31. Collectively, these considerations may contribute to findings indicating that older age is associated with poorer MDD diagnostic and symptom trajectoryCitation32.
Although older adults are more likely to have comorbidities that correspond to higher medication burden which may impact adherence to ADT, there is limited evidence regarding the prevalence of comorbidities, medication burden, and the ADT treatment patterns observed in older adults being treated for MDD. Furthermore, no studies have assessed if treatment patterns differ between those affected by the most commonly occurring comorbidities and those without prevalent comorbidities in this demographic. Leveraging real-world data, this study sought to assess the clinical characteristics, comorbidity burden, MDD-related healthcare resource utilization (HCRU), medication burden, and ADT treatment patterns among older adults with MDD being treated with ADT between those with ≥1 of the 5 common selected comorbidities and those without selected comorbidities.
Methods
Data source
The study was conducted using real-world administrative claims data from Komodo’s Healthcare MapTM database from January 1, 2016, to September 30, 2022. Komodo’s Healthcare MapTM is a real-world dataset that integrates disparate sources of patient-level data to map longitudinal patient journeys for more than 325 million patients that are well-distributed geographically across the US. Though both open and closed sources are integrated into the Healthcare Map, this study utilized closed data, which provide information on all patient healthcare interactions (including inpatient, outpatient, and prescription services) during plan enrollment. According to patient characteristic data from the Center for Disease Control and Prevention’s 2019 National Health Interview SurveyCitation33, the closed claim datasets from Komodo reflect the patient characteristics for the geographical, age, and sex distribution of insured patients in the United States.
Study design
This retrospective observational study leveraged real-world data from January 1, 2016, to September 30, 2022. Patients were required to be ≥65 years of age (i.e. older adults) at the time of the index date and to have prescription claims for ≥1 ADT during the identification period (January 1, 2018 - September 30, 2021). The index date was defined as the first observed ADT prescription claim (including SSRI, serotonin–norepinephrine reuptake inhibitor [SNRI], atypical antidepressant, tricyclic antidepressant, and monoamine oxidase inhibitor) during the identification period. Claims data were analyzed for patients who had ≥730 days of continuous medical and pharmacy enrollment prior to the index date (baseline period) and ≥365 days following the index date (follow-up period) (). Patients were considered for inclusion if they had an MDD diagnosis during the baseline period or within 30 days after the index date. MDD diagnosis was defined as: ≥1 MDD medical claim (ICD-10-CM: F32.0–F32.5, F32.8, F32.9, F33.0–F33.4, F33.8, F33.9) in an inpatient or emergency room setting, or ≥2 MDD medical claims in an outpatient setting, or ≥1 MDD medical claim and ≥1 depression medical claim in an outpatient setting.
Figure 1. Study timeline indicating identification period and example index date with baseline and follow-up periods.
Abbreviations: ADT, antidepressant treatment; MDD, major depressive disorder; Dx, diagnosis
Notes. Index date could occur any time during the identification period but had to be within 30 days prior to the MDD diagnosis date or anytime after this date to be eligible.
a≥1 MDD medical claim in an inpatient or emergency room setting OR ≥ 2 MDD medical claims in an outpatient setting, OR ≥1 MDD medical claims and ≥1 depression medical claim in an outpatient setting during the baseline period or within 30 days of the index date.
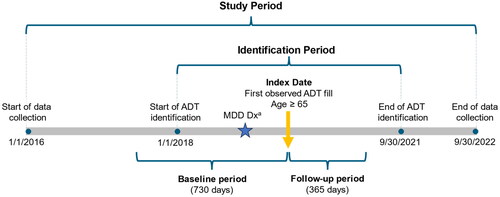
Patients were excluded if they had a diagnosis of bipolar disorder, schizophrenia, other psychosis-related disorders (Supplementary Table 1), dementia, or mild cognitive disorder or prescriptions of lithium during the baseline period. Additionally, patients were excluded if they had prescriptions claims initiated on the index date for the following: atypical or typical antipsychotics (Supplementary Table 2).
Eligible older adults with MDD who met the inclusion/exclusion criteria were then separated into two cohorts: those who experienced ≥1 of the 5 common selected comorbidities identified in the total patient cohort (with selected comorbidities) and those who did not experience any of the 5 selected comorbidities (without selected comorbidities). The 5 selected comorbidities were chosen based on the most prevalent comorbidities in the studied patient population (identified using ICD-10-CM codes) that coincide with comorbidities included in the Charlson Comorbidity Index (CCI) or other relevant conditions that were highly prevalent (i.e. hypertension and anxiety disorders). Of note, comorbidities were not mutually exclusive, so patients in the with selected comorbidities cohort could have more than one of the listed comorbidities.
