ABSTRACT
Previous research has shown that Evarcha culicivora, using only olfaction (i.e. airborne volatile compounds), discriminates between opposite-sex and same-sex conspecific individuals and also discriminates between opposite-sex conspecific and heterospecific individuals. However, there has been no evidence to date of E. culicivora also using olfaction alone to discriminate between virgin and previously mated opposite-sex conspecifics. Results from the present study suggest that males of E. culicivora may gain this information from contacting draglines and show a preference for virgin females. Males displayed significantly more often when they contacted the draglines from a virgin female instead of from a previously mated female. Males also escalated conflict with a same-sex rival (their own mirror image) significantly more often when in the presence of draglines from virgin females than when in the presence of draglines from previously mated females.
Introduction
Robert Jackson has enriched our understanding of spiders (especially jumping spiders: Salticidae) in so many ways, and challenged long-held views that complex, flexible behaviour is only relevant to big-brained animals (Jackson & Cross Citation2011). It is not surprising that vision-based behaviour is often emphasised in salticids, as these spiders are known for having unique, complex eyes and for having greater spatial acuity compared with similar-sized animals (Harland et al. Citation2012). However, early in his career, Robert also considered how salticids deploy other sensory modalities. For example, he found that when male Phidippus johnsoni (Peckham and Peckham, 1883) encounter females within nests, they successfully perform non-visual courtship displays even in complete darkness by probing and vibrating the nest silk (Jackson Citation1977). A consideration of sensory modalities other than vision has since inspired many insightful studies using salticids (e.g. VanderSal & Hebets Citation2007; Elias et al. Citation2010; Peckmezian & Taylor Citation2015) and here, in my Festschrift contribution, I consider how draglines influence the behaviour of a salticid.
Numerous studies have highlighted the diverse ways in which salticids use chemoreception (Clark & Jackson Citation1994, Citation1995; Taylor Citation1998; Clark et al. Citation1999), especially in the context of touching web, nest or dragline silk. Males, in particular, of many species launch into non-visual courtship behaviour after contacting nest or dragline silk from conspecific females (see Jackson Citation1987; Pollard et al. Citation1987). Findings from these studies imply that it is primarily chemical cues that mediate silk-based species and sex discrimination by salticids, as males stop responding after a female salticid's silk has been washed in alcohol or left in the open for a week (Jackson Citation1987). Olfaction (i.e. detecting airborne volatile compounds) has also been experimentally demonstrated for salticids (Gaskett Citation2007; Foelix Citation2011; Nelson et al. Citation2012).
Evarcha culicivora Wesolowska and Jackson, 2003, the salticid spider I consider here, is known for predatory and mating strategies based on a distinctive interplay of vision and olfaction. This East African salticid is proficient at identifying its preferred prey, blood-carrying female mosquitoes, by vision alone and by olfaction alone (Jackson et al. Citation2005; Jackson & Cross Citation2015). Yet E. culicivora also has an unusual mating strategy. Unlike the prevalent pattern among salticids, where males adopt a more active role in courtship and females place more emphasis on mate choice (Jackson & Pollard Citation1997), mutual mate choice is pronounced for E. culicivora and both sexes choose mates on the basis of active courtship (Cross et al. Citation2008) and on the basis of body size (Cross et al. Citation2007).
Previous research has also shown that, even when they are restricted to using only olfactory cues, males and females of E. culicivora can discriminate between opposite-sex and same-sex conspecific individuals (Cross & Jackson Citation2009a) and can also discriminate between opposite-sex conspecific and heterospecific individuals (Cross & Jackson Citation2013). There is also an important male–female difference whereby, after mating, females (but not males) become less responsive to the odour of potential mates (Cross & Jackson Citation2009a). Yet there has been no evidence that either sex can discern the odour of opposite-sex conspecifics that have, or have not, previously mated.
