Abstract
Objective
The aim of this study was to evaluate the impact of sex on disease activity in axial spondylitis (axSpA).
Method
Data were extracted from the Ankylosing Spondylitis Registry of Ireland (ASRI). In this cross-sectional study, patients were analysed on the basis of sex, with a series of comparison analyses performed.
Results
Overall, 886 participants were enrolled in the ASRI [232 (26.2%) women, 644 (72.6%) men]. Females recorded significantly worse Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (4.57 vs 3.83, p < 0.01) and Ankylosing Spondylitis Quality of Life questionnaire (ASQoL) (7.51 vs 6.12, p < 0.01) scores than males. There was a stronger correlation in the Bath Ankylosing Spondylitis Functional and Metrology Indices (BASFI and BASMI) in females (rs = 0.619, p < 0.01) than in males (rs = 0.572, p < 0.01). Analysis of factors in BASDAI revealed that the higher total scores in females compared to males were due not to any single component, but to worse scores in all six components of the BASDAI combined. Ranking of components by severity between sexes revealed identical ranking in four of the six components of the BASDAI.
Conclusions
Women with axSpA reported significantly worse disease activity, quality of life, and functional ability than men. However, the BASDAI capturedsimilar patterns of disease activity. Limitation of spinal mobility in women with axSpA corresponded to greater impairment in functional ability. Further evaluation of disease monitoring tools is required to ensure that disease activity is accurately captured in men and women with axSpA.
Axial spondyloarthropathy (axSpA) is an autoinflammatory arthritis predominantly involving the axial spine (Citation1). The defining feature is inflammatory sacroiliitis, which can be detected on X-ray in radiographic axial spondyloarthropathy (r-axSpA), or on magnetic resonance imaging (MRI) in non-radiographic axial spondyloarthropathy (nr-axSpA) (Citation2). This causes symptoms of inflammatory back pain such as nocturnal pain and prolonged early morning stiffness (EMS). Several articular and extra-articular manifestations (psoriasis, inflammatory colitis, and uveitis) are recognized, which can aid in clinical identification of axSpA. Onset is typically in the third decade, with a higher incidence in Caucasians and an association with human leucocyte antigen (HLA)-B27 (Citation3).
There has been a considerable shift in the sex distribution of axSpA over time. Historically described as strongly male-predominant disease with male to female sex ratios reported as high as 10:1 (Citation4), more recent studies have reported ratios closer to 2–3:1 (Citation5). One of the major factors contributing to this dramatic shift is the Assessment of SpondyloArthritis international Society (ASAS) classification criteria for axSpA, which recognized not only r-axSpA, also called ankylosing spondylitis (AS), but also nr-axSpA (Citation6). While initially believed to represent an early stage of disease, longitudinal studies have demonstrated that only 10–40% of nr-axSpA will advance to r-axSpA over time (Citation7). As women with axSpA are more likely to have non-radiographic disease (Citation8), the formal recognition of nr-axSpA allowed these women not only to have a diagnosis of their condition but also to be eligible for treatment previously reserved for r-axSpA only.
Despite the evolution of classification and epidemiology of axSpA, much of what is known about axSpA is based on research into men with AS (Citation9). As a result, there remains a considerable underrepresentation of axSpA in women in published research. Combined with the underrecognition of the disease in women (Citation10), there is a significant need for increased awareness and focused research on the sex-specific impact and disease manifestations of axSpA (Citation11). Previous research into women with axSpA has demonstrated significantly worse quality of life, functional ability, and disease activity, as well as a poorer response to treatment compared to men (Citation9). Detailed analysis and characterization of axSpA in women is the first step in optimizing management and outcomes in this significant proportion of the population.
The aim of this study is to carry out a focused analysis of disease activity, reporting of disease, and impact of disease in women compared to men with axSpA. This sex-based analysis goes beyond summary comparisons, to capture how axSpA affects women, examine the relationship between functional ability and spinal mobility, and highlight directions for future research.