Patients were required to have at least two claims for ≥1 of the 5 selected comorbidities to be included in the with selected comorbidities cohort, to increase specificity of the studied comorbidities as described previouslyCitation34. Patients in the without selected comorbidities cohort had 0 claims for each of the 5 selected comorbidities, and patients with only 1 claim for any of the selected comorbidities were not considered for analysis. In addition, differences between males and females with or without selected comorbidities were also evaluated.
Outcome measures
Patient demographics were assessed at the time of the index date, including the patient’s age, sex, region, and payer. Clinical characteristics, including the CCI and selected comorbidities, were assessed during the baseline period. MDD-related HCRU was also assessed during the baseline period, defined as using claims for outpatient, inpatient, and emergency room visits with an MDD ICD-10 diagnosis code in any field (e.g. primary, secondary, etc.).
All-cause, MDD-related (type and number of MDD medications), and comorbidity-specific medication burden were assessed over the baseline and follow-up periods. All-cause medication burden was based on the total number of prescription claims, and MDD-related medication burden was defined as the number of ADT claims in the pharmacy data for each patient. The medication burden was calculated as the mean number of prescription claims on a per-month basis. Other medications with psychiatric or psychotropic effects (Supplementary Table 3) were also assessed during baseline and follow-up.
Treatment patterns associated with the index treatment were assessed during follow-up. Index treatments could be monotherapies or combination therapies (ADT initiated on the index date and any additional ADT added in the next 7 days). Treatment patterns included adherence, persistence, discontinuation, switching, augmentation therapy, and add-on therapy. Definitions of treatment patterns are provided in ; of note, the claims data used in this study do not indicate the reason behind such treatment changes, so these characterizations do not indicate why patients changed or discontinued treatment.
Table 1. Treatment pattern definitions.
Descriptive analysis
Descriptive analyses were performed to assess patient characteristics during the baseline period and outcomes during the follow-up period. Means, medians, standard deviations (SDs), interquartile ranges (IQRs), minimums, and maximums were reported for continuous variables, and counts and proportions were reported for categorical variables. Adherence was evaluated using PDC, and treatment patterns, including discontinuation, switching, add-on therapy, and augmentation, were assessed within 3, 6, 9, and 12 months after the index date.
Ethical approval
This study did not require ethical approval, as it used only de-identified claims data for adult patients.
Results
Patient characteristics
A total of 417,643 older adults (≥65 years of age) with MDD met all of the inclusion criteria during the identification period. Of the total population cohort, 405,531 (97.1%) had ≥2 claims for ≥1 of the 5 selected comorbidities and 12,112 (2.9%) had 0 claims for selected comorbidities (). The mean (SD) age at the index date for those with selected comorbidities was 73.3 (6.0) and was 70.9 (5.5) for those without selected comorbidities; both cohorts were predominantly female (). The majority of both groups (81.9% and 65.6% of those with and without selected comorbidities, respectively) had Medicare coverage.
Figure 2. Attrition flowchart for cohorts of older adults with MDD treated with ADT.
Abbreviations: SSRI, selective serotonin reuptake inhibitors; SNRI, serotonin–norepinephrine reuptake inhibitors; TCA, tricyclic antidepressant; MAOI, monoamine oxidase inhibitor; MDD, major depressive disorder
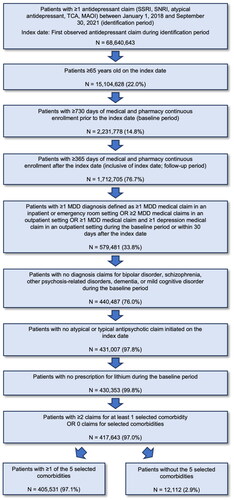
Table 2. Characteristics of patients at index.
Selected comorbidities
Based on the prevalence of comorbidities using ICD-10-CM codes that coincide with comorbidities included in the CCI or other relevant conditions that were highly prevalent (i.e. hypertension and anxiety disorders), the 5 selected comorbidities identified in this patient cohort were hypertension, hyperlipidemia, diabetes (type 1 and type 2), anxiety disorders, and chronic obstructive pulmonary disease (COPD, ). The majority of patients in both cohorts received MDD-related care during the baseline period, meeting our inclusion criteria. During the baseline period, nearly all patients in both groups (96.0% with selected comorbidities, 96.2% without selected comorbidities) had MDD-related outpatient visits, with smaller proportions in each group with emergency room or inpatient visits (). Given our use of a two-year baseline period and the fact that 75-80% of patients had prior treatment before the index, it is likely that they would have an MDD-related visit during that time period.
Figure 3. 5 selected comorbidities of interest in older adults with MDD treated with ADT.
