ABSTRACT
Irritable Bowel Syndrome (IBS) is a common, chronic and distressing functional gastrointestinal disorder (FGID) which is currently considered a dysregulation of the brain-gut axis (BGA). Multiple biological systems have been implicated in the pathophysiology of IBS, however there is currently no reliable biomarkers for this disorder and no cure. Observations that the gut microbiota of IBS patients differs from healthy controls have been made for decades as well as the role of the gut microbiota in the pathophysiology of multiple disease. The use of probiotics, antibiotics and elimination diets have been proven to be efficacious in the treatment of IBS symptoms. A subset of IBS patients develop chronic IBS symptoms after contraction of infectious gastroenteritis or after antibiotic use. Furthermore, the gut microbiota are known to modulate the BGA. We review here the history and current state of knowledge of the role that the gut microbiota plays in the pathophysiology of IBS.
Introduction
IBS is a common, chronic, and distressing FGID characterised by pain and a change in bowel habit in the absence of an obvious physical cause (Tack and Drossman Citation2017). Diagnosis is based on the presentation of these symptoms according to the validated Rome IV criteria (Drossman Citation2016). Based on patients’ predominant bowel habit, IBS is subtyped into diarrhoea (IBS-D), constipation (IBS-C), or a mixed phenotype (IBS-M) (Tack and Drossman Citation2017). Post infectious (Pi-) IBS, in which patients develop ongoing IBS symptoms after contracting an infectious gastroenteritis is also considered an IBS subtype (Spiller and Lam Citation2012). The global prevalence of IBS is estimated to be around 11% (Lovell and Ford Citation2012; Sperber et al. Citation2017) and constitutes a major health problem in the western world due to significant direct and indirect health care costs and a decreased quality of life (Buono et al. Citation2017). However, due to its multifactorial pathophysiology there is neither consensus concerning the aetiology nor reliable biological markers for IBS diagnosis. Patients’ responses to treatment are also variable (Mujagic et al. Citation2017). There is evidence of multiple biological system dysfunction in IBS patients compared to healthy controls, but current understanding of this disorder is that symptoms arise from dysregulation of the BGA (Quigley Citation2018). The BGA describes the bidirectional communication between the cognitive and emotional areas of the central nervous system (CNS) and the peripheral functions of the gastrointestinal tract (GIT). This is mediated by neurons, hormones, neurotransmitters, and the immune system (Bonaz et al. Citation2018; Florens et al. Citation2019; Parker et al. Citation2019) as well as the gut microbiota (Pusceddu et al. Citation2018). The GIT contains more than 100 different bacterial species which perform different functions in the gut, including developing and maintaining the gut epithelial barrier, protecting the host from pathogen colonisation, modulation of the immune and nervous systems, and digestion of dietary components (Sánchez et al. Citation2017). There is significant inter-individual variation of the gut microbiota, which is governed by the environment and host genetics (Rothschild et al. Citation2018; Johnson et al. Citation2019), as well as differences between healthy individuals and those with disease (Zhuang et al. Citation2017). Recently, the functions of the microbiota in healthy individuals have been shown to be compromised in IBS patients (Sundin et al. Citation2017).
IBS and the brain gut axis
Discovering IBS
After the First and Second World Wars, there were numerous reports of soldiers who had contracted infectious dysentery and then experienced ongoing gastrointestinal (GI) symptoms which could remit and recur over weeks and months. These symptoms were not due to strictures, or other organic causes, and there was no evidence of ongoing infection. Today, these soldiers would be described as likely experiencing Pi-IBS. Interestingly, the physicians of the day also recorded the personalities and mental states of their patients who exhibited these symptoms. It was noted that the majority of IBS patients were anxious and depressed (Osler and McCrae Citation1892; Ryle Citation1928; Jordan and Kieder Citation1929; White and Jones Citation1940; Hurst and Barber Citation1943; Alvarez Citation1949; Bargen Citation1950; Stewart Citation1950; Whorton Citation2000; Drossman Citation2016) and it was theorised at the time that symptoms in patients may be a physical reaction to these emotions. This was subsequently reinforced by a prospective study in 1996 that found patients hospitalised for enteric infection who scored higher on psychometric tests for anxiety, depression, somatisation and neuroses, were more likely to go on to develop Pi-IBS (Gwee et al. Citation1996). Today anxiety and depression are the most commonly reported non-GI co-morbidities of IBS (White et al. Citation2010), and their association with worse GI and non-GI symptoms in IBS as well as worse quality of life (Midenfjord et al. Citation2019) may, in part, reflect altered processing within the CNS of IBS symptoms (Elsenbruch et al. Citation2010).
The BGA: symptoms common to IBS patients
Visceral hypersensitivity
Visceral hypersensitivity, or an abnormal pain response to a normal stimulus, was elegantly demonstrated in IBS patients in 1973, when James Ritchie established that inflating a balloon in the colon resulted in pain in a disproportionate number of IBS patients when compared to healthy controls (Ritchie Citation1973). This finding is suggestive of a significantly lower pain threshold in this group (Whitehead et al. Citation1990). In the absence of a detectable biological cause it was postulated that the altered bowel function, disturbed motility, secretion, and absorption in IBS patients (Baird Citation1946, p. 503) could all potentially be explained by changes in the autonomic nervous system (ANS) brought on by an over-exaggerated stress response mediated through the HPA axis (Selye Citation1950). However, whereas IBS patients were shown to exhibit abnormal GI motility in response to stressful situations (Almy Citation1951), animal studies found no association between the adrenal and/or the pituitary gland, and visceral hypersensitivity in response to stress (Williams et al. Citation1988). Collectively, these findings suggested that while the HPA axis is not involved, abnormal communication between the brain and periphery in IBS patients was likely to play a role in triggering of IBS symptoms.
Current research suggests that decreased pain thresholds in IBS patients likely contributes to visceral hypersensitivity but the underlying mechanism within the CNS remains unknown. Intriguingly, functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) brain scans of IBS patients identify functional alterations in various regions of the CNS that include the thalamus, hippocampus, and prefrontal cortex (Silverman et al. Citation1997; Mertz et al. Citation2000). These are areas that are important in the perception of sensory and motor signals, the relay of these signals between the CNS and the periphery, as well as memory, emotion and behaviour (Mertz et al. Citation2000; Naliboff et al. Citation2001; Ma et al. Citation2015). Functional changes to the way that these areas of the brain communicate are thought to be important in the pathophysiology of IBS. These functional changes potentially include an inability to effectively modulate sensory information from being brought to the brain or increased ‘attention’ being paid to ‘normal’ sensations in the GIT. Both of these changes could be the root of visceral hypersensitivity, where ‘normal’ sensations are relayed to the brain as painful sensations (Icenhour et al. Citation2017).
Corticotrophin Releasing Hormone (CRH), a vital mediator of the HPA axis and the stress response, is also thought to have a role in mediating IBS symptoms in the gut via modification of the inflammasome. The inflammasome is a component of the immune system that responds to the presence of pathogens via inflammatory responses, resulting in visceral hypersensitivity and increased GI barrier permeability (Ren et al. Citation2017). An increase in barrier permeability that exposes the enteric immune system (ENS) to luminal antigens then has the potential to perpetuate a positive feedback loop of inflammation, visceral pain, and altered colonic motility.
