Abstract
Background
Osteoarthritis (OA) is a common chronic joint disease that significantly affects an individual’s quality-of-life and frailty has become one of the common complications in OA patients as the disease progresses. The relationship between dietary patterns is not clear.
Methods
All participants are from the National Health and Nutrition Examination Survey (NHANES) and have been diagnosed with OA. The dietary inflammation index (DII) is calculated based on the dietary intake reported by the participants. Logistic regression analysis is used to investigate the relationship between DII and frailty. Restricted cubic splines are utilised to explore their nonlinear relationship. Mediation analysis is conducted to explore the role of inflammation in this relationship.
Results
A total of 2,530 OA patients were included in the study, with an average age of 64.46 (12.67) years. After adjusting for covariates, for each one standard deviation increase in DII, the risk of frailty increased by 15% (OR = 1.15, 95% CI = 1.03–1.28). Compared to patients with DII < –1, patients with DII > 1 had a significantly higher risk of frailty (OR = 1.50, 95% CI = 1.05-2.14).
Conclusions
The findings of this study indicate a positive association between DII and the risk of frailty in OA patients. These results underscore the potential impact of dietary interventions in improving the quality-of-life for OA patients.
Introduction
Osteoarthritis (OA) is a widely prevalent chronic joint condition that afflicts millions of people worldwide (Jiang Citation2022). One of the primary characteristics of OA is joint pain and impaired function, significantly affecting the quality-of-life of affected individuals (Conaghan et al. Citation2019). Despite OA emerging as a major health concern among the elderly population, its pathogenesis remains incompletely understood, and clinical preventive and treatment methods have limitations (Cho et al. Citation2021). Consequently, the search for proactive and effective approaches to treat OA is of paramount importance.
In recent years, there has been increasing focus on the close connection between diet and inflammation. Inflammation plays a significant role in the pathophysiology of OA, contributing to joint pain and tissue damage (Trouvin and Perrot Citation2018). Therefore, by improving dietary habits, it is possible to alleviate the inflammatory response in arthritis patients, subsequently improving their condition. However, diet is a complex concept because it is influenced by social determinants such as ethnicity, economic status, and education level (Groth et al. Citation2016). Simply evaluating the intake of certain substances can easily lead to bias. The DII is a tool used to assess the relationship between diet and inflammation, quantitatively representing the inflammatory potential of the diet by accounting for various nutrients and bioactive compounds in food (Shu et al. Citation2022). Growing evidence suggests that a high DII is associated with various chronic diseases, including cardiovascular diseases and cancer (Fowler and Akinyemiju Citation2017; Hariharan et al. Citation2022). Previous studies have also indicated a relationship between the DII and the occurrence of OA. Wang et al. (Citation2022a) suggested that a higher pro-inflammatory potential in the diet among older adults is associated with a higher risk of OA. Therefore, the DII is considered a highly promising indicator for evaluating the risk of developing chronic diseases.
Frailty is a complex concept, typically encompassing a reduction in muscle mass and function, coupled with an increased vulnerability of the body (Kojima et al. Citation2018). In OA patients, frailty is often associated with muscle wasting, decreased physical capacity, and impaired activities of daily living (Wang et al. Citation2022b). However, to date, research on the relationship between DII and frailty in OA patients remains limited, and it is not yet clear whether improving dietary inflammation status can alleviate frailty symptoms in OA patients. Therefore, this study aims to explore the association between the DII and frailty in OA patients, with the goal of providing further insights into how dietary interventions can enhance the quality-of-life for OA patients.
Method
Study design and participants
This study employed a cross-sectional research design using the National Health and Nutrition Examination Survey (NHANES) database (2007–2018) to obtain nationally representative population data, ensuring the external validity of the research findings. NHANES is a nationally representative health survey project conducted by the National Centre for Health Statistics (NCHS) in the United States (Liu et al. Citation2023). The NHANES database includes samples from different regions, age groups, and ethnicities across the country. All participants in this study received ethical approval from the NCHS Institutional Review Board and provided informed consent. The study procedures and design were in accordance with the Helsinki Declaration (https://www.cdc.gov/nchs/nhanes/index.htm) (Johnson et al. Citation2023).