Abbreviations: COPD, Chronic obstructive pulmonary disease; MDD, major depressive disorder; ADT, antidepressant treatment
Notes: The 5 selected comorbidities were identified based on the most prevalent comorbidities in the studied patient population (identified using ICD-10-CM codes) that coincide with comorbidities included in the Charlson Comorbidity Index (CCI) or other relevant conditions that were highly prevalent (i.e., hypertension and anxiety disorders). Results reported in are among patients in the study cohorts (with and without selected comorbidities; N= 417,643) to depict the prevalence of these conditions in older adult patients treated with MDD. Patients in the without comorbidities cohort did not experience any of the selected comorbidities.
The gray dots in represent the published prevalence rates of the selected comorbidities in older adults. These values were identified from the literature and have been shown as estimates where a range of values were provided in the existing literature. The data used in generating the points plotted above are as follows: An estimated 77.1% of US adults aged ≥65 were identified as having hypertension (2017–2020) [Citation35]. An estimated 54.6% of US males aged 65-74, 55.7% of US males aged 75+, 55.4% of US females aged 65-74, and 52.6% of US females aged 75+ were identified as having hypercholesterolemia (2015–2018) [Citation36]. An estimated 29.2% of US adults ≥65 have diagnosed or undiagnosed diabetes (2017–2020) [Citation37]. An estimated 20.2% of US adults aged 60-69 years, 15.1% of US adults aged 70-79 years, and 15.7% of US adults aged ≥80 years reported symptoms of anxiety disorder (October 18-30, 2023) [Citation38]. An estimated 10.8% of US adults aged ≥65 reported having ever been diagnosed with chronic obstructive pulmonary disease, C.O.P.D., emphysema, or chronic bronchitis (2020) [Citation39].
![Figure 3. 5 selected comorbidities of interest in older adults with MDD treated with ADT.Abbreviations: COPD, Chronic obstructive pulmonary disease; MDD, major depressive disorder; ADT, antidepressant treatmentNotes: The 5 selected comorbidities were identified based on the most prevalent comorbidities in the studied patient population (identified using ICD-10-CM codes) that coincide with comorbidities included in the Charlson Comorbidity Index (CCI) or other relevant conditions that were highly prevalent (i.e., hypertension and anxiety disorders). Results reported in Figure 3 are among patients in the study cohorts (with and without selected comorbidities; N= 417,643) to depict the prevalence of these conditions in older adult patients treated with MDD. Patients in the without comorbidities cohort did not experience any of the selected comorbidities.The gray dots in Figure 3 represent the published prevalence rates of the selected comorbidities in older adults. These values were identified from the literature and have been shown as estimates where a range of values were provided in the existing literature. The data used in generating the points plotted above are as follows: An estimated 77.1% of US adults aged ≥65 were identified as having hypertension (2017–2020) [Citation35]. An estimated 54.6% of US males aged 65-74, 55.7% of US males aged 75+, 55.4% of US females aged 65-74, and 52.6% of US females aged 75+ were identified as having hypercholesterolemia (2015–2018) [Citation36]. An estimated 29.2% of US adults ≥65 have diagnosed or undiagnosed diabetes (2017–2020) [Citation37]. An estimated 20.2% of US adults aged 60-69 years, 15.1% of US adults aged 70-79 years, and 15.7% of US adults aged ≥80 years reported symptoms of anxiety disorder (October 18-30, 2023) [Citation38]. An estimated 10.8% of US adults aged ≥65 reported having ever been diagnosed with chronic obstructive pulmonary disease, C.O.P.D., emphysema, or chronic bronchitis (2020) [Citation39].](/cms/asset/14534894-f432-47d3-8873-3d365e0967d2/icmo_a_2348603_f0003_c.jpg)
Table 3. Clinical characteristics extracted within the baseline period.
Medication burden
Regarding the ADTs initiated on the index date, SSRIs comprised the largest ADT category, with 54.6% of patients with selected comorbidities and 53.2% of patients without selected comorbidities initiating SSRI treatment on the index date (Supplementary Table 4). This was followed, in both groups, by atypical antidepressants, SNRIs, SSRI + atypical antidepressants, and TCAs. Of the total study patient cohort, 20.4% did not receive any MDD pharmacotherapy during the baseline period (Supplementary Table 4).
The all-cause per-month mean medication burden during the baseline period was 3.8 for patients with selected comorbidities and 1.7 for patients without a selected comorbidity; these values rose to 4.5 and 2.3, respectively, during the follow-up period (). The comorbidity-specific per-month mean medication burden for those with selected comorbidities was similar over the baseline and follow-up periods for each selected comorbidity (). Both total and comorbidity-specific medication burdens were highly variable across the study population, with high standard deviation values relative to the mean medication burden ( and ).