Low-grade inflammation potentially underpins visceral hypersensitivity in IBS patients via the increased number of histamine-producing mast cells (MCs) in the myenteric plexus of patients with IBS (). There is evidence to suggest that visceral pain positively correlates with the physical closeness of MCs to enteric neurons (Törnblom et al. Citation2002; Wouters et al. Citation2016). However, while inflammation potentially has a role in triggering visceral hypersensitivity, it is not necessarily involved in its maintenance (Balemans et al. Citation2017). Low-grade inflammation in the gut is likely to be linked to the gut microbiota and this is emphasised by depolarisation of enteric neurons following the application of bacterial supernatants from enteric mucosal biopsies (Balemans et al. Citation2017). Colonic biopsy supernatant from patients with IBS-D (but not IBS-C) are also shown to cause neuronal excitement via the protease signalling pathway, PAR2 (Valdez-Morales et al. Citation2013). This demonstrates that while both IBS-D and IBS-C patients experience abdominal pain by Rome IV definition, the mechanisms behind visceral hypersensitivity are likely heterogeneous in nature, despite being part of the communication between the periphery of the gut and the pain centres of the brain. More recently a genetic predisposition for visceral hypersensitivity has been demonstrated in Pi-IBS patients due to a polymorphism which produces increased expression of ion channels known to be involved in the transmission of nociceptive signals to the CNS (Lashermes et al. Citation2018). Intriguingly these are the same channels that become sensitised during inflammation (Burnstock Citation2016).
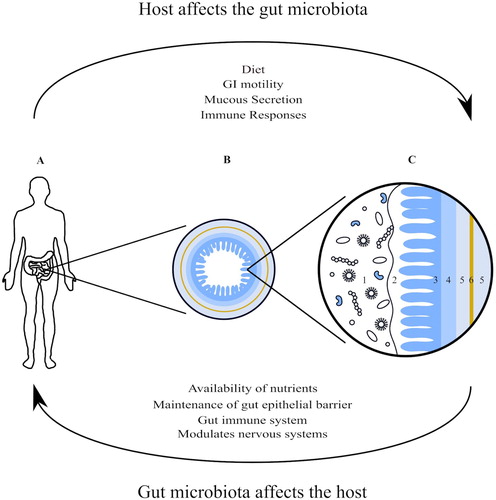
Figure 1. Anatomy of the gastrointestinal tract; the myenteric plexus (6), a part of the enteric nervous system, sits between the two layers of smooth muscle that form the muscularis (5). The gut microbiota live in and on the mucous layer (2) that covers the mucosa (3) as well as within the lumen of the gastrointestinal tract (1). The gut microbiota affect and are effected by the enteric, autonomic and central nervous systems as well as being effected by the life style of the host through a variety of mechanisms. (A) The position of the small and large intestines in the human body; (B) an enlarged cross section of the small intestine; (C) a magnification of B. (1) Gut microbiota in the GI lumen; (2) mucous layer; (3) mucosa; (4) submucosa; (5) muscularis; (6) myenteric plexus.
Altered colonic transit
The other defining symptom common to all IBS patients is altered colonic transit. Originally, it was thought that IBS patients exhibit abnormal colonic motility that, in turn, translated to both visceral pain and altered transit (Almy Citation1957), and early studies suggested that IBS was primarily a disorder of the motility of the gut (Snape Jr et al. Citation1977; Taylor et al. Citation1978). However, altered GI motility was also shown to correspond poorly with visceral pain (Mayer and Helen Citation1990). Current evidence, however, shows that while altered transit is observed in IBS patients (i.e. altered bowel habit), only a subset of IBS patients’ exhibit abnormal colonic motility, and that there is no overriding pattern that defines these patients (Simrén et al. Citation2000; Agrawal et al. Citation2009). While the exact mechanisms by which symptoms correlate to colonic motility remain unknown, patients who exhibit this abnormal colonic motility (particularly IBS-C patients), tend to respond well to pharmacological interventions that stimulate gut motility (Caldarella et al. Citation2002; Malagelada et al. Citation2017).
GI hormones and neurotransmitters
Abnormalities in the metabolism of gut hormones and neurotransmitters observed in IBS patients could be a common mechanism for both altered transit and visceral hypersensitivity. A significantly lower density of enteroendocrine cells (ECs) have been demonstrated in IBS patients (Gunawardene et al. Citation2011; El-Salhy Citation2012; El-Salhy and Gilja Citation2017). ECs produce and secrete GI hormones and neurotransmitters in response to the luminal environment as well as signals from the CNS, ANS and ENS (Mawe et al. Citation2006; Camilleri Citation2012; El-Salhy Citation2012; El-Salhy et al. Citation2012). ECs also mediate the communication between the ENS, ANS and CNS and are therefore involved in both the modulation of GI sensation and motility, this has been reviewed in detail elsewhere (Wade et al. Citation1996; May and Kaestner Citation2010; Gunawardene et al. Citation2011; El-Salhy Citation2012; El-Salhy et al. Citation2012; Gershon Citation2013). One of the predominant neurotransmitters produced by ECs is serotonin. Serotonin has a vital role in the regulation of colonic motility as well as secretion and sensation, and is a vital neurotransmitter within and between the CNS and the ENS (Sikander et al. Citation2009). Abnormal metabolism of serotonin and its precursor, 5-hydroxytryptophan, have been associated with IBS pathophysiology, as well as the different IBS subtypes, for a number of years (Pata et al. Citation2002; Dunlop et al. Citation2005). Interestingly a recent study conducted in Norway has found that modulating the gut microbiota of IBS patients through various different methods increased EC cell density towards the level of healthy controls, which was associated with improvements to symptoms and quality of life (Mazzawi et al. Citation2013, Citation2016).
All IBS patients, by Rome IV definition, exhibit altered transit and visceral hypersensitivity. Therefore pathophysiological heterogeneity makes IBS particularly hard to study. Even the most stringent study inclusion criteria, if based on clinical symptoms alone, may still result in a heterogeneous pool of IBS participants. Given this heterogeneity it is not surprising that current guidelines describe IBS pathophysiology as dysregulation of the BGA, an ‘umbrella’ term under which all of the symptoms and mechanisms of IBS can sit. It is interesting to note however that apart from its direct roles in the pathophysiology of IBS, the gut microbiota has the ability to regulate the BGA from the CNS to the gut epithelial barrier.
Gut microbiota and IBS
The gut microbiota is believed to be one of the main contributors to the aetiology of IBS. However, past and current research has been unable to determine exactly what components of the microbiota play a part in IBS development. An early study found that IBS patients had a greater proportion of facultative anaerobes cultured from stool samples compared to healthy controls (Bayliss et al. Citation1984). More recently, a systematic review and meta-analysis of IBS studies using culturing techniques found consistent reductions in Bifidobacterium and Lactobacillus in IBS patients compared to healthy controls (Zhuang et al. Citation2017). However, anaerobic culturing techniques are no longer considered appropriate for studying the complex ecology of the gut microbiota. Instead genetic sequencing techniques are now widely used, particularly 16S rRNA sequencing, which allows the investigation of complex microbial communities by amplifying the evolutionarily conserved 16S rRNA gene (Larsen et al. Citation1993). This sequencing technique is able to identify bacterial colonies that grow poorly in culture as well as detect species of bacteria previously uncultured. Subsequent analyses comparing culture and sequencing techniques found that 60%–80% of bacteria present in human stool are unculturable and only 24% of the sequencing results identify species that corresponded to previously described organisms (Suau et al. Citation1999). Despite these advances studies investigating differences in the gut microbiota in IBS populations are still contradictory.