A recent study (Cross & Jackson Citation2013) has highlighted that E. culicivora responds especially strongly to draglines by performing typical visual courtship displays (e.g. by hunching and other leg posturing) even in the absence of a visible conspecific individual. In that study, males sometimes launched into display when they were only presented with female odour, but significantly more displayed in trials where draglines could be touched. A normal preliminary to E. culicivora displaying on silk is to ‘palpate’ a dragline by using its forelegs to pull the silk thread closer and then by moving its palps across the silk. My hypothesis is that E. culicivora gains more precise information about potential mates from draglines than it does from odour alone. Here I investigate in two experiments: 1. whether E. culicivora males discriminate between virgin and previously mated conspecific females on the basis of draglines; and 2. whether E. culicivora males interact more aggressively with each other when they are presented with draglines from virgin conspecific females. This includes considering a prediction, from evolutionary game theory, that conspecific individuals become more willing to escalate their level of aggression with each other when they detect the presence of a limiting resource (Maynard Smith & Harper Citation2003; Bradbury & Vehrencamp Citation2011); the ‘limiting resource’ in this case being a virgin female. ‘Escalating’ implies that an individual adjusts its behaviour in ways that will subject it to a higher risk of injury or death. Earlier research has shown that males and females of E. culicivora escalate to a higher level of aggression with a virtual rival (their own mirror image) when they are presented with odour from opposite-sex conspecifics instead of opposite-sex heterospecifics or same-sex conspecifics (Cross & Jackson Citation2009b). Whether E. culicivora also escalates when presented with draglines from virgin, instead of from previously mated, opposite-sex conspecifics, has not been investigated before now.
Materials and methods
In both experiments, I used spiders from laboratory cultures (F2–F3 generation) that were started from E. culicivora collected in Mbita Point, Western Kenya (for field site details, see Cross et al. Citation2008). As my basic methods for rearing, maintaining and testing spiders were as in earlier studies (e.g. Cross & Jackson Citation2013), only essential details are provided here.
Adult males (‘test spiders’) were tested with draglines from adult females (‘source spiders’). Source spiders were either virgins or were previously mated and had already deposited an egg sac before being used in experiments. In both experiments, I adopted procedures whereby the test spider could contact draglines on all surfaces of the testing apparatus (‘arena’). It is common practice in experiments with lycosid spiders to line an arena with filter paper (e.g. Persons & Rypstra Citation2001), as lycosids tend to remain only on the floor of an arena covered with paper. Salticids, by contrast, are more inclined to walk on all surfaces of an arena.
For standardisation, all test and source spiders had reached maturity 1–3 weeks before being used in experiments and no test spiders had mated or had any experience with conspecific female draglines. All test and source spiders were fed the day before being used in experiments and all trials began between 0800 and 1400 h (laboratory photoperiod 12L:12D, lights on at 0700 h). All spiders were, to the nearest 0.5 mm, of standardised body length (5 mm) and no spider was used more than once in experiments.
In both experiments, the test spider was kept in a holding tube (length 40 mm, diameter 10 mm) for 5 min before each trial, with both ends of the tube plugged with stoppers. After 5 min, one of the stoppers was removed and the test spider was introduced to the arena from the open end of the tube. If the test spider had not spontaneously walked out of the tube and into the arena 5 min later, then the other stopper was removed and, using a soft brush, the spider was lightly prodded, whereupon it promptly moved into the arena. To minimise the possibility of draglines or chemical traces influencing the outcome of subsequent trials, the arena was always wiped with 80% ethanol and then dried between trials.