Method
Study population
The Ankylosing Spondylitis Registry of Ireland (ASRI) is a large, observational, national registry of patients with axSpA in Ireland. Currently, the ASRI contains data on 887 patients recruited from 12 rheumatology centres nationally, representing all geographic regions of Ireland. This study includes data on all axSpA patients captured within the ASRI since enrolment began in 2013. To be considered for enrolment in the ASRI, participants must have been diagnosed with axSpA by a rheumatologist and must meet the 2009 ASAS classification criteria for axSpA. This enabled capture of both radiographic and non-radiographic disease. Additional eligibility criteria included: age over 18 years, fluency in English, and presence of full capacity to consent to participation.
Once identified, eligible patients were invited to participate in the ASRI. To limit the possibility of selection bias, investigators recruited as many patients as possible from each centre, including participants across the spectrum of disease severity, socioeconomic status, and geographic regions in the country. Prior to enrolment, trained investigators discussed what participation in the ASRI would involve, the purpose of the ASRI, and how the collected information would be used and protected. Participants were encouraged to ask questions of the investigator prior to signing a written consent form. Ethical approval for the ASRI was obtained from local hospital ethics committees from all participating centres, including the Joint Research Ethics Committee for St James’ and Tallaght University Hospital. Trained investigators at each centre were responsible for local oversight of data collection. All data were collected and stored in compliance with national data protection legislation.
Outcomes and assessment
Enrolment consisted of a single clinical visit involving a structured interview, a series of questionnaires to capture patient-reported outcomes (PROs), a focused clinical examination, and chart review. This captured information on baseline demographics (age, sex, ethnicity), details of disease history (age at symptom onset, delay to diagnosis, disease duration), pattern of disease (articular and extra-articular manifestations), and treatments [non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying anti-rheumatic drugs (DMARDs), and biological agents]. Information on imaging (conventional radiographs, MRI) and serology (inflammatory markers, HLA-B27) was also captured via a medical records review.
Multiple PROs were recorded for each participant. These outcome measures are validated for use in axSpA and capture information on numerous aspects of participants’ daily living (Citation12). PROs captured via self-administered questionnaires were the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (Citation13), Bath Ankylosing Spondylitis Function Index (BASFI) (Citation14), Health Assessment Questionnaire (HAQ) (Citation15), and Ankylosing Spondylitis Quality of Life questionnaire (ASQoL) (Citation16). Focused clinical examination to assess spinal mobility allowed for calculation of the Bath Ankylosing Spondylitis Metrology Index (BASMI) (Citation17). Responses were recorded along a numerical rating system of a visual analogue score. For all outcome measures, higher scores indicated a greater level of impairment or severity.
Statistical analysis
Data were extracted from the ASRI and cleaned for analysis. A series of descriptive comparison analyses was carried out on all participants captured in the ASRI, stratified on the basis of sex. Participants who did not have sex recorded were excluded from the analysis. A Shapiro–Wilk test was used to assess normality of distribution for all included variables. Differences between continuous variables in the two groups were tested for significance using an independent two-tailed t-test or a Mann–Whitney U-test. A chi-squared test for independence was used to assess statistical significance in differences in categorical variables between sexes.
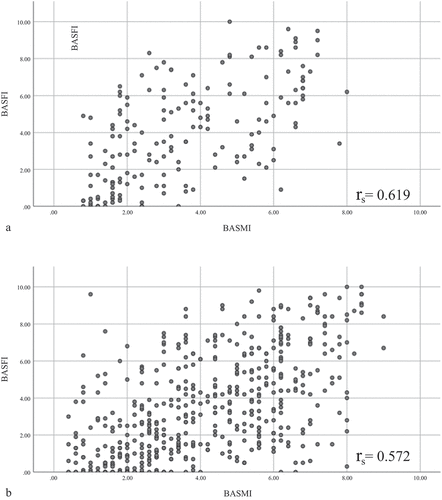
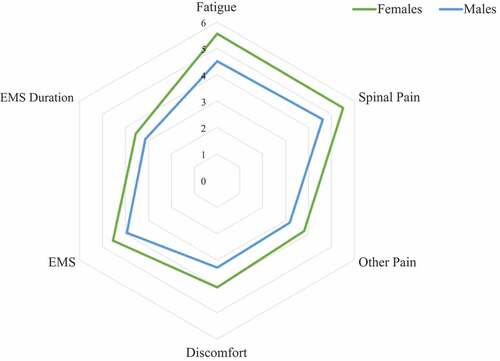
Focused analysis of the BASDAI involved comparison of mean total score and mean component scores between sexes. In addition to statistical testing for significance of differences, components were ranked by average scores within each sex, with average component scores plotted in a spider chart for visualization of results.