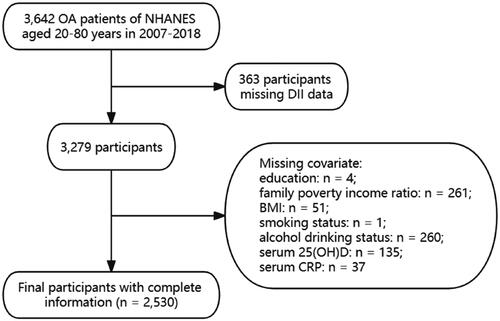
The study included a total of 3,642 adult patients diagnosed with OA between 2007 and 2018. The diagnosis of OA was based on participants’ self-reports data. Exclusion criteria included: (1) lack of sufficient dietary information or weakly related data (n = 363); and (2) participants lacking sufficient covariate information (n = 749). Finally, a total of 2,530 participants with complete data were included ().
Study variables
The NHANES data collection involved face-to-face interviews, physical examinations, and laboratory measurements. Dietary data were collected through the 24-h dietary recall questionnaire and the dietary supplement questionnaire to assess the participants’ dietary intake. This process was typically conducted in two phases, with the first dietary recall interview being conducted in person at a mobile examination centre, and the second interview conducted via phone between 3–10 days later (Ahluwalia et al. Citation2016). We calculated the DII based on the average of dietary data from the two occasions. The calculation of DII followed the definition proposed by Shivappa et al. (Citation2014), which primarily involves computing the inflammatory score for 45 nutrients (either pro-inflammatory or anti-inflammatory). The specific formula used is: (Daily intake of each dietary component – global mean daily intake)/standard deviation (SD) of the global mean daily intake for that particular dietary component * overall inflammatory effect score of that dietary component. In this study, we selected 26 nutrients from the DII calculation, which have been demonstrated to possess reliable predictive capabilities (Supplementary Methods 1) (Tan et al. Citation2022).
Outcome
The Frailty Index comprises 49 health questions covering various systems (Hakeem et al. Citation2021), including one question on cognition, 15 questions on activities of daily living, seven questions pertaining to mental health and depressive symptoms according to the patient’s patient health questionnaire-9 questionnaire (Levis et al. Citation2019), 13 questions related to chronic diseases, five questions regarding self-rated health, two anthropometric indicators, and six laboratory measurements (Supplementary Methods 2). Each of these health problems is scored on a scale of 0–1. The data collected from participants who completed a comprehensive health assessment with 30 or more questions are deemed sufficient to accurately evaluate adverse outcomes (Blodgett et al. Citation2015). The frailty score equals the ratio of the sum of health problem scores to the total number of health problems. A higher frailty score indicates a poorer prognosis for the participants. Based on prior research, we set the cut-off point for the frailty score at 0.212, with scores below 0.21 classified as non-frailty and scores equal to or above 0.21 classified as frailty (Blodgett et al. Citation2015).
Covariates
Sociodemographic characteristics of study participants were assessed, including age, sex (male, female), race and ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), health insurance status (yes, no), education level (below high school, above high school), and household poverty-to-income ratio (total household income divided by poverty line; < 1.3, 1.3–3.5, ≥ 3.5) (Zhang et al. Citation2021). Lifestyle factors such as smoking status (never, previous, present) and leisure-related activities (MET/wk) were collected through face-to-face interviews, relying on self-reports provided by participants. In addition, the participants’ body mass index (BMI) was divided into three groups: < 25, 25.0–30, and > 30, representing normal weight, overweight, and obese, respectively. Haematological indicators such as C-reactive protein (CRP) and 25(OH)D are measured in the laboratory by trained personnel because these indicators have been widely proven to be significantly associated with the occurrence, progression, and prognosis of OA (Xiao et al. Citation2022; Dainese et al. Citation2023).