Experiment 1. Dragline testing in two-sector Petri dishes
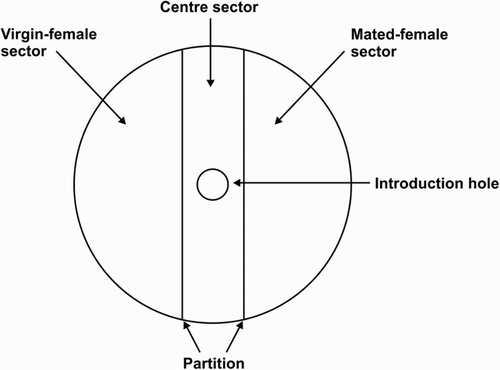
The arena () was a transparent plastic Petri dish (diameter 90 mm, height 15 mm) divided into three sectors. The ‘centre sector’ (20 mm wide), which did not contain any draglines, was straddled by two sectors containing draglines, a ‘virgin female sector’ and a ‘mated female sector’ (both 35 mm wide). Whether the virgin female sector was to the left or right of the centre sector was determined at random. Before testing began, a partition (1 mm thick paper card, folded to create two walls reaching from the bottom to the top of the arena) lined the centre sector and was secured with sticky tape. With the partition in place, each source spider was free to move around in its respective sector for 1 h.
After this 1 h interval, I removed the two source spiders and the partition from the arena. The test spider was then introduced to the arena through a 10 mm hole centred in the lid (plugged with a rubber stopper after introducing the test spider). Once the spider entered the arena, the trial began and lasted for 30 min, during which time I recorded how long the test spider stayed in each sector of the arena. I also recorded whether the test spider displayed during the trial. Most displays were brief (3–5 s in duration) and so, instead of recording interaction duration, I recorded the sector the male was occupying when it first displayed as well as the number of times it displayed. ‘Display’ refers to behaviour used during courtship, such as leg posturing, hunching and zigzag dancing, which has already been defined and described in detail elsewhere (Cross et al. Citation2008). A criterion for a successful trial was that the test spider had to palpate draglines, but there were no individuals that failed to do so.
Experiment 2. Dragline testing of mirror-image response
In this experiment, test spiders encountered their own mirror images on two successive days in conjunction with draglines from different source spiders. On one day the male encountered draglines from a virgin female and on the next or previous day the male encountered draglines from a mated female (order of presentation decided at random; no spider used in more than one test pair). Salticids readily respond to their own mirror images in much the same way as they respond to living same-sex conspecific rivals (Harland et al. Citation1999; Cross & Jackson Citation2009b), and this was important because it meant I could ascertain the test spider's decisions to escalate or desist without the decisions of a living, responsive rival introducing confounding variables.
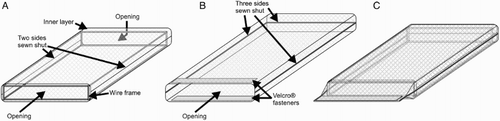
The arena () was a transparent Perspex box (140 mm long × 110 mm wide × 20 mm high) that included a removable glass top and a mirror at one end. Before testing began, a source spider was placed inside a ‘netting cage’ () for 16 h to deposit draglines. Each netting cage included an inner layer (140 × 115 × 20 mm; A) and an outer layer (160 × 115 × 20 mm; B), these being rectangular pouches made from white nylon mosquito netting (mesh diameter 2 mm). The cage also included a rectangular wire frame (130 × 110 × 20 mm; wire thickness 1.5 mm) which fitted securely inside the inner layer (narrow sides of inner layer open; other two sides sewn shut; A). During the 16 h interval, the outer layer held the inner layer and wire frame (C), and it was closed at each end to keep the source spider inside (one narrow side of outer layer secured with Velcro fasteners [120 × 15 mm]; other three sides sewn shut). The inner layer served as a substrate on which the source spider deposited draglines while moving inside the netting cage.
Figure 2. Testing arena for Experiment 2 (not drawn to scale), as seen from above, made from transparent Perspex. Glass lid not shown. All side walls were held in place with plastic clamps.