A recent systematic review collated 16S rRNA studies and found that there are consistent detectable alterations in the gut microbiota of IBS patients, which may have a significant association with IBS pathophysiology. In particular the study identified a reduction in the gut microbiota α diversity and a shift in the Firmicutes:Bacteroidetes ratio. The Firmicutes and Bacteroidetes are the two anaerobic bacterial phyla in that dominate the human gut, with IBS patients demonstrating a higher abundance of Firmicutes and lower abundance of Bacteroidetes compared to healthy controls (Duan et al. Citation2019). While this finding has been consistent among different IBS populations, it is unknown how it is related to IBS pathophysiology and aetiology. It has been speculated that increases in Firmicutes could lead into increased gut barrier permeability and acid production (driven by Clostridia species) which could lead to low-grade inflammation and visceral hypersensitivity (Ringel-Kulka et al. Citation2015; Duan et al. Citation2019). In contrast, a lower abundance of Bacteroidetes could lead to increased inflammation through decreases in bacterial metabolite production of anti-inflammatory compounds, such as the short chain fatty acid (SCFA) butyrate (Pozuelo et al. Citation2015; Borycka-Kiciak et al. Citation2017).
There is also contradictory evidence for differences in the composition of the gut microbiota between different IBS subtypes. Some studies have associated IBS-C, not IBS-D, with an increased rate of breath methane production (Ghoshal et al. Citation2016). Breath methane has been suggested as a potential diagnostic test for IBS-C (Hwang et al. Citation2010), however methane is also detectable in healthy people and is not produced by all IBS-C patients. Methane has been shown to delay intestinal transit, and while a causative role has not yet been established it is believed that methane may act as a neuromuscular transmitter (Triantafyllou et al. Citation2014). There has been no consensus on a microbial signature for IBS-D, although several studies have observed decreases in the relative abundance of Lactobacillus species and Faecalibacterium prausnitzii (both generally considered ‘good’ bacteria) and an increase in the Enterobacteriaceae family (generally considered pathogenic) (Carroll et al. Citation2011, Citation2012; Liu et al. Citation2017). This has led to speculation that a disruption in the ratio of ‘good’ and ‘bad’ bacteria in IBS-D could contribute to the pathophysiology of IBS. However there is currently no mechanistic evidence for this, nor is this disruption considered sufficient in and of itself to explain the symptoms and altered physiology of IBS-D (Maharshak et al. Citation2018).
The ability of commensal bacteria to alter the expression of genes involved in the construction of the gut epithelial barrier is another potential mechanism of IBS pathophysiology (Hooper et al. Citation2001; Ma et al. Citation2004; Jacob et al. Citation2005). IBS patients have significantly lower mRNA expression of tight junction proteins involved in the construction of the gut epithelial barrier when compared to healthy controls (Piche et al. Citation2009). The resulting increased exposure of neuromuscular junctions to bacterial antigens leads to induction of an immune response (Collins Citation2002) which produces low grade inflammation and CNS mediated visceral hypersensitivity (O’Mahony et al. Citation2005; Zhou et al. Citation2009).
Gut microbiota and the BGA
Evidence for microbial involvement in the BGA dates back to the 1970s when anatomical and immune system abnormalities of the GIT were noted in germ-free mouse models (Gustafsson and Maunsbach Citation1971; Wannemuehler et al. Citation1982), suggesting that the gut microbiota was directly affecting the tissues it came in close contact with. Altered GI motility was also noted in germ-free animal models which was ‘normalised’ by the introduction of conventional gut microbiota (Caenepeel et al. Citation1989; Husebye et al. Citation2001). Subsequently bacterial components, such as Flagellin, have been shown to induce an immune system reaction as well as ANS mediated altered motility (Vrees et al. Citation2002; Rhee et al. Citation2004). Another notable anatomical difference found in germ-free animal models in is the significantly increased EC density (Uribe et al. Citation1994), which could potentially explain the differences in GI motility observed in these models.
The far-reaching effects of the gut microbiota have been demonstrated on the CNS and on the pathogenesis of depression and anxiety. Morphological differences in regions of the brain that control fear, anxiety and stress have been demonstrated in germ-free animal models, as well as reduced anxiety-like behaviour highlighting the vital role that the microbiota play in the development of these structures(Heijtz et al. Citation2011; Luczynski et al. Citation2016). Furthermore, GI infections have been demonstrated to induce anxiety-like behaviour in mouse models; activation of brain regions involved with mood, fear, and anxiety have been noted in these animals (Lyte et al. Citation2006; Goehler et al. Citation2007). Modulation of the gut microbiota by probiotics (Bravo et al. Citation2011), diet (Ledochowski et al. Citation2000) and antibiotics (Bercik et al. Citation2011) have all been shown to modulate anxiety- and depressive-like behaviours in mouse models. In human trials, participants reported reduced depression symptoms when taking probiotics which correlated decreased amygdala and limbic system activation on fMRI. This suggests that a probiotic organism could modulate the CNS and the HPA axis in humans (Pinto-Sanchez et al. Citation2017). Furthermore some components of the inflammasome complex, which as mentioned previously, mediates immune responses to the presence of pathogens as well as being a key mediator of physical and psychological stress, have recently been shown to be altered in patients with depression. When caspase-1, a mediator of the inflammasome, was pharmacologically inhibited in mice, decreased exhibition of depressive behaviour was observed. Increases in gut bacteria known to ameliorate inflammation were also noted in these animals (Wong et al. Citation2016).
Diet
Diet plays an important role in the generation of symptoms in IBS patients, around 70% of IBS patients report that their symptoms are triggered by food (Rej et al. Citation2018). Abnormal small intestinal motility after food ingestion has been observed in a subset of IBS patients however the mechanism of this is unknown (Manousos et al. Citation1967; Deiteren et al. Citation2010). Early studies implicated reductions in fibre intake to IBS symptoms (Thompson Citation1974). This observation was supported by studies that reported the beneficial effects of wheat bran expediting GI transit (Kirwan and Smith Citation1977). However, IBS patients often report an intolerance to fibre containing foods (Floch Citation2005; Ford et al. Citation2008; Heizer et al. Citation2009) and insoluble fibre may worsen IBS symptoms in some patients (Bijkerk et al. Citation2004). Levels of butyrate (an end product of fibre fermentation in the gut) are reportedly decreased in IBS patients (Farup et al. Citation2016), which could reflect low fibre intake and/or fewer butyrate-producing bacteria in IBS patients (Duan et al. Citation2019). Reductions in butyrate production are thought to effect GI motility and sensation as well as increase gut barrier permeability (Pryde et al. Citation2002; Soret et al. Citation2010; Wang et al. Citation2012). Non-coeliac gluten sensitivity has been suggested as a driver of symptoms in some patients (Barmeyer et al. Citation2017). However, recent work has elegantly demonstrated that many IBS patients are intolerant to fructans, a member of a group of carbohydrates called FODMAPs (Fermentable Oligosaccharide, Disaccharide, Monosaccharide, and Polyols) (Skodje et al. Citation2018). Significantly, fructans are often present in gluten containing food.