For the correlation analysis, data from patients with both BASFI and BASMI scores were included. Variables were assessed with a Shapiro–Wilk test for normal distribution, in addition to visual inspection of a scatterplot to determine the presence of a monotonic relationship between the two variables. Once established, a Spearman’s rank-order correlation was run between the two PROs. Records were then split by sex and a Spearman’s rank-order correlation was undertaken to assess the strength of correlation of scores within each sex. An alpha level of p < 0.05 was deemed significant. SPSS version 26 (IBM Corp., Armonk, NY, USA) was used to carry out all statistical analyses in this study.
Results
At the time of data extraction, 886 participants were enrolled in the ASRI. The patient population comprised 232 females (26.2%) and 644 males (72.6%), with a mean ± sd age of 45.9 ± 12.6 years (range 18–85 years), mean disease duration of 19.4 ± 12.3 years, and mean delay to diagnosis of 8 ± 8.5 years. Mean scores for the ASRI population were: ASQoL 6.47 [95% confidence interval (CI) 6.07, 6.95], HAQ 0.53 (95% CI 0.55, 0.6), BASDAI 4.02 (95% CI 3.81, 4.19), BASFI 3.67 (95% CI 3.47, 3.89), and BASMI 4 (3.85, 4.17). The majority of the population was Caucasian (90.2%), with a high prevalence of HLA-B27 positivity (88.7%).
No significant differences between sexes were noted in delay to diagnosis (females vs males: 7.42 vs 8.18; 95% CI −2.03, 0.52; p = 0.24) or age at symptom onset (25.9 vs 26.6; 95% CI −2.49, 1.02; p = 0.41) (). r-axSpA was more commonly encountered in males than females (78.1% vs 70.7%, p = 0.02), while nr-axSpA was significantly more prevalent in females (21.9% vs 29.3%, p = 0.02).
Table 1. Analysis of patient characteristics on the basis of sex.
In terms of articular manifestations, females had a significantly higher prevalence of dactylitis (9.9% vs 5%, p = 0.01) and a trend towards an increased prevalence of peripheral arthritis (36.2% vs 28.3%, p = 0.08). For extra-articular manifestations (EAMs), females reported a significantly higher prevalence of both uveitis (40.5% vs 35.7%, p = 0.01) and colitis (17.2% vs 7.9, p < 0.01) compared to males.
Females with axSpA recorded significantly higher ASQoL (7.51 vs 6.12; 95% CI 0.56, 2.22; p < 0.01) and BASDAI (4.57 vs 3.83; 95% CI 0.37, 1.10; p < 0.01) scores compared to males (). They also recorded trends towards worse mean HAQ (0.59 vs 0.51; 95% CI −0.002, 0.16; p = 0.05) and BASFI scores (3.85 vs 3.63; 95% CI −0.22, 0.66; p = 0.32), but these differences were not significant. Spinal mobility, as captured in the BASMI, was significantly less impaired in females than in males (3.58 vs 4.16; 95% CI −0.94, −0.22; p < 0.01).
Table 2. Breakdown of Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) total and component scores by sex, with ranking of average component scores by severity within each sex.
BASDAI analysis
Females with axSpA reported worse mean total BASDAI (4.6 vs 3.83; 95% CI 0.37, 1.10; p < 0.01), compared to males. Within the BASDAI, females scored significantly worse than males across all components (Fatigue: 5.56 vs 4.51, p < 0.01; Spinal pain: 5.51 vs 4.63, p < 0.01; Other pain: 3.82 vs 3.19, p = 0.01; Discomfort: 4.05 vs 3.29, p < 0.01; EMS: 4.55 vs 3.94, p = 0.01), with the exception of duration of EMS, which demonstrated a non-significant trend towards higher average scores in females (3.54 vs 3.12, p = 0.07) (). Ranking of the BASDAI components by mean scores in order of overall severity revealed identical ranking in both sexes for four of the six components of the BASDAI, with the only variation observed in the component ranked as the most severe. For males this was spinal pain, while for females it was fatigue. Plotting of average component scores for each sex revealed a similar pattern in disease activity ().