Table 4. Medication burden during baseline and follow-up periods for those with and without selected comorbidities.
Table 5. Medication burden specific to treating comorbidities in those with selected comorbidities (n = 405,531).
The prevalence of patients taking medications with psychiatric or psychotropic effects was generally lower in the without selected comorbidities cohort (). For the baseline period, the largest numerical differences in prevalence between the with and without selected comorbidities cohorts were observed with benzodiazepines (37.0% and 19.6%, respectively), followed by anticonvulsants (30.9% and 17.2%); for the follow-up period, the largest numerical differences were for anticonvulsants (28.7% and 17.2%, respectively), followed by benzodiazepines (29.1% and 18.2%; ).
Treatment patterns
Overall, treatment patterns were largely similar between the with and without selected comorbidities cohorts (). The percentage of adherent patients during the first 3 months of the follow-up period was 78.2% for patients with selected comorbidities and 77.6% for patients without selected comorbidities, which decreased to 54.9% and 54.8%, respectively, by 12 months. The percent of patients who did not undergo a treatment pattern change throughout the 12-month follow-up period was 39.0% in patients with selected comorbidities and 40.5% without selected comorbidities. The largest contributor to treatment pattern changes was discontinuation. For patients with selected comorbidities, 16.2% had discontinued treatment by 3 months in the follow-up period, and 32.9% had discontinued by 12 months; 17.1% and 24.4% of patients without selected comorbidities had discontinued treatment by 3 months and 12 months, respectively. The second largest contributor to treatment pattern changes was patients who initiated add-on therapy during the follow-up period. Smaller percentage of patients, both with and without selected comorbidities, augmented or switched ADTs. Treatment patterns were largely similar between males and females with and without selected comorbidities (Supplementary Table 5).
Table 6. Treatment patterns of ADTs in older adults with MDD.
Discussion
This study provides a robust and comprehensive analysis of comorbidity burden, MDD-related HCRU, medication burden, and treatment patterns among older adults (≥65 years) with MDD treated with ADT. We found that nearly all patients had at least one of the selected comorbidities, and these patients had numerically higher rates of MDD-related emergency room (ER) and inpatient visits at baseline, suggesting a potential association between comorbidity burden and the need for MDD-related care. By evaluating ADT treatment patterns, we also identified that MDD treatment in this population may pose challenges and require treatment changes, as were required by the majority of patients in this study.
Here we highlight the extent of comorbidities experienced by older adults with MDD treated with ADTs, as 97.1% of assessed patients had at least one of the 5 selected comorbidities, including hypertension, hyperlipidemia, diabetes, anxiety disorder, and COPD. The prevalence of 4 of the 5 selected comorbidities (hyperlipidemia, diabetes, anxiety disorder, and COPD) were higher in the studied cohort compared to reports on the prevalence of these conditions in the broader older adult population. Hypertension prevalence was similar to estimates provided by the Center for Disease Control (CDC) for the general population of older adults (80.3% in our study vs. 77.1% reported by the CDC)Citation35. In contrast, hyperlipidemia prevalence was higher in the studied cohort (75.4%) compared to values reported by the CDC in older adults (∼55%)Citation36, as was the prevalence of diabetes (54.2% vs 29.2%, respectively)Citation37. Anxiety disorders were also more prevalent in the studied cohort (39%) than observed in a national survey (15.1% and 20.2% of adults aged ≥60 years reported experiencing symptoms of anxiety)Citation38. This discrepancy between the study cohort and a broader older adult population was also observed for COPD, with the CDC estimating that 10.8% of adults 65 or older had been diagnosed with COPD, while 19.5% of our study cohort had claims for COPDCitation39.
Collectively, these findings support prior observations that MDD may increase the incidence of comorbidities, and indicate that hypertension, hyperlipidemia, diabetes, anxiety disorder, and COPD are more prevalent among older adults with MDD treated with ADTs compared to the general older adult population. These findings underscore the importance of future research investigating the link between MDD and comorbidity pathology. Such future research could also investigate how links between MDD and comorbidity burden influence HCRU among this population, as we also observed that patients with selected comorbidities had a numerically higher percentage of MDD-related emergency room (ER) (13.9% vs. 6.0%) and inpatient (13.5% vs. 4.1%) visits during the baseline period.
This study also reveals novel information regarding real-world ADT treatment patterns for older adults and how they compare to clinical guidance. Following successful treatment of a major depressive episode, the American Psychiatric Association (APA) practice guidelines indicate that treatment should be maintained 4-9 months prior to discontinuationCitation40. However, the current findings show that only ∼32.9% of older adults discontinued ADT treatment as their first pattern change by 12 months of follow-up. Although observations on discontinuation frequencies do not provide causal insight for the reason of discontinuation (e.g. remission), the fact that 67.1% of patients with selected comorbidities had either no change in treatment patterns or experienced a different type of treatment change by 12 months may indicate that the majority of older adult MDD patients did not experience remission of MDD symptoms following the observed first-line treatment. Indeed, prior studies have demonstrated that robust challenges exist for achieving remission of MDD by revealing the extent of treatment pattern changes that occur with existing therapeuticsCitation8,Citation41.