Disease histories of participants in this study were collected from multiple sources. First, patients self-reported the use of related drugs, such as hypoglycaemic drugs, antihypertensive drugs, etc. Second, participants reported that they had been diagnosed with these conditions during a previous physical exam and informed of their condition by a health care professional. Third, during their participation in the NHANES screening programme, the test results confirmed the presence of the disease according to established diagnostic criteria. Diseases that were considered covariates in this study included high blood pressure and diabetes.
Statistical analysis
NHANES employs a stratified and multi-stage sampling approach, necessitating the application of appropriate sampling weights in our statistical analysis. This step ensures that our findings accurately represent the broader population.
To summarise the data, we used descriptive statistics. Normally distributed variables are presented as mean values with accompanying SD, while skewed variables are expressed as medians with interquartile ranges or as frequencies with percentages. In cases where the data exhibited skewness, we applied natural logarithm transformations. To investigate the linear relationship between DII and frailty score, we conducted Pearson’s correlation analysis. The correlation coefficient is denoted by r-value, and is used to measure the degree of linear association between two variables. The value of r ranges from −1 to +1. The closer the r-value is to 0, the weaker the relationship between the two variables. Additionally, we assessed the association between DII and frailty, through restrictive cubic spline regression (RCS) and weighted logistic regression analysis. The outcomes of these analyses were reported as odds ratios (OR) along with 95% confidence intervals (CI).
Interaction analysis was used to explore the role of potential modifiers in this relationship. These modifiers included variables such as age, sex, BMI, smoking, diabetes, and hypertension. Subgroup analyses were carried out based on these factors to explore the relationship between DII and frailty across different strata. In addition, we conducted a joint analysis to further understand the role of these modifiers in the interaction effect.
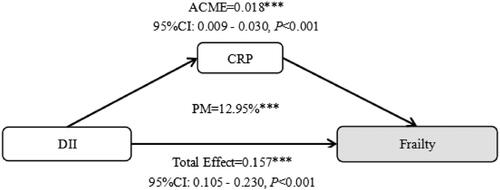
In addition, we also conducted a mediation analysis to explore the relationship between inflammation and frailty in OA patients. We reported the direct effects, indirect effects, and the proportion of mediation. The direct effect refers to the total effect of the independent variable on the dependent variable, while the indirect effect refers to the effect of the independent variable on the dependent variable through the mediator variable, as illustrated in Supplementary Figure S1.
To assess the robustness of our findings, we conducted sensitivity analyses involving excluding participants with a history of cancer, as tumour burden can significantly impact inflammation levels, potentially affecting frailty, excluded participants older than 70 years and included data from four examination cycles conducted between 2007 and 2014 as internal validation in these sensitivity analyses. All statistical analyses were performed by R 4.2.3 software, with statistical significance defined as a two-sided p-value of less than 0.05.
Results
Baseline characteristics
A total of 2,530 OA patients were included in the study, with an average age of 64.46 (12.67) years, including 911 men. Among them, 1,150 patients exhibited frailty. Compared to non-frail patients, frail individuals were more likely to be women, older, had a higher BMI, lower income, and lower education levels. They were also more likely to have comorbid conditions such as hypertension, diabetes, and cancer. In terms of lifestyle, frail patients were more likely to be smokers and engaged in lower levels of physical activity. Laboratory test results indicated that frail patients had higher levels of CRP and 25(OH)D ().
Table 1. Baseline characteristics.