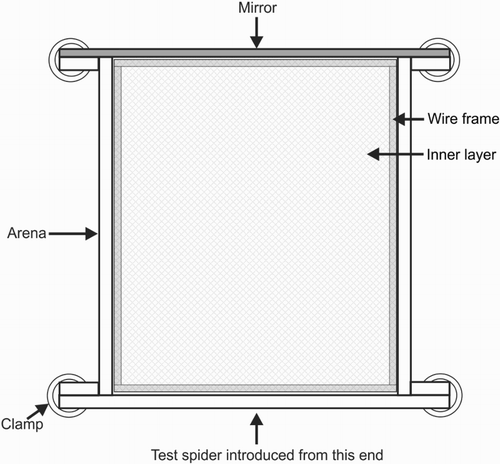
Figure 3. Netting cage, consisting of a wire frame, and two layers (inner layer and outer layer) made from white mosquito netting. A, Inner layer surrounding the wire frame; B, outer layer, fully open, before the inner layer with wire frame was inserted through the opening; C, outer layer holding inner layer and wire frame.
After the 16 h interval, the source spider was removed from the netting cage and the outer layer was taken off the inner layer and frame. The inner layer and frame were then placed inside the testing arena. One of the open ends of the inner layer faced the mirror, whereas the other end, on the opposite side, was used as an opening for introducing the test spider onto the netting inside the arena. Netting cages were rinsed with 80% ethanol and then dried before being re-used.
Before a trial began, the side wall furthest from the mirror was raised, and the beginning of a trial was defined as when the test spider walked out of the glass tube and onto the netting inside the arena. I aborted testing if the test spider entered the arena by running, instead of walking calmly, out of the tube and also if, after calmly walking into the arena, 60 min elapsed without the test spider displaying at the mirror. Adopting these rules resulted in fewer than 5% of the trials being aborted. Spiders in aborted trials were not used again and data for these spiders were omitted from analysis. In successful trials, the spider displayed and I recorded the rank (see below) reached in the interaction with the mirror and also the time elapsing between the first display and the end of the interaction (interaction duration). I ended trials when test spiders retreated from the mirror and did not return or display again for 30 s. Whenever a spider interacted with its mirror image for as long as 30 min, I terminated the trial, recorded the highest rank reached (see below) and recorded 30 min as interaction duration.
Ranking
As in earlier studies (e.g. Cross & Jackson Citation2014), I recognised three levels of aggression (‘ranks’). I based these ranks on discrete categories of behaviour and the rank I recorded was the highest reached before the test spider retreated and failed to return. ‘Rank’ can be envisaged as being the level of risk the spider was willing to accept in a given contest, with rank 1 being the lowest and rank 3 the highest. Below I provide a general characterisation of the three ranks (for further details, see Cross et al. Citation2008).
Rank 1 (posturing from a distance): while facing the mirror, the spider adopted distinctive positioning of forelegs (e.g. highly flexed and held out to the side), chelicerae (e.g. spread apart with fangs extended) and abdomen (e.g. elevated), sometimes accompanied by a distinctive walking pattern (e.g. stepping more abruptly than during normal locomotion).
Rank 2 (propulsive displays): the spider ran, lunged or leapt toward the mirror, but with little or no contact being made.
Rank 3 (face-to-face contact): the spider pushed against the mirror, with the front of its cephalothorax touching the mirror and with its forelegs usually extending out to the side and touching the mirror.
Data analysis
Unless otherwise stated, I analysed data using Wilcoxon tests for paired comparisons because data for both experiments did not meet the assumptions for parametric data analyses. As these were paired comparisons, I displayed the distributions of ‘scores’, not the separate distributions of rank or duration for when a particular dragline type (from a virgin or mated female) was presented. For example, in Experiment 2 I calculated a score by subtracting the interaction duration recorded when a test spider was presented with mated-female draglines from the interaction duration recorded when the same test spider was presented with virgin-female draglines (i.e. a positive score indicated that the test spider interacted for longer when presented with virgin females; null hypothesis: score = 0). For further details about statistical procedures, see Howell (Citation2002). In both experiments, n = 50.
In Experiment 1, besides considering how long the test spider spent in the virgin female sector compared with how long he spent in the mated female sector, I also considered the proportion of time he spent displaying in these two sectors. I calculated ‘proportion’ by dividing the number of times the male displayed in a sector of the arena with the total length of time the male spent in that sector of the arena. I also compared where males were located in the dish when they initiated display using a chi-square test of goodness of fit (null hypothesis: probability of initiating display in the virgin female sector the same as the probability of initiating display in the mated female sector).