Notably, higher percentages of patients in the current study (39% and 40.5% of older adults with and without selected comorbidities, respectively) also had no ADT treatment pattern changes through a 1-year follow-up as compared to previous studies. Zhu et al. found that among adults (≥18 years) initiating monotherapy ADT for MDD, only 19.3% of patients had no changes in their ADT treatment patterns through a 1-year follow-upCitation8. Gauthier et al. observed that only 3.5% of patients had no changes in their treatment patterns with an overall mean follow-up duration of 4.1 yearsCitation41. Rates of discontinuation and switching were also higher in Zhu et al. than in the current study, with discontinuation rates of 56.2% (vs. 32.9% in patients with selected comorbidities) and switching rates of 24.5% (vs. 8.8%)Citation8.
A likely contributor to the discrepancies observed between the current findings and prior studies is that the majority of patients in the current study were not treatment-naïve prior to indexing. In this regard, 79.7% and 74.2% of those with and without selected comorbidities had prior MDD treatment claims before observations were made during the follow-up period in the current study. Since the majority of patients in our study had prior MDD treatment, they may have been more likely than treatment-naive adults to persist on the treatment regimen observed during the study period. These individuals’ experiences with MDD treatment and knowledge of which pharmacotherapies had previously been well-tolerated or effective may have informed their treatment. This potential impact on treatment decisions may have influenced the comparably higher percentage of our study population who experienced no treatment changes compared to the literature.
It is possible that the patients who experienced no treatment changes were satisfied with their ADT regimen and continued treatment through the end of the follow-up period. However, this population’s lack of treatment discontinuation may also suggest that a sizable percentage of our study population was at risk of recurrence and so kept on maintenance phase treatmentCitation40, or that the effects of their ADT were insufficient to induce remission and support treatment discontinuation.
Although this characteristic of our study population is a limitation for fully understanding the treatment pattern sequences in treatment-naïve older adults with MDD, this observation highlights a likely reality for many older adults with MDD; achieving remission of MDD symptoms is a challenging endeavor that can persist throughout older age. It is important to note that a large portion of older adult patients underwent treatment pattern changes, despite ∼79% of these patients having been exposed to MDD treatments prior to the follow-up observation period. These findings underscore the difficulty of achieving remission of MDD symptoms in older adults that may have greater experience with ADT treatments. Moreover, these observations do not vary between genders, as treatment patterns were largely similar between both males and females. Of note, despite the majority of patients not being treatment naïve, SSRIs were still the predominant ADT identified at the index date for both cohorts. The predominant use of SSRIs in the current study is similar to findings by Gauthier et al. and Zhu et al. likely reflecting the first-line use of SSRIsCitation8,Citation41.
Prior evidence has indicated that comorbid conditions may increase the medication burden of patients and that high medication burden (i.e. polypharmacy) is associated with poor medication adherenceCitation28,Citation29. We found that treatment patterns were largely similar between those with and without selected comorbidities, though numerically, the all-cause medication burden was higher in those with selected comorbidities than those without selected comorbidities (4.5 vs 2.3 claims per month). In both cohorts, we also observed high variability in both all-cause and comorbidity-specific medication burden, suggesting that this assessment is dependent on heavy users of health care resources, as is common among claims dataCitation42. Future studies could consider stratifying patients by healthcare use in order to assess how medication burden and HCRU are distributed within the population.
In terms of HCRU, both cohorts had the highest prevalence of MDD-related outpatient visits (96.0% [with selected comorbidities] vs 96.2% [without selected comorbidities]), though patients with selected comorbidities had a numerically higher prevalence of inpatient and emergency room visits during the baseline period than those without selected comorbidities. Nonetheless, there were no apparent differences across any of the treatment patterns assessed. However, patients were only required to have ≥1 of the 5 selected comorbidities to be included in the with selected comorbidities cohort, and this study did not make determinations on the severity of the comorbidity. Hence, it is plausible that this approach did not provide the needed granularity to determine if differences exist in treatment patterns between those experiencing multimorbidity or a more severe condition that would result in a higher medication burden. Future studies may be warranted to determine if patients with MDD receiving ADT coinciding with polypharmacy have altered treatment pattern sequences compared to those without polypharmacy. Similarly, future studies could investigate the impacts of comorbidities which are less common, but may require a higher volume of care, such as cancer, to characterize the HCRU and medication burdens associated with these conditions among older adults with MDD.