The relationship between DII and frailty
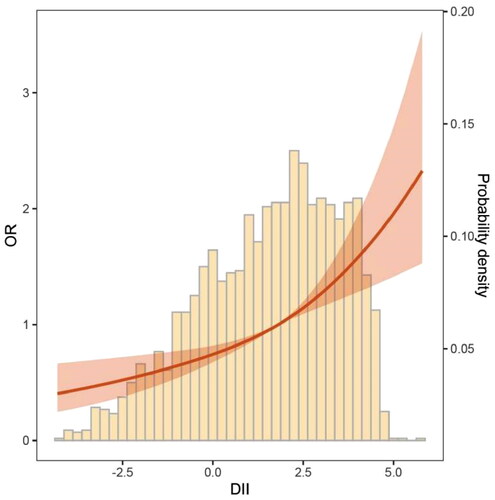
Correlation analysis revealed (Supplementary Figure S2) a positive correlation between DII and frailty scores in all OA patients (R = 0.19, p < 0.001). Further stratifying patients by gender (Men, r = 0.2, p < 0.001; Women, r = 0.18, p < 0.001) and BMI categories (BMI < 25, r = 0.17, p < 0.001; 25 ≤ BMI < 30, r = 0.20, p < 0.001; BMI ≥ 30, r = 0.17, p < 0.001) confirmed the persistence of this relationship. The RCS results demonstrated that, as DII increased, the risk of frailty exhibited an exponential rise (). further elucidates the results of three models regarding the relationship between DII and frailty risk. When adjusting only for age differences and treating DII as a continuous variable, for each one standard deviation increase in DII, the risk of frailty increased by 39% (OR = 1.39, 95% CI = 1.25–1.53). When considering DII as a categorical variable, patients in the remaining two groups had significantly higher frailty risks compared to those with DII < –1 (T2: OR = 1.54, 95% CI = 1.04–2.27; T3: OR = 2.42, 95% CI = 1.72–3.39). After adjusting for factors such as age, sex, race and ethnicity, education, family poverty income ratio, BMI, smoking status, hypertension, diabetes, CRP, 25(OH)D, and others, for each one standard deviation increase in DII, the risk of frailty increased by 15% (OR = 1.15, 95% CI = 1.03–1.28). Compared to patients with DII < –1, patients with DII > 1 had a significantly higher risk of frailty (OR = 1.50, 95% CI = 1.05–2.14).
Table 2. The association between Dietary Inflammatory Index (DII) and frailty.
Interaction analysis revealed (Supplementary Figure S3) that the relationship between DII and frailty was influenced by age (P for interaction = 0.045) and smoking (P for interaction = 0.004). Further detailed subgroup analyses for these two factors showed that the negative correlation between DII and frailty was only present in patients younger than 65 years (OR per SD = 1.26, 95% CI = 1.08–1.47) and in smokers (OR per SD = 1.33, 95% CI = 1.11–1.60).
Additionally, we conducted joint analyses of DII with these two factors (Supplementary Table S1). Compared to patients younger than 65 years with low DII, patients younger than 65 years with high DII had a higher risk of frailty (OR = 2.05, 95% CI = 1.20–3.52). Compared to non-smokers with low DII, smokers with high DII had a higher risk of frailty (OR = 1.70, 95% CI = 1.07–2.69).
illustrates the mediation effect of inflammation in the relationship between DII and frailty. The results indicate that inflammation mediated approximately 12.95% of the relationship between DII and frailty in OA patients.
Sensitivity analysis
Sensitivity analysis was conducted to assess the robustness of this study (Supplementary Table S2). After excluding elderly and tumour patients, as they are susceptible populations to frailty, the results indicated that, for each one SD increase in DII, the risk of frailty increased by 24% and 17%, respectively. Furthermore, internal validation was performed. Initially, we compared DII levels between different time periods (Supplementary Figure S4). Subsequently, we excluded patients from the last two rounds (2015–2018). For each one SD increase in DII, the risk of frailty increased by 14%. Compared to patients with low DII, those with high DII had a significantly higher risk of frailty (OR = 1.53, 95% CI = 1.02–2.30).
Discussion
To the best of our knowledge, this is the first study to investigate the relationship between the DII and frailty among OA patients. The findings of this study indicate a positive association between DII and the risk of frailty in OA patients. Furthermore, the results suggest that these relationships may be influenced by age and smoking, with a more pronounced effect observed in patients under the age of 65 and those who have a history of smoking. Additionally, mediation analysis reveals that inflammation plays a significant mediating role in this process.