Food intolerances have been studied intensely in terms of IBS, which are generally produced by Immunoglobulin E (IgE) mediated immune reactions, but studies of IgE in IBS patients are inconclusive (Lessof et al. Citation1980; Jun et al. Citation2006; Choung and Murray Citation2019). Instead, what has been shown is that the direct application of food antigens to the gut mucosa results in increased intra-epithelial lymphocyte (IEL) recruitment and loss of epithelium integrity (Fritscher-Ravens et al. Citation2014) that enables luminal bacterial antigens to enter the systemic circulation and induce a low grade inflammatory response (through MC and IEL recruitment and activation), impacting visceral hypersensitivity and GI motility.
The idea that food intolerances are an important trigger in the pathogenesis of IBS has stemmed from the efficacy of elimination diets in controlling IBS symptoms (Ong et al. Citation2010). However, despite elimination diet studies consistently demonstrating a reduction in GI symptoms, food intolerance as a trigger for IBS is still controversial in part because of small study numbers, not taking into account IBS subtype and/or inappropriate controls. Moreover, when participants are blinded and challenged with their ‘trigger’ foods, a significant placebo effect is demonstrated (Farah et al. Citation1985). This may, in part, reflect the inter-individual differences in gut microbiota that, in turn, reflect how people might respond differently to potential dietary triggers. IBS patients have consistently demonstrated significantly higher breath hydrogen and methane concentrations, a proxy of gut microbiota fermentation, which is reduced after elimination diets (King et al. Citation1998). It was hypothesised that changes in gas production rates were caused by altered bacterial fermentation in the gut but that other factors associated with bacterial fermentation, for example local effects of SCFAs or amines may drive GI symptoms such as altered motility (King et al. Citation1998).
FODMAPs
Over the last 15 years there has been increasing evidence concerning the efficacy of elimination diets for the treatment of IBS symptoms. In particular the elimination of FODMAPs has shown consistent alleviation of IBS symptoms (Nanayakkara et al. Citation2016). FODMAPs are thought to induce GI symptoms through two central mechanisms. Firstly, through osmotic actions in the small intestine (Madsen et al. Citation2006; Barrett et al. Citation2010) and secondly through rapid fermentation by the gut microbiota in the proximal colon (Major et al. Citation2017). This causes increased water content and gas production and, therefore, changes in colonic transit and pain in patients with visceral hypersensitivity. A high FODMAP diet has been shown to increase gut epithelial barrier permeability in animal models, potentially through increased abundance of inflammation causing bacterial antigens. Decreases in the abundance of these bacteria have been observed in humans undertaking low FODMAP diets (Zhou et al. Citation2018).
However the restriction of FODMAPs in the diet has ramifications on the gut microbiota. Breath hydrogen excretion has been demonstrated to be significantly reduced after undertaking the low FODMAP diet indicating a shift in gut microbiota fermentation patterns (Staudacher et al. Citation2012). One study has shown that a 50% reduction in FODMAP ingestion led to a 6 fold reduction in the relative abundance of Bifidobacteria compared to controls (Staudacher et al. Citation2012) and other studies showed a reduction in Bifidobacteria, Faecalibacterium prausnitzii and Clostridium cluster IV during the low FODMAP diet (Staudacher et al. Citation2012, Citation2016). Most low FODMAP diet studies are for less than six months duration and the long term consequences of this disruption to the composition and potentially the metabolism to gut microbiota are unknown (Staudacher and Whelan Citation2017).
Antibiotics
Antibiotic use has long been associated with short-term GI symptoms that may become chronic, despite the cessation of treatment (Goldin and Gorbach Citation1984). Clostridium difficile infection is most commonly associated with antibiotic use and has become a major cause for morbidity and mortality in hospitalised patients (Ambrose et al. Citation1985). These observations support to the idea that antibiotic-mediated disruption of the normal gut microbiota has the potential to facilitate the growth of opportunistic pathogenic bacteria in at least a subset of patients with IBS. This is illustrated by the increased incidence of IBS in women given (Balsari et al. Citation1982) peri-operative metronidazole before undergoing hysterectomy (Alun Jones et al. Citation1984), and in Italians (Mendall and Kumar Citation1998) who received antibiotics for an outbreak of Salmonella enteritis (Barbara et al. Citation2000). A recent study confirmed the association between antibiotic use and IBS incidence in a Danish population (Krogsgaard et al. Citation2018). Mechanistic information is still lacking but animal studies suggest transient alterations in the gut microbiota triggered by the antibiotics are associated with altered GI motility (Corinaldesi et al. Citation1999; Barbara et al. Citation2005), increased inflammatory activity, and increased release of Substance P, a neurotransmitter associated with increased visceral hypersensitivity to colonic distension (Verdu et al. Citation2006).
Intriguingly, there have been consistent observations that antibiotic usage can lead to clinical improvement in some IBS patients, suggesting antibiotics have potential as a therapeutic as opposed to a trigger (Afdhal et al. Citation1986). This may reflect an effect of antibiotics on small intestine bacterial overgrowth (SIBO) and associated excessive colonic carbohydrate fermentation (Pimentel et al. Citation2000). Rifaximin, a nonabsorbed oral antibiotic was found to be associated with a significant reduction in IBS symptoms compared to placebo without the systemic effects produced by other antibiotics (Pimentel et al. Citation2006). Whilst SIBO is not considered a primary pathophysiological cause of IBS, the consistent reduction of symptoms associated with antibiotic treatment supports a role for the gut microbiota in IBS symptom generation in at least a subset of patients.
Probiotics
Probiotics also offer the ability to modulate IBS symptoms through their capacity to alter the gut microbiota. Defined as ‘live microorganisms which when administered in adequate amounts confer a health benefit on the host’ (Hotel and Cordoba Citation2001), probiotics have been used to modulate GI health for at least 100 years, predating knowledge that gut bacteria are influenced by diet. In 1907 Elie Metchnikoff advocated the health benefits of adding specific bacterial cultures to the diet (Metchnikoff Citation1907). He hypothesised that fermented food decreased the amount of ‘putrefactive’ proteolytic bacteria in the gut, and increased ‘good’ saccharolytic bacteria. Metchnikoff identified a bacterium (now known as Lactobacillus acidophilus) in his early experiments that reduced colonic pH as well as the number of bacteria excreted in the stool, both considered at the time to be beneficial for the health. Rettger and Cheplin went on to show that it was possible to change the faecal microbiota by ingesting L. acidophilus cultures, although their study did not record GI symptom improvement (Rettger and Cheplin Citation1921). The first use of probiotics for the treatment of IBS was reported in the early 1960s (Beck and Necheles Citation1961), although it has taken the advent of sequencing technologies to more fully appreciate how oral administration of probiotic strains exert their positive effect in the gut. There is evidence to suggest that IBS patients (sorted by constipation or diarrhoea predominant subtype) are characterised by a relative reduction in Lactobacillus and Bifidobacterium species compared to healthy controls, both of which are common probiotic species (Malinen et al. Citation2005; Kerckhoffs et al. Citation2009). Early trials of probiotics in IBS found that abdominal pain could be modulated by oral administration of Lactobacillus species (Niedzielin et al. Citation2001; Sinn et al. Citation2008). The mechanism of pain reduction was postulated to be due to Lactobacillus induced expression of endogenous opioid and cannabinoid receptors in the GIT (Rousseaux et al. Citation2007). Further trials of probiotics containing Bifidobacterium infantis led to global improvements in IBS symptom scores (O’Mahony et al. Citation2005), with increased and reduced plasma concentrations of anti-inflammatory (IL-10) and pro-inflammatory (IL-12) cytokines, respectively, suggesting the immunomodulatory effect of some probiotics. However, other studies found no differences in cytokine levels between IBS patients and healthy controls after probiotic ingestion (O’Sullivan and O’Morain Citation2000; Niv et al. Citation2005). Systematic reviews at the time confirmed significant heterogeneity between studies and evidence of publication bias; but the overall consensus was that probiotics are likely beneficial in the treatment of IBS symptoms (Saggioro Citation2004). A finding reinforced by more recent evidence that certain probiotic organisms, singly or in combination, are associated with reduction of global symptom severity in specific subsets of IBS patients (Distrutti et al. Citation2016; Ford et al. Citation2018; Principi et al. Citation2018). It is not yet possible to conclusively show that probiotics are efficacious in the treatment of IBS (Ford et al. Citation2018), or whether different probiotics would be more beneficial in different IBS subtypes (Principi et al. Citation2018; Shapiro et al. Citation2019). However, there is evidence that profiling volatile organic compounds in the stool of IBS patients shows high predictive value for probiotic efficacy which may be an initial step in resolving these questions (Rossi et al. Citation2018).