Limitations
This study leveraged real-world evidence from hospital and pharmacy claims data to explore treatment patterns, healthcare resource utilization, and comorbidities within the studied cohort. While these data offer valuable insights, several limitations should be acknowledged. First, though this study provides a robust view of patient history by extending the baseline period for 730 days, events and conditions occurring before this time remain unknown, potentially impacting our understanding of long-term treatment patterns. Since the selection of first-line ADT may be influenced by factors such as past medical history, patient healthcare coverage, or prior use of ADT, any uncaptured effects of these variables may limit the depth of our analysis. Additionally, in order to identify MDD-related HCRU from claims data, we considered medical visit claims that had an MDD code in any diagnosis field (e.g. primary, secondary, tertiary, etc.). As such, it is possible that MDD may not have been the primary cause of some claims that were assessed as MDD-related and was instead included as an additional diagnosis code.
There are also limitations that arise due to this study’s reliance on pharmacy claims data. For example, filled prescriptions that were collected without insurance are not included, and patients in the cohort may have received off-label medications that were not directly captured in the data. As such, the actual medication burden among this patient population may be higher than captured here. Claims data are also limited in the amount of additional context they can provide regarding patient experiences. For example, this data set can indicate claims that were filled, but it does not indicate actual medication usage. While assessments of adherence using proportion of days covered are common in the literatureCitation43,Citation44, such values do not indicate actual medication usage and therefore serve as estimated measures of adherence. Similarly, it is challenging to confirm via claims data why patients discontinued their medication, as the data do not indicate whether discontinuation occurred due to patients reaching remission, experiencing adverse events, lacking access to medication, or utilizing alternative means to fill prescriptions, such as discount cards. This uncertainty may impact the interpretation of treatment patterns, though the high proportion of patients who did not discontinue their ADT suggests that this effect may be limited.
Finally, despite rigorous data analysis, the presence of unmeasured or unknown confounding variables cannot be ruled out. One area for further investigation includes that the comorbidities identified in the dataset are not mutually exclusive, which may affect the precise evaluation of their individual impact on the study outcomes. Future research on this group of selected comorbidities could investigate whether any particular comorbidity is associated with a greater medication burden. Additionally, future research could increase the generalizability of these study findings to other populations. For example, this study population was predominantly female (72.2%-75.4%); while MDD diagnosis tends to be more common among women in all age groupsCitation45, future studies investigating populations that are less well-represented in this study would build upon these findings in other demographic groups. Similarly, this study focused primarily on patients with Medicare Advantage plans; further studies on the medication and comorbidity burden among patients with Medicare fee-for-service coverage could expand these findings, which are currently limited in their generalizability.
Conclusions
This study provides novel insights on the clinical characteristics, comorbidity burden, MDD-related HCRU, medication burden and treatment patterns among older adults (≥65 years) with MDD treated with ADT. The findings from this study shed light on the high comorbidity burden in older adults with MDD and reveals that this patient cohort experiences diabetes, anxiety disorders, and COPD at higher rates than found in the overall older adult population. Such findings suggest the link between MDD and higher incidence of comorbidities. Insights from this study can thus inform the optimization of treatment outcomes and improved medication management in older adults with MDD by emphasizing the need for effective treatments and integrated care that attends to comorbidities and polypharmacy.
Observation of treatment patterns in this cohort also indicates that many of these patients do not quickly achieve remission following first-line therapies, and that the majority of patients require either changes to their treatment regimen or continuation of observed first-line ADT treatment throughout 12-months of follow-up. Collectively, these findings underscore the difficult treatment landscape for adults ≥65 years of age with MDD and shed light on the challenges faced by this specific population. Addressing the challenges of successful MDD treatment is critical given the growing prevalence of MDE in older adultsCitation6, warranting further improvements in patient monitoring, identification of effective treatment regimens to support symptom management and remission, and development of novel therapeutics.
Transparency
Declaration of financial/other relationships
LG, MW, and SH are employees of Sage Therapeutics, Inc, and may own stock and/or stock options. FD, LP and SL are employees of Komodo Health, Inc., which received fees from Sage Therapeutics, Inc. and Biogen Inc. MP is an employee of Biogen Inc. and may own stock, and MP owns stock in Pfizer Inc. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
LG, FD, LP, SL, MW, MP, and SH participated in the design of this study. Data analysis was performed by FD, LP, and SL. LG, FD, LP, SL, MW, MP, and SH contributed to the writing and editing of the manuscript. All authors approved of the final version of the manuscript.