DII is a promising tool for exploring the relationship between diet and chronic diseases. It offers a comprehensive approach to assess the impact of individual diets on inflammation levels and the progression of chronic diseases from different angles. This contributes to a deeper understanding of the relationship between diet and health (Shakya et al. Citation2021). Multiple studies have confirmed the relationship between DII and chronic diseases, including prognosis. Regarding metabolic diseases, Han et al. (Citation2023) demonstrated a significant association between DII and hyperlipidaemia. A higher DII was found to exponentially increase the risk of participants developing hyperlipidaemia. Conversely, maintaining a lower DII, indicative of a diet rich in anti-inflammatory foods, may play a crucial role in the early prevention and treatment of hyperlipidaemia (Han et al. Citation2023). When it comes to cancer, a meta-analysis incorporating 15 studies involving nearly 55,000 individuals revealed a significant association between DII scores and the risk of developing colorectal cancer. The analysis showed that, as DII scores increased, the risk of colorectal cancer also increased (RR = 1.16; 95% CI = 1.05–1.27). For each one-point increase in DII score, the risk of developing colorectal cancer increased by 1.34 times. Specific dietary patterns in certain cultures may contribute to regional variations in colorectal cancer incidence. Therefore, adjusting dietary patterns using DII may play a crucial role in reducing the high incidence rates of colorectal cancer in certain regions (Shivappa et al. Citation2017). In terms of prognosis, DII also plays a crucial role (Shivappa et al. Citation2018a). Wang et al. (Citation2023) demonstrated that, in Cox proportional hazards regression models, DII was significantly associated with all-cause mortality, cardiovascular disease mortality, and cancer mortality. Compared to individuals with the lowest DII, those with the highest DII had a 49% higher risk of all-cause mortality, a 58% higher risk of cardiovascular disease mortality, and a 56% higher risk of cancer mortality (Wang et al. Citation2023).
With the global trend of ageing populations and the accelerated development of chronic diseases, frailty, as a commonly overlooked physiological and functional state, is gradually garnering attention in the medical and public health field (Proietti and Cesari Citation2020). This is a complex clinical condition characterised by the physiological decline in several organ systems, leading to increased sensitivity to stressors (Dent et al. Citation2019). Chronic diseases and nutritional deficiencies resulting from dietary changes are significant contributors to frailty (McIsaac et al. Citation2020). OA, as a common chronic disease, is closely associated with frailty. Clinical studies have shown that OA is often accompanied by joint pain and limited mobility, leading patients to reduce physical exercise and daily activities, so this reduction in physical activity can result in decreased muscle mass and function among patients (Abramoff and Caldera Citation2020). It is this decline in muscle function that can expedite the development of frailty, as one of its hallmark features is decreased physical strength. Furthermore, OA patients often experience chronic inflammation and the accumulation of inflammatory factors. Research indicates that upregulated cytokines in OA include IL-1β, tumour necrosis factor-α, and IL-6. These inflammatory factors accumulate locally in the joints and throughout the body, resulting in decreased muscle function, metabolic abnormalities, and weight loss. All of these factors contribute to an increased risk and progression of frailty (Motta et al. Citation2023). Moreover, these relationships have been observed in epidemiological studies. A study from the UK Biobank, involving a cohort of 450,000 individuals, indicated that individuals with OA were more likely to develop or be afflicted by frailty. Timely intervention and control of frailty among OA patients may reduce the risk of late-stage disability (Cook et al. Citation2022). Effective dietary strategies are among the best approaches to treat frailty. Shivappa et al. (Citation2018b) demonstrated the impact of the DII on the incidence of frailty through a longitudinal study, showing that a higher DII score, indicating a more pro-inflammatory diet, is associated with a higher incidence of frailty. However, this study did not involve patients with OA, who are a high-risk group for frailty. This study provides critical evidence for the role of DII in preventing and treating frailty in OA patients. Mechanistically, a well-balanced dietary structure can fundamentally inhibit the production and development of inflammation (Malesza et al. Citation2021). For instance, Omega-3 fatty acids (one of the components of DII) are widely recognised as anti-inflammatory agents. A plethora of research studies have confirmed that Omega-3 fatty acids, when present in the body, can reduce the synthesis of inflammatory mediators such as prostaglandin E2 and leukotrienes, additionally, they inhibit the activation of the critical inflammatory pathway, NF-κB (Ishihara et al. Citation2019; Singh Citation2020). Furthermore, Omega-3 fatty acids competitively bind to the receptor sites of omega-6 fatty acids on cell membranes. This competitive binding reduces the metabolism of omega-6 fatty acids, which are typically pro-inflammatory in nature (Simopoulos Citation2002). Therefore, a well-designed dietary pattern based on DII may significantly improve the inflammatory environment within the body, aiming to control the risk of frailty in OA patients.