Spinal mobility and function
Data on both BASMI and BASFI scores were available for 680 patients, and were included in the analysis. Variables were not normally distributed, as assessed by the Shapiro–Wilk test (p < 0.01). The relationship between the two measures was monotonic (as the BASFI score increases, so too does the BASMI score), as determined by visual inspection of the scatterplot.
There was a statistically significant, strong positive correlation between BASMI and BASFI scores in axSpA patients [rs(678) = 0.568, p < 0.01], based on calculations by a Spearman’s rank-order correlation. A Spearman’s rank-order correlation analysis following splitting of records by sex showed that the correlation became stronger when assessed within each sex [females rs(144) = 0.619, p < 0.01; males rs(509) = 0.572, p < 0.01] and had a slightly stronger association with in females ().
Discussion
This analysis of real-world data from a large cohort of axSpA patients captured in the ASRI outlines the significant burden of disease in women with axSpA. This is reflected by worse PROs, with a higher prevalence of articular and extra-articular manifestations noted in women compared to men with axSpA. The analysis contained within this study provides valuable insight into the impact of axSpA and how this varies between men and women.
Overall, women with axSpA reported significantly worse disease activity and greater impairment of their quality of life compared to men, captured via worse average BASDAI and ASQoL scores. There were also non-significant trends towards worse HAQ and BASFI, representing greater impairment of function. However, women recorded significantly better spinal mobility, captured via the BASMI, with a lower proportion classified as r-axSpA than males with axSpA, although the majority of the ASRI population had radiographic disease. These findings are supported by studies in other large international axSpA cohorts, which reported similar differences between sexes (Citation8, Citation18–21).
Women with axSpA recorded significantly worse total BASDAI scores compared to men, consistent with results from other large axSpA cohorts (Citation8, Citation19, Citation20). Further focused analysis of the individual components of the BASDAI demonstrated that this was due to worse scores across all six components of the BASDAI in women, and not driven by higher scores in any single component. Ranking of mean component scores in order of severity between sexes revealed identical ranking of four of the six BASDAI components. The main difference observed in this ranking was the symptom ranked as the most severe within each sex, which was spinal pain in men and fatigue in women. However, when examining the mean scores for these two components within each sex, minimal difference was noted. This suggests that despite differences in total scores, the overall pattern of disease activity, as captured by the BASDAI, is strikingly similar in men and women.
The question of why women record worse PROs, including the BASDAI, still remains. The findings of our analysis demonstrated that women with axSpA had a significantly higher burden of a number of both articular and extra-articular manifestations. This could explain the higher average scores across a number of items within the BASDAI, with a greater burden of inflammatory symptoms potentially contributing to the increased severity of fatigue observed in women with axSpA. Of interest, a previous study examined the topography of pain reporting in axSpA and reporting of symptoms in the BASDAI (Citation22). This revealed that the level of pain corresponded to BASDAI total scores in men, while in women the level of pain was linked to assessment of peripheral and axial symptoms when considered separately. These findings led to the recommendation of sex-specific approaches when considering the results of the BASDAI.
The elegance of the BASDAI is in the simplicity of the design, allowing both ease of use for patients and ease of interpretation for clinicians. It is one of the most commonly used outcome measures in clinical practice for monitoring disease activity in axSpA. For this reason, it is essential to ensure that the wealth of information captured by this tool is interpreted in a way that adequately reflects the clinical picture in both men and women with axSpA.
It has been well established that men with axSpA tend to have greater limitation of spinal mobility, as captured in the BASMI (Citation23, Citation24), although this finding seems contradictory to the worse outcomes observed in women with the disease. It stands to reason that level of function would have a strong positive correlation with spinal mobility in axSpA; in clinical terms, patients with greater limitation of spinal mobility tend to have an increased level of functional impairment and vice versa. The results of this analysis by sex indicate that this correlation is more significant if compared separately within each sex, and is notably stronger in females.
Men with axSpA have previously been shown to have a higher prevalence of radiographic disease and a worse severity of radiographic disease over time (Citation21, Citation25, Citation26). It would be expected that this would result in greater impact on functional ability; however, males in the ASRI demonstrated a non-significant trend towards better BASFI scores compared to females. The stronger correlation noted in women with axSpA in our study indicates that as the level of spinal mobility worsens, a larger change is observed in deterioration of functional ability compared to men. In practical terms, deterioration of spinal mobility appears to have less of an impact on restriction of function in men compared to women with axSpA. Why this variation occurs is not immediately apparent; however, research and advances in motion capture technology may offer further insights (Citation27).