Faecal microbial transfer
Faecal microbial transfer (FMT) is emerging as a promising means of modulating the gut microbiota in IBS patients, where faecal microbial content of a ‘healthy’, non-symptomatic individual is given to a diseased individual either orally, via colonoscopy or enema. The exact mechanism of action of FMT is not known; putative mechanisms include direct effects such as nutrient competition with the recipients’ gut microbiota and/or the production of bacteriocins and other anti-microbials, as well as indirect effects that include SCFA production, bile acid metabolism and/or maintenance of the GI epithelial barrier (De Palma et al. Citation2017). However, while clinical trials of FMT in IBS patients have been performed since the 1980s (Borody et al. Citation1989), inter-study heterogeneity with regards to methodology, screening of faecal microbial donors, and the subtype of IBS patients as well as a lack of long term follow-up have led to inconsistent findings (Aguilar et al. Citation2015; Mazzawi et al. Citation2016; Mizuno et al. Citation2017). Consequently, there is currently no clear understanding of the long-term efficacy of FMT in the treatment of IBS.
A well-designed multicentre, double blind, randomised controlled trial who had carefully selected IBS-D patients reported (Holvoet et al. Citation2018) lower symptom severity after 24 weeks in patients given a FMT compared to those treated with placebo. Interestingly, IBS patients who responded well to FMT were found to have a higher Bacteroidetes: Firmicutes ratio and larger Prevotella fractions in their faeces than non-responders (Holvoet et al. Citation2018). A recent study explored the effect that repeated FMT or increasing the dose of FMT would have on IBS patients that had not responded to FMT in a previous trial using the same donor. While this trial only involved 10 participants who had not previously responded to FMT, 70% of these participants responded to the repeated transplant. Improvements to GI and non-GI symptoms were also observed (El-Salhy et al. Citation2019). This suggests that a large proportion of IBS patients may respond positively to modulation of the gut microbiota via FMT. However, more large-scale studies with long-term follow up are required. It is important to note that not all IBS patients respond positively to FMT (Holvoet et al. Citation2018), and other factors within the recipient and host, such as genetics, need to be considered (Abadi Citation2017; Holvoet et al. Citation2018).
Conclusions
The aetiology of IBS is multifactorial. Convincing evidence for the dysfunction of the immune system, the CNS, the ENS, and the gut microbiota has been found in different subsets of IBS patients. Whether any one pathophysiological mechanism predates the others is unknown, however evidence of a temporal relationship between enteric infection and Pi-IBS development provides an interesting insight into the importance of the disruption of the gut microbiota in the development of IBS symptoms (Collins Citation2014). IBS patients exhibit a compositionally and metabolically different gut microbiota compared to healthy controls and the functional ramifications of this are unknown, as is the cause. The differences in the composition of gut microflora seen between IBS patients and controls is not consistent across studies and marked heterogeneity remains a feature. Additionally, the drivers of dysbiosis in IBS are not clear but may include host genetics, changes in colonic transit time, or changes in gut hormone secretion. However it seems that a reliable diagnostic gut microbiota ‘fingerprint’ for IBS, or even between IBS subtypes is currently not feasible.
Regardless of pathophysiology, the modulation of the gut microbiota presents exciting possibilities for the treatment of this disorder. Probiotics and antibiotics have been used in IBS treatments for many years, with varying levels of success. The low FODMAP diet has a high level of success in controlling IBS symptoms; however the ramifications for the gut microbiota of adhering to this diet long term are unknown. Increasingly FMT has been trialled for the treatment of IBS symptoms; however long-term follow-up studies are required to assess the efficacy of FMT over long periods of time. Validation tools that could predict the responsiveness of an individual to treatments involving the modulation of the gut microbiota would provide a powerful clinical tool for the treatment of IBS. Initial studies examining faecal biomarkers that have shown initial success in predicting IBS patients responses to probiotic treatments (Rossi et al. Citation2018) are a promising beginning.
It is likely that a systems biology approach that measures all of the variables discussed in this review may offer the best hope of understanding the aetiology of FGIDs including IBS. However, such an approach will require carefully phenotyped cohorts and significant computational power to understand this complex system.
Acknowledgement
The authors would like to acknowledge Dr Catherine Wall for her input into the final draft of this review as well as funding from High Value Nutrition, National Science Program, Ministry of Business Innovation and Employment, New Zealand.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Phoebe E. Heenan http://orcid.org/0000-0003-2350-6306
Simone Bayer http://orcid.org/0000-0002-5880-2095
Richard B. Gearry http://orcid.org/0000-0002-2298-5141
Additional information
Funding
References
- Abadi ATB. 2017. Fecal microbiota transplantation against irritable bowel syndrome? Rigorous randomized clinical trials are required. World Journal of Gastrointestinal Pharmacology and Therapeutics. 8(4):208–209.
- Afdhal N, Piggott C, Long A, O’Donoghue D. 1986. Carbohydrate handling by colonic flora—is it pathogenic in the irritable bowel syndrome? Irish Journal of Medical Science. 155(6):197–201.
- Agrawal A, Houghton LA, Reilly B, Morris J, Whorwell PJ. 2009. Bloating and distension in irritable bowel syndrome: the role of gastrointestinal transit. The American Journal of Gastroenterology. 104(8):1998.
- Aguilar CR, Buch T, Bajbouj M, Schmid R, Wantia N, Thöringer C. 2015. Fecal microbiota transplantation as a novel therapy for irritable bowel syndrome with predominant diarrhea: 002. Neurogastroenterology & Motility. 27:110.
- Almy TP. 1951. Experimental studies on the irritable colon. The American Journal of Medicine. 10(1):60–67.
- Almy TP. 1957. What is the “irritable colon”? The American Journal of Digestive Diseases. 2(3):93–97.
- Alun Jones V, Wilson AJ, Hunter JO, Robinson RE. 1984. The aetiological role of antibiotic prophylaxis with hysterectomy in irritable bowel syndrome. Journal of Obstetrics and Gynaecology. 5(1):S22–S23.
- Alvarez WC. 1949. Hysterical type of nongaseous abdominal bloating. Archives of Internal Medicine. 84(2):217–245.