In Experiment 2, I compared the rank observed when males were presented with draglines from virgin females with the rank observed when males were presented with draglines from previously mated females. I also compared the interaction duration observed when males were presented with draglines from virgin females with the interaction duration observed when males were presented with draglines from previously-mated females.
Results
Experiment 1. Dragline testing in two-sector Petri dishes
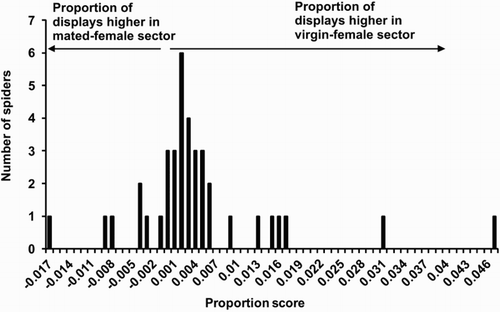
The length of time males spent in the virgin female sector was not significantly different from the length of time males spent in the mated female sector (Z = 1.36, P = 0.175). However, 76% of males launched into display while they were in the arena and these males spent a significantly higher proportion of their time displaying while in the virgin female sector than in the mated female sector (Z = 2.90, P = 0.004; ). Moreover, the number of males that initiated display in the virgin female sector (n = 29) was significantly higher than the number of males that initiated display in the mated female sector (n = 9; χ² = 10.53, P = 0.001).
Experiment 2. Dragline testing of mirror-image response
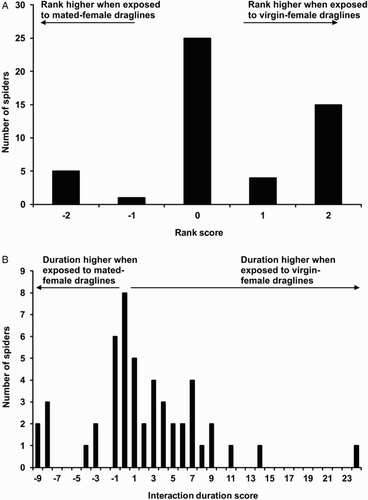
Ranks were significantly higher when males were presented with virgin female draglines than when they were presented with mated female draglines (Z = 2.21, P = 0.027; A). Interaction duration was also significantly higher when males were presented with virgin female draglines than when they were presented with mated female draglines (Z = 2.32, P = 0.020; B).
Figure 5. Dragline testing of mirror-image response (Experiment 2) using males of Evarcha culicivora (n = 50). A, Rank scores: rank attained while exposed to draglines from virgin females minus rank attained while exposed to draglines from mated females; B, duration scores: interaction duration while exposed to draglines from virgin females minus interaction duration while exposed to draglines from mated females.
Discussion
The results of both experiments suggest that E. culicivora males discriminate between draglines from virgin and previously mated conspecific females. In Experiment 1, males displayed significantly more often when contacting the silk from a virgin female. In Experiment 2, males escalated conflict with a same-sex rival significantly more often when in the presence of draglines from virgin females instead of when in the presence of draglines from previously mated females. Earlier studies on intraspecific interactions using E. culicivora had focused on using rank (Cross & Jackson Citation2009b) or interaction duration (Cross & Jackson Citation2014) as a measure of conflict escalation. Here, I have shown that both rank and interaction duration increased when E. culicivora males were presented with draglines from virgin conspecific females, suggesting that these females are a more valuable resource than previously mated females.