Previous studies have proposed that the higher prevalence of centralized sensitization (Citation20) and fibromyalgia (Citation28) in women could be significant contributors to the differences detected in patient outcomes, as both can lead to chronic widespread pain (CWP) (Citation29, Citation30). Although these issues are more common in women in the general population, they are clinical diagnoses of exclusion (Citation31, Citation32). The importance of thorough investigation of CWP was highlighted in an Israeli study of known fibromyalgia patients, which found that 10.2% met ASAS criteria for axSpA while up to 25% had imaging findings suggestive of inflammatory disease (Citation33). In patients with a known underlying axSpA, distinguishing between disease activity and CWP can be quite difficult (Citation34, Citation35). To further complicate this diagnostic dilemma, symptoms of inflammatory pain can vary by sex, with women more likely to describe widespread pain while men report back pain (Citation36). However, it is crucial to ensure adequate treatment of axSpA prior to attributing symptoms to CWP.
Recent increased use of the Ankylosing Spondylitis Disease Activity Score (ASDAS) has demonstrated less variation between sexes (Citation37, Citation38) when assessing disease activity in axSpA. A meta-analysis examining the performance of the ASDAS and BASDAI concluded that the ASDAS may not be sensitive enough in the detection of active peripheral disease (Citation39). As peripheral involvement is more common in females with axSpA (Citation5), this introduces a significant sex bias. The ASDAS has proven to be a remarkable tool in detecting disease activity and even in predicting response to biological therapies in males with axSpA (Citation40). However, given the known variation in patterns of disease activity between axSpA in women and men, it may be time to reconsider our evaluation of disease activity in females with axSpA.
As suggested by the experience with the ASDAS, differences in reporting of disease activity could be due to the methods used to capture this information. Tools such as the BASDAI and BASFI were developed and validated for AS in predominantly male cohorts (Citation13, Citation41). The development of sex-specific scores indicative of active disease could help to better compare scores between men and women. In addition, evaluation of outcome measures regarding their ability to capture the burden of articular and extra-articular manifestations in both sexes may provide further insights into the variation in outcomes observed between sexes.
Despite the higher prevalence of several disease manifestations and worse PROs in women with axSpA, medication use was statistically similar between the sexes. This pattern of medication use is not unique to the ASRI, with similarities in the frequency of use of DMARDs and biological therapy in men and women observed in other large cohort studies (Citation8, Citation18). A 2020 publication examining medication use before and after the recognition of nr-axSpA found no change in use of DMARDs or biologics between males and females (Citation42). This raises the question: Are women with axSpA less likely to be offered treatment? If so, why? Could it be that women’s symptoms are less likely to be considered inflammatory and thus less responsive to treatment? Most importantly, are women with axSpA being undertreated?
The most obvious but least understood cause for variations between men and women is hormonal differences (Citation43). Oestrogens have been shown to inhibit production of tumour necrosis factor-α and decrease differentiation of Th17 cells (Citation44), which has significant effects on the inflammatory pathway. However, studies examining the effect of female sex hormone supplementation have failed to show any effect on outcomes in axSpA (Citation45). In addition, we must consider the significant hormonal shifts that the female body undergoes over the course of a lifetime and how they affect disease activity. These include menstruation, pregnancy, and menopause, as data suggest that the overall effects of hormones are concentration dependent (Citation46). Studies on pregnancy in axSpA vary widely, but a high prevalence of active disease during pregnancy, peaking in the second trimester, has been reported (Citation47). Research on menstrual variation in disease activity is incredibly limited; however, one study reported an inverse relationship between oestrogen level and erythrocyte sedimentation rate (Citation48). Further research into the effect of sex hormones in axSpA has the potential to offer significant insight into observed variations in outcomes between sexes and could potentially lead to improved management of axSpA in women.