This study has several limitations. Firstly, it is a cross-sectional study and, despite conducting numerous sensitivity analyses, there is still an inherent risk of unclear causality. Secondly, the dietary data in this study are solely based on participant recall. While we incorporated the average of dietary reports from participants on two occasions, the dietary data reported by participants may still be subject to recall bias. Lastly, the determination of OA patients was based on previous physician diagnoses and lacked relevant imaging data. This may have resulted in the omission of certain individuals who already had OA but had not yet been diagnosed, potentially introducing sample bias.
Conclusion
The findings of this study demonstrate a significant association between elevated DII and an increased risk of frailty among OA patients, with a more pronounced relationship observed in patients under 65 years of age and those with a history of smoking. Additionally, mediation analysis suggests that inflammation mediates this relationship. Assessing the dietary status of OA patients and reducing DII through dietary modifications play a crucial role in lowering the risk of future frailty in OA patients.
Ethical approval and consent to participate
The research adhered to the principles of the Declaration of Helsinki. Participants provided their informed consent, and the study protocol for the NHANES was approved by the ethics committee of the National Centre for Health Statistics (NCHS) (Protocol #2011-17).
Author contributions
Conceptualisation, F. Z.; methodology, F. Z. and F. P.; software, F. P. and F. Z.; validation, F. Z. and F. P.; formal analysis, F. Z. and F. P.; investigation, F. Z. and J. L.; resources, F. Z. and F. P.; data curation, F. Z. and F. P.; writing – original draft preparation, F. Z. and F. P.; writing – review and editing, F. Z. and J. L.; visualisation, J. L.; supervision, F. Z.; project administration, F. Z.
| Abbreviations | ||
| BMI | = | Body Mass Index |
| CI | = | Confidence Intervals |
| CRP | = | C-reactive Protein |
| DII | = | Dietary Inflammatory Index |
| NHANES | = | National Health and Nutrition Examination Survey |
| OA | = | Osteoarthritis |
| OR | = | Odds Ratio |
| RCS | = | Restrictive Cubic Spline |
| RR | = | Relative Risk |
| SD | = | Standard Deviation |
Supplemental Material
Download ()Acknowledgements
We are grateful to all the staff and participants who have contributed to NHANES.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
All raw data related to this study can be obtained for free here: https://www.cdc.gov/nchs/nhanes/.
Additional information
Funding
References
- Abramoff B, Caldera FE. 2020. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 104(2):1–8. doi: 10.1016/j.mcna.2019.10.007.
- Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. 2016. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. 7(1):121–134. doi: 10.3945/an.115.009258.
- Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. 2015. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatr. 60(3):464–470. doi: 10.1016/j.archger.2015.01.016.
- Cho Y, Jeong S, Kim H, Kang D, Lee J, Kang S-B, Kim J-H. 2021. Disease-modifying therapeutic strategies in osteoarthritis: current status and future directions. Exp Mol Med. 53(11):1689–1696. doi: 10.1038/s12276-021-00710-y.