- Ambrose N, Johnson M, Burdon D, Keighley M. 1985. The influence of single dose intravenous antibiotics on faecal flora and emergence of Clostridium difficile. Journal of Antimicrobial Chemotherapy. 15(3):319–326.
- Baird M. 1946. Chronic or recurrent diarrhœa. Canadian Medical Association Journal. 55(3):273.
- Balemans D, Mondelaers S, Cibert-Goton V, Stakenborg N, Aguilera-Lizarraga J, Dooley J, Liston A, Bulmer D, Berghe PV, Boeckxstaens G. 2017. Evidence for long-term sensitization of the bowel in patients with post-infectious-IBS. Scientific Reports. 7(1):13606.
- Balsari A, Ceccarelli A, Dubini F, Fesce E, Poli GJM. 1982. The fecal microbial population in the irritable bowel syndrome. Microbiologica. 5(3):185–194.
- Barbara G, Stanghellini V, Berti-Ceroni C, De Giorgio R, Salvioli B, Corradi F, Cremon C, Corinaldesi R. 2000. Role of antibiotic therapy on long-term germ excretion in faeces and digestive symptoms after salmonella infection. Alimentary Pharmacology and Therapeutics. 14(9):1127–1131.
- Barbara G, Stanghellini V, Brandi G, Cremon C, Di Nardo G, De Giorgio R, Corinaldesi R. 2005. Interactions between commensal bacteria and gut sensorimotor function in health and disease. The American Journal of Gastroenterology. 100(11):2560.
- Bargen JA. 1950. Psychosomatic relationships in the digestive system. Gastroenterology. 15(4):581–591.
- Barmeyer C, Schumann M, Meyer T, Zielinski C, Zuberbier T, Siegmund B, Schulzke J-D, Daum S, Ullrich R. 2017. Long-term response to gluten-free diet as evidence for non-celiac wheat sensitivity in one third of patients with diarrhea-dominant and mixed-type irritable bowel syndrome. International Journal of Colorectal Disease. 32(1):29–39.
- Barrett JS, Gearry RB, Muir JG, Irving PM, Rose R, Rosella O, Haines M, Shepherd SJ, Gibson PR. 2010. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Alimentary Pharmacology & Therapeutics. 31(8):874–882.
- Bayliss CE, Houston AP, Jones VA, Hishon S, Hunter JO. 1984. Microbiological studies on food intolerance. Proceedings of the Nutritional Society. Oxon: CAB Iinternational.
- Beck C, Necheles H. 1961. Beneficial effects of administration of Lactobacillus acidophilus in diarrheal and other intestinal disorders. The American Journal of Gastroenterology. 35:522–530.
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD. 2011. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 141(2):599–609.e593.
- Bijkerk C, Muris J, Knottnerus J, Hoes A, De Wit NJ. 2004. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 19(3):245–251.
- Bonaz B, Bazin T, Pellissier S. 2018. The vagus nerve at the interface of the microbiota-gut-brain axis. Frontiers in Neuroscience. 12:49.
- Borody TJ, George L, Andrews P, Brandl S, Noonan S, Cole P, Hyland L, Morgan A, Maysey J, Moore-Jones D. 1989. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Medical Journal of Australia. 150(10):604–604.
- Borycka-Kiciak K, Banasiewicz T, Rydzewska G. 2017. Butyric acid – a well-known molecule revisited. Gastroenterology Review. 12(2):83–89.
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. 2011. Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences. 108(38):16050–16055.
- Buono JL, Carson RT, Flores NM. 2017. Health-related quality of life, work productivity, and indirect costs among patients with irritable bowel syndrome with diarrhea. Health and Quality of Life Outcomes. 15(1):35.
- Burnstock G. 2016. Purinergic mechanisms and pain. Advances in Pharmacology. 75:91–137.
- Caenepeel P, Janssens J, Vantrappen G, Eyssen H, Coremans G. 1989. Interdigestive myoelectric complex in germ-free rats. Digestive Diseases and Sciences. 34(8):1180–1184.
- Caldarella MP, Serra J, Azpiroz F, Malagelada JR. 2002. Prokinetic effects in patients with intestinal gas retention. Gastroenterology. 122(7):1748–1755.
- Camilleri M. 2012. Peripheral mechanisms in irritable bowel syndrome. New England Journal of Medicine. 367(17):1626–1635.
- Carroll IM, Ringel-Kulka T, Keku TO, Chang Y-H, Packey CD, Sartor RB, Ringel Y. 2011. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhoea-predominant irritable bowel syndrome. Neuroregulation and Motility. 301(5):799–807.
- Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. 2012. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterology & Motility. 24(6):521.
- Choung RS, Murray JA. 2019. The role for food allergies in the pathogenesis of irritable bowel syndrome: understanding mechanisms of intestinal mucosal responses against food antigens. Gastroenterology. 157(1):15–17.
- Collins SM. 2002. A case for an immunological basis for irritable bowel syndrome. Gastroenterology. 122(7):2078–2080.
- Collins SM. 2014. A role for the gut microbiota in IBS. Nature Reviews Gastroenterology & Hepatology. 11:497–505.
- Corinaldesi R, Barbara G, De Giorgio R, Stanghellini V. 1999. Irritable bowel syndrome and enteric flora. Gastroenterol Int. 12(Suppl. 2):38–39.
- Deiteren A, Camilleri M, Burton D, McKinzie S, Rao A, Zinsmeister AR. 2010. Effect of meal ingestion on ileocolonic and colonic transit in health and irritable bowel syndrome. Digestive Diseases and Sciences. 55(2):384–391.
- De Palma G, Lynch MD, Lu J, Dang VT, Deng Y, Jury J, Umeh G, Miranda PM, Pastor MP, et al. 2017. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Science Translational Medicine. 9(379):eaaf6397.
- Distrutti E, Monaldi L, Ricci P, Fiorucci S. 2016. Gut microbiota role in irritable bowel syndrome: new therapeutic strategies. World Journal of Gastroenterology. 22(7):2219.
- Drossman DA. 2016. Functional gastrointestinal disorders: history, pathophysiology, clinical features, and Rome IV. Gastroenterology. 150(6):1262–1279.e1262.
- Duan R, Zhu S, Wang B, Duan L. 2019. Alterations of gut microbiota in patients with irritable bowel syndrome based on 16S rRNA-targeted sequencing: a systematic review. Clinical and Translational Gastroenterology. 10(2):e00012.
- Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. 2005. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 3(4):349–357.
- El-Salhy M. 2012. Irritable bowel syndrome: diagnosis and pathogenesis. World Journal of Gastroenterology. 18(37):5151–5163.
- El-Salhy M, Gilja OH. 2017. Abnormalities in ileal stem, neurogenin 3, and enteroendocrine cells in patients with irritable bowel syndrome. BMC Gastroenterology. 17.
- El-Salhy M, Hausken T, Hatlebakk JG. 2019. Increasing the dose and/or repeating faecal microbiota transplantation (FMT) increases the response in patients with irritable bowel syndrome (IBS). Nutrients. 11(6):1415.
- El-Salhy M, Ostgaard H, Gundersen D, Hatlebakk JG, Hausken T. 2012. The role of diet in the pathogenesis and management of irritable bowel syndrome (review). International Journal of Molecular Medicine. 29:723–731.
- El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. 2012. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Frontiers in Bioscience. E4:2683–2700.
- Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. 2010. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. 59:489–495.