This study has several potential limitations. Data for the analysis contained within this study were cross-sectional in nature owing to the current design of the ASRI. As such, it was not possible to comment on disease progression over time or treatment response. Plans to collect longitudinal data for the ASRI are in place and will further enrich this valuable epidemiological resource. At the time of analysis, ASDAS results were not available for a large proportion of the ASRI population, preventing comparative analysis of the performance of the ASDAS and the BASDAI in our population. This has been studied in detail in numerous axSpA populations previously, and thus sex-specific patterns in reporting of the ASDAS are well established. As fluency in English was required for participation, it is possible that this resulted in exclusion of certain smaller populations from enrolment in the ASRI; however, English fluency in Ireland is 97.5% so this is unlikely (Citation49). Ireland does not have centralized medical records, so it was not feasible to report the percentage of axSpA patients in Ireland captured within the ASRI. However, recruitment was from all major geographic regions of the country and included all levels of the socioeconomic spectrum to reflect the true population as accurately as possible and limit selection bias.
Conclusion
In this large registry-based study of axSpA, women had a higher prevalence of a number of articular and extra-articular manifestations compared to men. They also reported significantly worse disease activity and quality of life. However, the overall pattern of symptom severity as captured by the BASDAI was similar in men and women. Despite better mean spinal mobility overall compared to men with axSpA, limitation of spinal mobility in women with axSpA corresponded to a greater impairment in functional ability. These results suggest that further evaluation of the tools and outcome measures currently used to monitor disease activity are needed to accurately assess the burden of axSpA in women. In addition, further studies are required to examine the impact of significant hormonal changes on disease activity and disease progression in women with axSpA.
Supplemental Material
Download MS Word (50 KB)Acknowledgements
SM is the recipient of the Gilead Inflammation fellowship. All other authors have no relevant financial interests to disclose. The ASRI is supported by unrestricted funding from AbbVie, Pfizer, and UCB.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/03009742.2021.2007609.
Additional information
Funding
References
- Robinson PC, van der Linden S, Khan MA, Taylor WJ. Axial spondyloarthritis: concept, construct, classification and implications for therapy. Nat Rev Rheumatol 2021;17:109–18.
- Braun J, Baraliakos X, Buehring B, Kiltz U, Fruth M. Imaging of axial spondyloarthritis. New aspects and differential diagnoses. Clin Exp Rheumatol 2018;36 Suppl 114:35–42.
- Marzo-Ortega H. Axial spondyloarthritis: coming of age. Rheumatology (Oxford) 2020;59:iv1–iv5.
- Gran JT, Husby G. Ankylosing spondylitis in women. Semin Arthritis Rheum 1990;19:303–12.
- Rusman T, van Bentum RE, van der Horst-bruinsma IE. Sex and gender differences in axial spondyloarthritis: myths and truths. Rheumatology (Oxford) 2020;59:iv38–iv46.
- Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83.
- Protopopov M, Poddubnyy D. Radiographic progression in non-radiographic axial spondyloarthritis. Expert Rev Clin Immunol 2018;14:525–33.
- de Jong H, Paramarta JE, de Winter J, Baeten D, van de Sande M. Differences between females and males in axial spondyloarthritis: data from a real-life cross-sectional cohort. Scand J Rheumatol 2020;49:28–32.
- Rusman T, van Vollenhoven RF, van der Horst-bruinsma IE. Gender differences in axial spondyloarthritis: women are not so lucky. Curr Rheumatol Rep 2018;20:35.
- Garrido-Cumbrera M, Poddubnyy D, Gossec L, Mahapatra R, Bundy C, Makri S, et al. Gender differences in patient journey to diagnosis and disease outcomes: results from the European Map of Axial Spondyloarthritis (EMAS). Clin Rheumatol 2021;40:2753–61.
- Ruiz-Cantero MT, Blasco-Blasco M. Perspectiva de género en epidemiología clínica. Aprendiendo con el caso de las espondiloartritis [Gender perspective in clinical epidemiology. Learning from spondyloarthritis]. Gac Sanit 2020;34:83–6.
- Zochling J. Measures of symptoms and disease status in ankylosing spondylitis: Ankylosing Spondylitis Disease Activity Score (ASDAS), Ankylosing Spondylitis Quality of Life Scale (ASQoL), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Global Score (BAS-G), Bath Ankylosing Spondylitis Metrology Index (BASMI), Dougados Functional Index (DFI), and Health Assessment Questionnaire for the Spondylarthropathies (HAQ-S). Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S47–58.
- Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91.
- Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994;21:2281–5.
- Kwan YH, Fong W, Lui NL, Yong ST, Cheung YB, Malhotra R, et al. Validity and reliability of the Health Assessment Questionnaire among patients with spondyloarthritis in Singapore. Int J Rheum Dis 2018;21:699–704.
- Doward LC, Spoorenberg A, Cook SA, Whalley D, Helliwell PS, Kay LJ, et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis 2003;62:20–6.
- Jenkinson TR, Mallorie PA, Whitelock HC, Kennedy LG, Garrett SL, Calin A. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J Rheumatol 1994;21:1694–8.
- Andreasen RA, Kristensen LE, Egstrup K, Baraliakos X, Strand V, Horn HC, et al. The impact of sex and disease classification on patient-reported outcome measures in axial spondyloarthritis: a descriptive prospective cross-sectional study. Arthritis Res Ther 2019;21:221.
- Tournadre A, Pereira B, Lhoste A, Dubost JJ, Ristori JM, Claudepierre P, et al. Differences between women and men with recent-onset axial spondyloarthritis: results from a prospective multicenter French cohort. Arthritis Care Res (Hoboken) 2013;65:1482–9.
- Mease PJ, McLean RR, Dube B, Liu M, Rebello S, Glynn M, et al. Comparison of men and women with axial spondyloarthritis in the US-based corrona psoriatic arthritis/spondyloarthritis registry. J Rheumatol 2021;48:1528–36.
- Wallman JK, Kapetanovic MC, Petersson IF, Geborek P, Kristensen LE. Comparison of non-radiographic axial spondyloarthritis and ankylosing spondylitis patients–baseline characteristics, treatment adherence, and development of clinical variables during three years of anti-TNF therapy in clinical practice. Arthritis Res Ther 2015;17:378.
- Swinnen TW, Westhovens R, Dankaerts W, de Vlam K. Widespread pain in axial spondyloarthritis: clinical importance and gender differences. Arthritis Res Ther 2018;20:156.
- Webers C, Essers I, Ramiro S, Stolwijk C, Landewé R, van der Heijde D, et al. Gender-attributable differences in outcome of ankylosing spondylitis: long-term results from the Outcome in Ankylosing Spondylitis International Study. Rheumatology (Oxford) 2016;55:419–28.
- Biallas RL, Dean LE, Davidson L, Hollick R, Pathan E, Robertson L, et al. The role of metrology in axSpA: does it provide unique information in assessing patients and predicting outcome? Results from the BSRBR-AS registry. Arthritis Care Res (Hoboken) 2020.
- Huang JX, Chung HY, Chui ETF, Lee KH, Chan SCW, Tsang HHL, et al. Intensity of spinal inflammation is associated with radiological structural damage in patients with active axial spondyloarthritis. Rheumatol Adv Pract 2020;4:rkz049.
- Baraliakos X, Braun J. Non-radiographic axial spondyloarthritis and ankylosing spondylitis: what are the similarities and differences? RMD Open 2015;1:e000053.
- Gardiner PV, Small D, Muñoz-Esquivel K, Condell J, Cuesta-Vargas A, Williams J, et al. Validity and reliability of a sensor-based electronic spinal mobility index for axial spondyloarthritis. Rheumatology (Oxford) 2020;59:3415–23.
- Rencber N, Saglam G, Huner B, Kuru O. Presence of fibromyalgia syndrome and its relationship with clinical parameters in patients with axial spondyloarthritis. Pain Physician 2019;22:E579–e85.
- Lubrano E, Perrotta FM, Manara M, D’Angelo S, Addimanda O, Ramonda R, et al. The sex influence on response to tumor necrosis factor-α inhibitors and remission in axial spondyloarthritis. J Rheumatol 2018;45:195–201.
- Lorenzin M, Ortolan A, Frallonardo P, Oliviero F, Punzi L, Ramonda R. Predictors of response and drug survival in ankylosing spondylitis patients treated with infliximab. BMC Musculoskelet Disord 2015;16:166.
- Marques AP, Santo A, Berssaneti AA, Matsutani LA, Yuan SLK. Prevalence of fibromyalgia: literature review update. Rev Bras Reumatol Engl Ed 2017;57:356–63.