Acknowledgements
The authors extend their gratitude to Tito Ghosh and Madhav Namjoshi who provided valuable support toward this manuscript. Medical writing and editorial support were provided by Hayden Hyatt, PhD, Julie Bevilacqua, MSc, and Francie Moehring-Moskal, PhD of Boston Strategic Partners, Inc. (Boston, MA, USA), funded by Sage Therapeutics, Inc. and Biogen Inc. The authors had full editorial control of the manuscript and provided final approval on all content. Results from this study were presented in an abstract and poster presentation at AMCP Nexus 2023 (October 16-19 2023, Orlando, FL).
MDD in Older Adults_Supplement_Clean.docx
Download MS Word (24.5 KB)Declaration of funding
This study was funded by Sage Therapeutics, Inc and Biogen Inc. Support for medical writing assistance was funded by Sage Therapeutics, Inc. and Biogen Inc.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5-TR. Fifth edition, text revision. ed. Washington, DC: American Psychiatric Association Publishing; 2022.
- Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2(1):16065. doi: 10.1038/nrdp.2016.65.
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1211–1259.
- Friedrich MJ. Depression is the leading cause of disability around the world. JAMA. 2017; 317(15):1517. doi: 10.1001/jama.2017.3826.
- Substance Abuse and Mental Health Services Administration (SAMHSA). Key substance use and mental health indicators in the United States: results from the 2021 National Survey on Drug Use and Health (HHS Publication No. PEP22-07-01-005, NSDUH Series H-57). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2022.
- Yang KH, Han BH, Moore AA, et al. Trends in major depressive episodes and mental health treatment among older adults in the United States, 2010-2019. J Affect Disord. 2022;318:299–303. doi: 10.1016/j.jad.2022.09.007.
- Rosenblat JD, Lee Y, McIntyre RS. The effect of pharmacogenomic testing on response and remission rates in the acute treatment of major depressive disorder: a meta-analysis. J Affect Disord. 2018;241:484–491. doi: 10.1016/j.jad.2018.08.056.
- Zhu L, Ferries E, Suthoff E, et al. Economic burden and antidepressant treatment patterns among patients with major depressive disorder in the United States. J Manag Care Spec Pharm. 2022;28(11–a Suppl):S2–S13. doi: 10.18553/jmcp.2022.28.11-a.s1.
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. AJP. 2006;163(11):1905–1917. doi: 10.1176/appi.ajp.163.11.1905.
- Pocklington C. Depression in older adults. Br J Med Pract. 2017;10(1):a1007.
- Kok RM, Reynolds CF. 3rd. Management of depression in older adults: a review. JAMA. 2017;317(20):2114–2122. doi: 10.1001/jama.2017.5706.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Vol. 5. Washington, DC: American Psychiatric Association; 2013.
- Serafini G, Adavastro G, Canepa G, et al. Abnormalities in kynurenine pathway metabolism in treatment-resistant depression and suicidality: a systematic review. CNS Neurol Disord Drug Targets. 2017;16(4):440–453.
- Costanza A, Vasileios C, Ambrosetti J, et al. Demoralization in suicide: a systematic review. J Psychosom Res. 2022;157:110788. doi: 10.1016/j.jpsychores.2022.110788.
- Conwell Y, Van Orden K, Caine ED. Suicide in older adults. Psychiatr Clin North Am. 2011;34(2):451–468. doi: 10.1016/j.psc.2011.02.002.
- De Carlo V, Calati R, Serretti A. Socio-demographic and clinical predictors of non-response/non-remission in treatment resistant depressed patients: a systematic review. Psychiatry Res. 2016;240:421–430. doi: 10.1016/j.psychres.2016.04.034.
- Divo MJ, Martinez CH, Mannino DM. Ageing and the epidemiology of multimorbidity. Eur Respir J. 2014;44(4):1055–1068. doi: 10.1183/09031936.00059814.
- Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155–162. doi: 10.4088/JCP.14m09298.
- Bangalore S, Shah R, Pappadopulos E, et al. Cardiovascular hazards of insufficient treatment of depression among patients with known cardiovascular disease: a propensity score adjusted analysis. Eur Heart J Qual Care Clin Outcomes. 2018;4(4):258–266. doi: 10.1093/ehjqcco/qcy023.
- Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2013;40(1):195–211. doi: 10.1016/j.pop.2012.11.003.
- Kannel WB. Hypertension as a risk factor for cardiac events–epidemiologic results of long-term studies. J Cardiovasc Pharmacol. 1993;21 Suppl 2(Suppl 2):S27–S37. doi: 10.1097/00005344-199321002-00006.
- Arnaud AM, Brister TS, Duckworth K, et al. Impact of major depressive disorder on comorbidities: a systematic literature review. J Clin Psychiatry. 2022;83(6):21r14328. doi: 10.4088/JCP.21r14328.