Earlier work (Cross & Jackson Citation2013) has shown that E. culicivora can discriminate between opposite-sex conspecifics and opposite-sex heterospecifics when presented with the odour of these individuals, or even the odour of their draglines alone, in an olfactometer. However, to date, there has been no evidence that E. culicivora can also discriminate between virgin and previously mated opposite-sex conspecifics in an olfactometer (Cross & Jackson Citation2009a). This may be surprising, considering that E. culicivora makes pronounced use of olfaction in a variety of contexts (e.g. Jackson et al. Citation2005) and considering that there is evidence of other animals determining mating status by odour alone (e.g. Bonduriansky Citation2001; Wyatt Citation2003; Carazo et al. Citation2004). Yet it is possible that E. culicivora acquires more information about mating status from contacting draglines than from airborne odour alone. Whether this ‘information’ includes differences in the structure of the silk (see Leonard & Morse Citation2006), as well as volatile compounds on the silk, is currently unknown.
It is also unknown, for now, whether E. culicivora females similarly discriminate between virgin and mated males on the basis of draglines. However, it is likely that making this discrimination is more important for E. culicivora males than it is for females. Evarcha culicivora males may readily re-mate but females are less likely to do so (e.g. Cross et al. Citation2007). A common pattern in the animal kingdom is of males preferring to mate with virgin females if these females prioritise the first male's sperm when re-mating (Andersson Citation1994). For spiders, there is ample evidence to suggest that this pattern of sperm usage by females predominates (Austad Citation1984). Although first-male sperm precedence has not yet been confirmed for E. culicivora, males are known to cohabit with sub adult females that are close to maturing (Cross et al. Citation2008). This form of mate guarding has been found in other salticids (Jackson Citation1986), suggesting that there are first-male priorities in sperm usage.
Moreover, salticids have genital morphology that makes first-male sperm precedence probable. Similar morphology is found in wolf spiders (Lycosidae) (Austad Citation1984) and a preference for silk from virgin females instead of from previously mated females has been confirmed for these spiders. For example, males of Pardosa milvina (Hentz, 1844) display more actively and are more likely to copulate when encountering a virgin female in conjunction with her silk instead of when encountering a previously mated female in conjunction with her silk (Rypstra et al. Citation2003). Males of P. milvina also escalate conflict when they contact the silk from a virgin female instead of when they contact the silk from a previously mated female (Rypstra et al. Citation2009).
It would be interesting to consider whether E. culicivora adults make adjustments to the silk they deposit when they are in the vicinity of a potential mate (see Havrilak et al. Citation2015; Khan & Persons Citation2015). Moreover, besides alerting E. culicivora individuals to the potential presence of a mate, draglines may assist individuals with avoiding encounters with salticids that are not potential mates. As both sexes of this species readily display to each other (Cross et al. Citation2008), a male's displays in the absence of a visual target may function by soliciting a reply by a not-yet-seen female, with the female's reply display rendering her more conspicuous to the male. In this way, displaying by the male in response to female draglines may function as a mate-locating tactic. This tactic has similarities to a predatory tactic called ‘speculative hunting’ (Curio Citation1976), which is known to be adopted by another salticid that Robert Jackson helped make famous, Portia fimbriata (Doleschall, 1859) (Clark et al. Citation2000).
Acknowledgements
I am grateful to Robert Jackson for the many ways in which he has inspired and encouraged my work over the years. I thank Cor Vink for inviting me to contribute to this Festschrift and I thank Simon Pollard for his comments on an earlier version of the manuscript. For technical assistance, I am grateful to Nick Etheridge, Aynsley Macnab (University of Canterbury) and Stephene Abok Aluoch (icipe). Maeve Cross gave me invaluable assistance with making the netting cages.
Associate Editor: Dr Cor Vink.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Andersson M. 1994. Sexual selection. Princeton: Princeton University Press.
- Austad SN. 1984. Evolution of sperm priority patterns in spiders. In: Smith RL, editor. Sperm competition and the evolution of animal mating systems. London: Academic Press; p. 223–249.
- Bonduriansky R. 2001. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev. 76:305–339. doi: 10.1017/S1464793101005693
- Bradbury JW, Vehrencamp SL. 2011. Principles of animal communication. 2nd ed. Sunderland, MA: Sinauer Associates.