- Farah D, Calder I, Benson L, MacKenzie J. 1985. Specific food intolerance: its place as a cause of gastrointestinal symptoms. Gut. 26(2):164–168.
- Farup PG, Rudi K, Hestad K. 2016. Faceal short-chain fatty acids – a diagnostic biomarker for irritable bowel syndrome?. BMC Gastroenterology. 16(51). doi:10.1186/s12876-016-0446z.
- Floch MH. 2005. Use of diet and probiotic therapy in the irritable bowel syndrome: analysis of the literature. Journal of Clinical Gastroenterology. 39(5):S243–S246.
- Florens MV, Van Wanrooy S, Dooley J, Aguilera-Lizarraga J, Vanbrabant W, Wouters MM, Van Oudenhove L, Peetermans WE, Liston A, Boeckxstaens GE. 2019. Prospective study evaluating immune-mediated mechanisms and predisposing factors underlying persistent postinfectious abdominal complaints. Neurogastroenterology & Motility. 31(4):e13542.
- Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. 2018. Systematic review with metaanalysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Alimentary Pharmacology & Therapeutics. 48:1044–1060.
- Ford AC, Talley NJ, Spiegel BM, Foxx-Orenstein AE, Schiller L, Quigley EM, Moayyedi P. 2008. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 337:a2313.
- Fritscher-Ravens A, Schuppan D, Ellrichmann M, Schoch S, Röcken C, Brasch J, Bethge J, Böttner M, Klose J, Milla PJ. 2014. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 147(5):1012–1020.e1014.
- Gershon MD. 2013. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Current Opinion in Endocrinology Diabetes and Obesity. 20(1):14–21.
- Ghoshal U, Shukla R, Srivastava D, Ghoshal UC. 2016. Irritable bowel syndrome, particularly the constipation-predominant form, involves an increase in Methanobrevibacter smithii, which is associated with higher methane production. Gut and Liver. 10(6):932–938.
- Goehler LE, Lyte M, Gaykema RP. 2007. Infection-induced viscerosensory signals from the gut enhance anxiety: implications for psychoneuroimmunology. Brain, Behavior, and Immunity. 21(6):721–726.
- Goldin BR, Gorbach SL. 1984. Alterations of the intestinal microflora by diet, oral antibiotics, and Lactobacillus: decreased production of free amines from aromatic nitro compounds, azo dyes, and glucuronides. Journal of the National Cancer Institute. 73(3):689–695.
- Gunawardene AR, Corfe BM, Staton CA. 2011. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. International Journal of Experimental Pathology. 92:219–231.
- Gustafsson BE, Maunsbach AB. 1971. Ultrastructure of the enlarged cecum in germfree rats. Zeitschrift für Zellforschung und Mikroskopische Anatomie. 120(4):555–578.
- Gwee KA, Graham JC, McKendrick MW, Collins SM, Marshall JS, Walters SJ, Read NW. 1996. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. The Lancet. 347(8995):150–153.
- Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. 2011. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences. 108(7):3047–3052.
- Heizer WD, Southern S, McGovern S. 2009. The role of diet in symptoms of irritable bowel syndrome in adults: a narrative review. Journal of the American Dietetic Association. 109(7):1204–1214.
- Holvoet T, Joossens M, Jerina B, Christiaens E, Heyerick L, Verhasselt B, De Vos M, Hindryckx P, Raes J, De Looze DJG. 2018. 617-fecal microbiota transplantation in irritable bowel syndrome with predominant abdominal bloating: results from a double blind, placebo-controlled clinical trial. Gastroenterology. 154(6):S-130.
- Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. 2001. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 291(5505):881–884.
- Hotel ACP, Cordoba A. 2001. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention. 5(1):1–10.
- Hurst AF, Barber HW. 1943. Medical diseases of war. London: E. Arnold & Co.
- Husebye E, Hellström PM, Sundler F, Chen J, Midtvedt T. 2001. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. American Journal of Physiology-Gastrointestinal and Liver Physiology. 280(3):G368–G380.
- Hwang L, Low K, Khoshini R, Melmed GY, Sahakian A, Makhani M, Pokkunuri V, Pimentel M. 2010. Evaluating breath methane as a diagnostic test for constipation-predominant IBS. Digestive Diseases and Sciences. 55(2):398–403.
- Icenhour A, Witt ST, Elsenbruch S, Lowen M, Engström M, Tillisch K, Mayer EA, Walter S. 2017. Brain functional connectivity is associated with visceral sensitivity in women with irritable bowel syndrome. NeuroImage: Clinical. 15:449–457.
- Jacob C, Yang P-C, Darmoul D, Amadesi S, Saito T, Cottrell GS, Coelho A-M, Singh P, Grady EF, Perdue M. 2005. Mast cell tryptase controls paracellular permeability of the intestine role of protease-activated receptor 2 and β-arrestins. Journal of Biological Chemistry. 280(36):31936–31948.
- Johnson AJ, Vangay P, Al-Ghalith GA, Hillmann BM, Ward TL, Shields-Cutler RR, Kim AD, Shmagel AK, Syed AN, Students PMC. 2019. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host & Microbe. 25(6):789–802.e785.
- Jordan SM, Kieder ED. 1929. The irritable colon. Journal of the American Medical Association. 93(8):592–595.
- Jun D-W, Less O-Y, Yoon H-J, Lee S-H, Lee H-L, Choi H-S, Yoon B-C, Lee M-H, Lee D-H, Cho S-H. 2006. Food intolerance and skin prick test in treated and untreated irritable bowel syndrome. World Journal of Gastroenterology. 12(15):2382–2387.
- Kerckhoffs AP, Samsom M, van der Rest ME, de Vogel J, Knol J, Ben-Amor K, Akkermans LM. 2009. Lower Bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World Journal of Gastroenterology. 15(23):2887.
- King T, Elia M, Hunter J. 1998. Abnormal colonic fermentation in irritable bowel syndrome. The Lancet. 352(9135):1187–1189.
- Kirwan W, Smith A. 1977. Colonic propulsion in diverticular disease, idiopathic constipation, and the irritable colon syndrome. Scandinavian Journal of Gastroenterology. 12(3):331–335.
- Krogsgaard LR, Engsbro AL, Bytzer P. 2018. Antibiotics: a risk factor for irritable bowel syndrome in a population-based cohort. Scandinavian Journal of Gastroenterology. 53(9):1027–1030.
- Larsen N, Olsen GJ, Maidak BL, McCaughey MJ, Overbeek R, Macke TJ, Marsh TL, Woese CR. 1993. The ribosomal database project. Nucleic Acids Research. 21(13):3021–3023.
- Lashermes A, Barbier J, Sion B, Gelot A, Barnich N, Ardid D, Carvalho FA. 2018. Adherent-Invasive E. coli enhances colonic hypersensitivity and P2X receptors expression during post-infectious period. Gut Microbes. 9(1):26–37.
- Ledochowski M, Widner B, Bair H, Probst T, Fuchs D. 2000. Fructose-and sorbitol-reduced diet improves mood and gastrointestinal disturbances in fructose malabsorbers. Scandinavian Journal of Gastroenterology. 35(10):1048–1052.
- Lessof M, Wraith D, Merrett T, Merrett J, Buisseret P. 1980. Food allergy and intolerance in 100 patients—local and systemic effects. QJM: An International Journal of Medicine. 49(3):259–271.