- Moore KL, Patel K, Boscardin WJ, et al. Medication burden attributable to chronic co-morbid conditions in the very old and vulnerable. PLoS One. 2018;13(4):e0196109. doi: 10.1371/journal.pone.0196109.
- Holt RI, de Groot M, Golden SH. Diabetes and depression. Curr Diab Rep. 2014;14(6):491. doi: 10.1007/s11892-014-0491-3.
- Grenard JL, Munjas BA, Adams JL, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011;26(10):1175–1182. doi: 10.1007/s11606-011-1704-y.
- Goldstein CM, Gathright EC, Garcia S. Relationship between depression and medication adherence in cardiovascular disease: the perfect challenge for the integrated care team. Patient Prefer Adherence. 2017;11:547–559. doi: 10.2147/PPA.S127277.
- Zivin K, Kales HC. Adherence to depression treatment in older adults: a narrative review. Drugs Aging. 2008;25(7):559–571. doi: 10.2165/00002512-200825070-00003.
- Sabaté E, World Health Organization. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization; 2003.
- Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. 2013;4:91. doi: 10.3389/fphar.2013.00091.
- Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818–1831. doi: 10.1001/jama.2015.13766.
- Fried TR, O'Leary J, Towle V, et al. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261–2272. doi: 10.1111/jgs.13153.
- Penninx BW, Nolen WA, Lamers F, et al. Two-year course of depressive and anxiety disorders: results from The Netherlands Study of Depression and Anxiety (NESDA). J Affect Disord. 2011;133(1–2):76–85. doi: 10.1016/j.jad.2011.03.027.
- National Center for Health Statistics. National Health Interview Survey. 2019. https://www.cdc.gov/nchs/nhis/2019nhis.htm
- Rector TS, Wickstrom SL, Shah M, et al. Specificity and sensitivity of claims-based algorithms for identifying members of medicare + choice health plans that have chronic medical conditions. Health Serv Res. 2004;39(6 Pt 1):1839–1857. doi: 10.1111/j.1475-6773.2004.00321.x.
- Centers for Disease Control and Prevention (CDC). Hypertension Cascade: Hypertension Prevalence, Treatment and Control Estimates Among US Adults Aged 18 Years and Older Applying the Criteria From the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES; 2017. [cited 20202023]. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html.
- Centers for Disease Control and Prevention (CDC). Table 23. Cholesterol among adults aged 20 and over, by selected characteristics: United States, selected years 1988–1994 through 2015–2018; 2019. https://www.cdc.gov/nchs/data/hus/2019/023-508.pdf.
- Centers for Disease Control and Prevention (CDC). National Diabetes Statistics Report: Prevalence of Both Diagnosed and Undiagnosed Diabetes; 2022. https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnosed-diabetes.html.
- National Center for Health Statistics; U.S. Census Bureau. Household Pulse Survey, 2020–2023. Anxiety and Depression; 2023. Generated interactively from https://www.cdc.gov/nchs/covid19/pulse/mental-health.htm.
- Centers for Disease Control and Prevention. National Center for Health Statistics; Analysis by American Lung Assocation Research and Program Services Division. National Health Interview Survey, 2019-2020. 2020. https://www.lung.org/research/trends-in-lung-disease/copd-trends-brief/copd-prevalence.
- Gelenberg AJ, Freeman MP, Markowitz JC, et al. American Psychiatric Association: Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. Am J Psychiatry. 2010;167(suppl):1–152.
- Gauthier G, Guérin A, Zhdanava M, et al. Treatment patterns, healthcare resource utilization, and costs following first-line antidepressant treatment in major depressive disorder: a retrospective US claims database analysis. BMC Psychiatry. 2017;17(1):222. doi: 10.1186/s12888-017-1385-0.
- Shih YT, Liu L. Use of claims data for cost and cost-effectiveness research. Semin Radiat Oncol. 2019;29(4):348–353. doi: 10.1016/j.semradonc.2019.05.009.
- Dalli LL, Kilkenny MF, Arnet I, et al. Towards better reporting of the proportion of days covered method in cardiovascular medication adherence: a scoping review and new tool TEN-SPIDERS. Br J Clin Pharmacol. 2022;88(10):4427–4442. doi: 10.1111/bcp.15391.
- Forbes CA, Deshpande S, Sorio-Vilela F, et al. A systematic literature review comparing methods for the measurement of patient persistence and adherence. Curr Med Res Opin. 2018;34(9):1613–1625. doi: 10.1080/03007995.2018.1477747.
- Girgus JS, Yang K, Ferri CV. The gender difference in depression: are elderly women at greater risk for depression than elderly men? Geriatrics (Basel). 2017;2(4):35. doi: 10.3390/geriatrics2040035.