- Carazo P, Sanchez E, Font E, Desfilis E. 2004. Chemosensory cues allow male Tenebrio molitor beetles to assess the reproductive status of potential mates. Anim Behav. 68:123–129. doi: 10.1016/j.anbehav.2003.10.014
- Clark RJ, Harland DP, Jackson RR. 2000. Speculative hunting by an araneophagic salticid spider. Behaviour. 137:1601–1612. doi: 10.1163/156853900502736
- Clark RJ, Jackson RR. 1994. Portia labiata, a cannibalistic jumping spider, discriminates between own and foreign eggsacs. Int J Comp Psychol. 7:38–43.
- Clark RJ, Jackson RR. 1995. Araneophagic jumping spiders discriminate between the draglines of familiar and unfamiliar conspecifics. Ethol Ecol Evol. 7:185–190. doi: 10.1080/08927014.1995.9522964
- Clark RJ, Jackson RR, Waas JR. 1999. Draglines and assessment of fighting ability in cannibalistic jumping spiders. J Insect Behav. 12:753–766. doi: 10.1023/A:1020900925863
- Cross FR, Jackson RR. 2009a. Mate-odour identification by both sexes of Evarcha culicivora, an East African jumping spider. Behav Process. 81:74–79. doi: 10.1016/j.beproc.2009.02.002
- Cross FR, Jackson RR. 2009b. How cross-modality effects during intraspecific interactions of jumping spiders differ depending on whether a female-choice or mutual-choice mating system is adopted. Behav Process. 80:162–168. doi: 10.1016/j.beproc.2008.11.001
- Cross FR, Jackson RR. 2013. The functioning of species-specific olfactory pheromones in the biology of a mosquito-eating jumping spider from East Africa. J Insect Behav. 26:131–148. doi: 10.1007/s10905-012-9338-4
- Cross FR, Jackson RR. 2014. Cross-modality effects of prey odour during the intraspecific interactions of a mosquito-specialist predator. Ethology. 120:598–606. doi: 10.1111/eth.12232
- Cross FR, Jackson RR, Pollard SD. 2007. Male and female mate-choice decisions by Evarcha culicivora, an East African jumping spider. Ethology. 113:901–908. doi: 10.1111/j.1439-0310.2007.01394.x
- Cross FR, Jackson RR, Pollard SD. 2008. Complex display behaviour of Evarcha culicivora, an East African mosquito-eating jumping spider. New Zeal J Zool. 35:151–187. doi: 10.1080/03014220809510112
- Curio E. 1976. The ethology of predation. Berlin: Springer-Verlag.
- Elias DO, Sivalinghem S, Mason AC, Andrade MCB, Kasumovic MM. 2010. Vibratory communication in the jumping spider Phidippus clarus: substrate-borne courtship signals are important for male mating success. Ethology. 116:990–998. doi: 10.1111/j.1439-0310.2010.01815.x
- Foelix RF. 2011. Biology of spiders. 3rd ed. Oxford: Oxford University Press.
- Gaskett AC. 2007. Spider sex pheromones: emission, reception, structures, and functions. Biol Rev. 82:27–48. doi: 10.1111/j.1469-185X.2006.00002.x
- Harland DP, Jackson RR, Macnab AM. 1999. Distances at which jumping spiders (Araneae: Salticidae) distinguish between prey and conspecific rivals. J Zool. 247:357–364. doi: 10.1111/j.1469-7998.1999.tb00998.x
- Harland DP, Li D, Jackson RR. 2012. How jumping spiders see the world. In: Lazareva O, Shimizu T, Wasserman EA, editors. How animals see the world: comparative behavior, biology, and evolution of vision. New York: Oxford University Press; p. 133–164.
- Havrilak JA, Shimmel KM, Rypstra AL, Persons MH. 2015. Are you paying attention? Female wolf spiders increase dragline silk advertisements when males do not court. Ethology. 121:345–352. doi: 10.1111/eth.12340
- Howell DC. 2002. Statistical methods for psychology. 5th ed. Belmont, CA: Wadsworth.