- Smith MT Jr., Remeniuk B, Finan PH, Speed TJ, Tompkins DA, Robinson M, et al. Sex differences in measures of central sensitization and pain sensitivity to experimental sleep disruption: implications for sex differences in chronic pain. Sleep 2019;42:1–15.
- Ablin JN, Eshed I, Berman M, Aloush V, Wigler I, Caspi D, et al. Prevalence of axial spondyloarthritis among patients with fibromyalgia: a magnetic resonance imaging study with application of the assessment of spondyloarthritis international society classification criteria. Arthritis Care Res (Hoboken) 2017;69:724–9.
- López-Medina C, Moltó A. Comorbid pain in axial spondyloarthritis, including fibromyalgia. Ther Adv Musculoskelet Dis 2020;12:1759720x20966123.
- Dougados M, Perrot S. Fibromyalgia and central sensitization in chronic inflammatory joint diseases. Joint Bone Spine 2017;84:511–3.
- Slobodin G, Reyhan I, Avshovich N, Balbir-Gurman A, Boulman N, Elias M, et al. Recently diagnosed axial spondyloarthritis: gender differences and factors related to delay in diagnosis. Clin Rheumatol 2011;30:1075–80.
- Salaffi F, De Angelis R, Carotti M, Gutierrez M, Sarzi-Puttini P, Atzeni F. Fibromyalgia in patients with axial spondyloarthritis: epidemiological profile and effect on measures of disease activity. Rheumatol Int 2014;34:1103–10.
- van der Horst-bruinsma IE, Zack DJ, Szumski A, Koenig AS. Female patients with ankylosing spondylitis: analysis of the impact of gender across treatment studies. Ann Rheum Dis 2013;72:1221–4.
- Blasco-Blasco M, Castrejón I, Jovaní V, Pascual E, Ruiz-Cantero MT. Reviewing disease activity indices in spondyloarthritis from the gender perspective: a systematic review and meta-analysis. J Rheumatol 2021;48:1395–404.
- Marona J, Sepriano A, Rodrigues-Manica S, Pimentel-Santos F, Mourão AF, Gouveia N, et al. Eligibility criteria for biologic disease-modifying antirheumatic drugs in axial spondyloarthritis: going beyond BASDAI. RMD Open 2020;6:e001145.
- Calin A, Nakache JP, Gueguen A, Zeidler H, Mielants H, Dougados M. Defining disease activity in ankylosing spondylitis: is a combination of variables (Bath Ankylosing Spondylitis Disease Activity Index) an appropriate instrument? Rheumatology (Oxford) 1999;38:878–82.
- Nieto RE, Plasencia Rodriguez C, Peiteado López D, Villalba Yllán A, Balsa Criado A, Navarro-Compán V. Are we treating women patients with real axial spondyloarthritis? Semin Arthritis Rheum 2020;50:432–5.
- Wright GC, Kaine J, Deodhar A. Understanding differences between men and women with axial spondyloarthritis. Semin Arthritis Rheum 2020;50:687–94.
- Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 2008;8:737–44.
- Mahendira D, Thavaneswaran A, Carty A, Haroon N, Anton A, Passalent L, et al. Analysis of the effect of the oral contraceptive pill on clinical outcomes in women with ankylosing spondylitis. J Rheumatol 2014;41:1344–8.
- Shepherd R, Cheung AS, Pang K, Saffery R, Novakovic B. Sexual dimorphism in innate immunity: the role of sex hormones and epigenetics. Front Immunol 2020;11:604000.
- Maguire S, O’Dwyer T, Mockler D, O’Shea F, Wilson F. Pregnancy in axial spondyloarthropathy: a systematic review & meta-analysis. Semin Arthritis Rheum 2020;50:1269–79.
- Jimenez-Balderas FJ, Tapia-Serrano R, Madero-Cervera JI, Murrieta S, Mintz G. Ovarian function studies in active ankylosing spondylitis in women. Clinical response to estrogen therapy. J Rheumatol 1990;17:497–502.
- Central Statistics Office (CSO). Census 2016 published reports [Website]. Cork, Ireland 2016 [ cited 2021]. Available from: https://www.cso.ie/en/census/census2016reports/.