- Conaghan PG, Cook AD, Hamilton JA, Tak PP. 2019. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat Rev Rheumatol. 15(6):355–363. doi: 10.1038/s41584-019-0221-y.
- Cook MJ, Verstappen SMM, Lunt M, O’Neill TW. 2022. Increased frailty in individuals with osteoarthritis and rheumatoid arthritis and the influence of comorbidity: an analysis of the UK Biobank Cohort. Arthritis Care Res (Hoboken)). 74(12):1989–1996. doi: 10.1002/acr.24747.
- Dainese P, Mahieu H, De Mits S, Wittoek R, Stautemas J, Calders P. 2023. Associations between markers of inflammation and altered pain perception mechanisms in people with knee osteoarthritis: a systematic review. RMD Open. 9(2):e002945. doi: 10.1136/rmdopen-2022-002945.
- Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. 2019. Management of frailty: opportunities, challenges, and future directions. Lancet. 394(10206):1376–1386. doi: 10.1016/S0140-6736(19)31785-4.
- Fowler ME, Akinyemiju TF. 2017. Meta-analysis of the association between dietary inflammatory index (DII) and cancer outcomes. Int J Cancer. 141(11):2215–2227. doi: 10.1002/ijc.30922.
- Groth SW, Simpson AH, Fernandez ID. 2016. The dietary choices of women who are low-income, pregnant, and African American. J Midwifery Womens Health. 61(5):606–612. doi: 10.1111/jmwh.12463.
- Hakeem FF, Bernabé E, Sabbah W. 2021. Association between oral health and frailty among american older adults. J Am Med Dir Assoc. 22(3):559–563.e552. doi: 10.1016/j.jamda.2020.07.023.
- Han Y, Jiang X, Qin Y, Zhao Y, Zhang G, Liu C. 2023. A cross-sectional study exploring the relationship between the dietary inflammatory index and hyperlipidemia based on the National Health and Nutrition Examination Survey (2005-2018). Lipids Health Dis. 22(1):140. doi: 10.1186/s12944-023-01908-x.
- Hariharan R, Odjidja EN, Scott D, Shivappa N, Hébert JR, Hodge A, de Courten B. 2022. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes Rev. 23(1):e13349. doi: 10.1111/obr.13349.
- Ishihara T, Yoshida M, Arita M. 2019. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int Immunol. 31(9):559–567. doi: 10.1093/intimm/dxz001.
- Jiang Y. 2022. Osteoarthritis year in review 2021: biology. Osteoarthritis Cartilage. 30(2):207–215. doi: 10.1016/j.joca.2021.11.009.
- Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR. 2013. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat. 2(161):1–24.
- Kojima G, Iliffe S, Walters K. 2018. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. 47(2):193–200. doi: 10.1093/ageing/afx162.
- Levis B, Benedetti A, Thombs BD, DEPRESsion Screening Data (DEPRESSD) Collaboration. 2019. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. 365:l1476. doi: 10.1136/bmj.l1476.
- Liu C-A, Liu T, Ge Y-Z, Song M-M, Ruan G-T, Lin S-Q, Xie H-L, Shi J-Y, Zheng X, Chen Y, et al. 2023. Muscle distribution in relation to all-cause and cause-specific mortality in young and middle-aged adults. J Transl Med. 21(1):154. doi: 10.1186/s12967-023-04008-7.
- Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, Bartkowiak-Wieczorek J, Mądry E. 2021. High-fat, western-style diet, systemic inflammation, and gut microbiota: a narrative review. Cells. 10(11):10. doi: 10.3390/cells10113164.
- McIsaac DI, MacDonald DB, Aucoin SD. 2020. Frailty for perioperative clinicians: a narrative review. Anesth Analg. 130(6):1450–1460. doi: 10.1213/ANE.0000000000004602.
- Motta F, Barone E, Sica A, Selmi C. 2023. Inflammaging and osteoarthritis. Clin Rev Allergy Immunol. 64(2):222–238. doi: 10.1007/s12016-022-08941-1.