- Jackson RR. 1977. Courtship versatility in the jumping spider, Phidippus johnsoni (Araneae: Salticidae). Anim Behav. 25:953–957. doi: 10.1016/0003-3472(77)90046-X
- Jackson RR. 1986. Cohabitation of males and juvenile females: a prevalent mating tactic of spiders. J Nat Hist. 20:1193–1210. doi: 10.1080/00222938600770791
- Jackson RR. 1987. Comparative study of releaser pheromones associated with the silk of jumping spiders (Araneae, Salticidae). New Zeal J Zool. 14:1–10. doi: 10.1080/03014223.1987.10422676
- Jackson RR, Cross FR. 2011. Spider cognition. Adv Insect Physiol. 41:115–174. doi: 10.1016/B978-0-12-415919-8.00003-3
- Jackson RR, Cross FR. 2015. Mosquito-terminator spiders and the meaning of predatory specialization. J Arachnol. 43:123–142. doi: 10.1636/V15-28
- Jackson RR, Nelson XJ, Sune GO. 2005. A spider that feeds indirectly on vertebrate blood by choosing female mosquitoes as prey. Proc Natl A Sci (USA). 102:15155–15160. doi: 10.1073/pnas.0507398102
- Jackson RR, Pollard SD. 1997. Jumping spider mating strategies: sex among cannibals in and out of webs. In: Choe JC, Crespi BJ, editors. The evolution of mating systems in insects and arachnids. Cambridge: Cambridge University Press; p. 340–351.
- Khan R, Persons MH. 2015. Female Pardosa milvina wolf spiders increase silk advertisements when in the presence of silk from courting males. J Arachnol. 43:168–173. doi: 10.1636/J14-70
- Leonard AS, Morse DH. 2006. Line-following preferences of male crab spiders, Misumena vatia. Anim Behav. 71:717–724. doi: 10.1016/j.anbehav.2005.08.004
- Maynard Smith J, Harper D. 2003. Animal signals. New York: Oxford University Press.
- Nelson XJ, Warui CM, Jackson RR. 2012. Widespread reliance on olfactory sex and species identification by lyssomanine and spartaeine jumping spiders. Biol J Linn Soc. 107:664–677. doi: 10.1111/j.1095-8312.2012.01965.x
- Peckmezian T, Taylor PW. 2015. Electric shock for aversion training of jumping spiders: towards an arachnid model of avoidance learning. Behav Process. 113:99–104. doi: 10.1016/j.beproc.2015.01.015
- Persons MH, Rypstra AL. 2001. Wolf spiders show graded antipredator behavior in the presence of chemical cues from different sized predators. J Chem Ecol. 27:2493–2504. doi: 10.1023/A:1013679532070
- Pollard SD, Macnab AM, Jackson RR. 1987. Communication with chemicals: pheromones and spiders. In: Nentwig W, editor. Ecophysiology of spiders. Berlin: Springer-Verlag; p. 133–141.
- Rypstra AL, Schlosser AM, Sutton PL, Persons MH. 2009. Multimodal signalling: the relative importance of chemical and visual cues from females to the behaviour of male wolf spiders (Lycosidae). Anim Behav. 77:937–947. doi: 10.1016/j.anbehav.2008.12.026
- Rypstra AL, Wieg C, Walker SE, Persons MH. 2003. Mutual mate assessment in wolf spiders: differences in the cues used by males and females. Ethology. 109:315–325. doi: 10.1046/j.1439-0310.2003.00874.x
- Taylor PW. 1998. Dragline-mediated mate-searching in Trite planiceps (Araneae, Salticidae). J Arachnol. 26:330–334.
- VanderSal ND, Hebets EA. 2007. Cross-modal effects on learning: a seismic stimulus improves color discrimination learning in a jumping spider. J Exp Biol. 210:3689–3695. doi: 10.1242/jeb.009126
- Wyatt TD. 2003. Pheromones and animal behaviour. Cambridge: Cambridge University Press.