- Proietti M, Cesari M. 2020. Frailty: what is it? Adv Exp Med Biol. 1216:1–7. doi: 10.1007/978-3-030-33330-0_1.
- Shakya PR, Melaku YA, Shivappa N, Hébert JR, Adams RJ, Page AJ, Gill TK. 2021. Dietary inflammatory index (DII®) and the risk of depression symptoms in adults. Clin Nutr. 40(5):3631–3642. doi: 10.1016/j.clnu.2020.12.031.
- Shivappa N, Godos J, Hébert JR, Wirth MD, Piuri G, Speciani AF, Grosso G. 2017. Dietary Inflammatory Index and Colorectal Cancer Risk-A Meta-Analysis. Nutrients. 9(9):9. doi: 10.3390/nu9091043.
- Shivappa N, Godos J, Hébert JR, Wirth MD, Piuri G, Speciani AF, Grosso G. 2018a. Dietary inflammatory index and cardiovascular risk and mortality-a meta-analysis. Nutrients. 10(2):10. doi: 10.3390/nu10020200.
- Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. 2014. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 17(8):1689–1696. doi: 10.1017/S1368980013002115.
- Shivappa N, Stubbs B, Hébert JR, Cesari M, Schofield P, Soysal P, Maggi S, Veronese N. 2018b. The relationship between the dietary inflammatory index and incident frailty: a longitudinal cohort study. J Am Med Dir Assoc. 19(1):77–82. doi: 10.1016/j.jamda.2017.08.006.
- Shu Y, Wu X, Wang J, Ma X, Li H, Xiang Y. 2022. Associations of dietary inflammatory index with prediabetes and insulin resistance. Front Endocrinol. 13:820932. doi: 10.3389/fendo.2022.820932.
- Simopoulos AP. 2002. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 56(8):365–379. doi: 10.1016/s0753-3322(02)00253-6.
- Singh JE. 2020. Dietary sources of omega-3 fatty acids versus omega-3 fatty acid supplementation effects on cognition and inflammation. Curr Nutr Rep. 9(3):264–277. doi: 10.1007/s13668-020-00329-x.
- Tan J, Liu N, Sun P, Tang Y, Qin W. 2022. A Proinflammatory diet may increase mortality risk in patients with diabetes mellitus. Nutrients. 14(10):14. doi: 10.3390/nu14102011.
- Trouvin AP, Perrot S. 2018. Pain in osteoarthritis. Implications for optimal management. Joint Bone Spine. 85(4):429–434. doi: 10.1016/j.jbspin.2017.08.002.
- Wang H, Liao R, Tang W, Su W, Zeng M, Yang J, Fan X, Xie J, Hu Y. 2022a. Dietary inflammation index and osteoarthritis in the elderly: is there a mediating role of physical activity? Br J Nutr. 128(11):2258–2266. doi: 10.1017/S0007114522000265.
- Wang H, Wang N, Wang Y, Li H. 2022b. Association between sarcopenia and osteoarthritis: a protocol for meta-analysis. PLoS One. 17(8):e0272284. doi: 10.1371/journal.pone.0272284.
- Wang X, Hu J, Liu L, Zhang Y, Dang K, Cheng L, Zhang J, Xu X, Li Y. 2023. Association of dietary inflammatory index and dietary oxidative balance score with all-cause and disease-specific mortality: findings of 2003-2014 national health and nutrition examination survey. Nutrients. 15(14):15. doi: 10.3390/nu15143148.
- Xiao Q, Cai B, Yin A, Huo H, Lan K, Zhou G, Shen L, He B. 2022. L-shaped association of serum 25-hydroxyvitamin D concentrations with cardiovascular and all-cause mortality in individuals with osteoarthritis: results from the NHANES database prospective cohort study. BMC Med. 20(1):308. doi: 10.1186/s12916-022-02510-1.
- Zhang Y-B, Chen C, Pan X-F, Guo J, Li Y, Franco OH, Liu G, Pan A. 2021. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. BMJ. 373:n604. doi: 10.1136/bmj.n604.