- Liu H-N, Wu H, Chen Y-Z, Chen Y-J, Shen X-Z, Liu T-T. 2017. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: a systematic review and meta-analysis. Digestive and Liver Disease. 49(4):331–337.
- Lovell RM, Ford AC. 2012. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clinical Gastroenterology and Hepatology. 10:712–721.
- Luczynski P, Whelan SO, O’Sullivan C, Clarke G, Shanahan F, Dinan TG, Cryan JF. 2016. Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. European Journal of Neuroscience. 44(9):2654–2666.
- Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE. 2006. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiology & Behavior. 89(3):350–357.
- Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. 2004. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. American Journal of Physiology-Gastrointestinal and Liver Physiology. 286(3):G367–G376.
- Ma X, Li S, Tian J, Jiang G, Wen H, Wang T, Fang J, Zhan W, Xu Y. 2015. Altered brain spontaneous activity and connectivity network in irritable bowel sybdrome patients: a resting-state fMRI study. Clinical Neurophysiology. 126:1190–1197.
- Madsen JL, Linnet J, Rumessen JJ. 2006. Effect of nonabsorbed amounts of a fructose–sorbitol mixture on small intestinal transit in healthy volunteers. Digestive Diseases and Sciences. 51(1):147–153.
- Maharshak N, Ringel Y, Katibian D, Lundqvist A, Sartor RB, Carroll IM, Ringel-Kulka T. 2018. Fecal and mucosa-associated intestinal mucosa in patients with diarrhoea-predominant irritable bowel syndrome. Digestive Diseases and Sciences. 63(7):1890–1899.
- Major G, Pritchard S, Murray K, Alappadan JP, Hoad CL, Marciani L, Gowland P, Spiller R. 2017. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate-related symptoms in individuals with irritable bowel syndrome. Gastroenterology. 152(1):124–133.e122.
- Malagelada C, Nieto A, Mendez S, Accarino A, Santos J, Malagelada JR, Azpiroz F. 2017. Effect of prucalopride on intestinal gas tolerance in patients with functional bowel disorders and constipation. Journal of Gastroenterology and Hepatology. 32(8):1457–1462.
- Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. 2005. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. The American Journal of Gastroenterology. 100(2):373.
- Manousos O, Truelove S, Lumsden K. 1967. Transit times of food in patients with diverticulosis or irritable colon syndrome and normal subjects. British Medical Journal. 3(5568):760.
- Mawe G, Coates M, Moses PL. 2006. Intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther. 23(8):1067–1076.
- May CL, Kaestner KH. 2010. Gut endocrine cell development. Molecular and Cellular Endocrinology. 323:70–75.
- Mayer EAR, Helen E. 1990. Role of visceral afferent mechanisms in functional bowel disorders. Gastroenterology. 99:1688–1704.
- Mazzawi T, El-Sahy M, Lied G, Gilja O, Hatlebakk J, Hausken T. 2016. Effect of fecal transplantation on the symptoms and duodenal endocrine cells in patients with irritable bowel syndrome. UEG J. 4:677.
- Mazzawi T, Hausken T, Gundersen D, El-Salhy M. 2013. Effects of dietary guidance on the symptoms, quality of life and habitual dietary intake of patients with irritable bowel syndrome. Molecular Medicine Reports. 8(3):845–852.
- Mendall MA, Kumar D. 1998. Antibiotic use, childhood affluence and irritable bowel syndrome (IBS). European Journal of Gastroenterology and Hepatology. 10(1):59–62.
- Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, Kessler R. 2000. Regional cerebral activiation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distension. Gastroenterology. 118(5):842–848.
- Metchnikoff E. 1907. The prolongation of life: optimistic studies. Mitchell PC, translator. New York: GP Putnam’s Sons.
- Midenfjord I, Polster A, Sjövall H, Törnblom H, Simrén M. 2019. Anxiety and depression in irritable bowel syndrome: exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neurogastroenterology & Motility. 31:e13619.
- Mizuno S, Masaoka T, Naganuma M, Kishimoto T, Kitazawa M, Kurokawa S, Nakashima M, Takeshita K, Suda W, Mimura M. 2017. Bifidobacterium-rich fecal donor may be a positive predictor for successful fecal microbiota transplantation in patients with irritable bowel syndrome. Digestion. 96(1):29–38.
- Mujagic Z, Jonkers D, Ludidi S, Keszthelyi D, Hesselink M, Weerts Z, Kievit R, Althof J, Leue C, Kruimel JW, et al. 2017. Biomarkers for visceral hypersensitivity in patients with irritable bowel syndrome. Neurogastroenterol Motil. 29(12):e13137.
- Naliboff B, Derbyshire SWG, Munakata J, Berman S, Mandelkern M, Chang L, Mayer E. 2001. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosomatic Medicine. 63(3):365–375.
- Nanayakkara WS, Skidmore PM, O’Brien L, Wilkinson TJ, Gearry RB. 2016. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: the evidence to date. Clinical and Experimental Gastroenterology. 9:131.
- Niedzielin K, Kordecki H, Birkenfeld B. 2001. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. European Journal of Gastroenterology & Hepatology. 13(10):1143–1147.
- Niv E, Naftali T, Hallak R, Vaisman N. 2005. The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome—a double blind, placebo-controlled, randomized study. Clinical Nutrition. 24(6):925–931.
- O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’Sullivan GC, Kiely B, Collins JK, Shanahan F. 2005. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 128(3):541–551.
- Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR, Muir JG. 2010. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. Journal of Gastroenterology and Hepatology. 25(8):1366–1373.
- Osler W, McCrae T. 1892. The principles and practice of medicine. 1st ed. New York: D. Appleton & Co.695.
- O’Sullivan M, O’Morain C. 2000. Bacterial supplementation in the irritable bowel syndrome. A randomised doubleblind placebo-controlled crossover study. Digestive and Liver Disease. 32(4):294–301.
- Parker CH, Naliboff BD, Shih W, Presson AP, Videlock EJ, Mayer EA, Chang L. 2019. Negative events during adulthood are associated with symptom severity and altered stress response in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 17:2245–2252.
- Pata C, Erdal ME, Derici E, Yazar A, Kanık A, Ulu O. 2002. Serotonin transporter gene polymorphism in irritable bowel syndrome. The American Journal of Gastroenterology. 97(7):1780.
- Piche T, Barbara G, Aubert P, Des Varannes SB, Dainese R, Nano J-L, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP. 2009. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 58(2):196–201.
- Pimentel M, Chow EJ, Lin HC. 2000. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 95(12):3503.
- Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. 2006. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 145(8):557–563.
- Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Martin F-P, Cominetti O, Welsh C, Rieder A. 2017. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 153(2):448–459.e448.
- Pozuelo M, Panda S, Santiago A, Mendez S, Accarino A, Santos J, Guarner F, Azpiroz F, Manichanh C. 2015. Reduction of butyrate- and methane- producing microorganisms in patients with irritable bowel syndrome. Scientific Reports. 5. doi:10.1038/srep12693(2015).
- Principi N, Cozzali R, Farinelli E, Brusaferro A, Esposito S. 2018. Gut dysbiosis and irritable bowel syndrome: the potential role of probiotics. J Infect. 76(2):111–120.
- Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. 2002. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 217(2):133–139.
- Pusceddu MM, Murray K, Gareau MG. 2018. Targeting the microbiota, from Irritable bowel syndrome to mood disorders: focus on probiotics and prebiotics. Curr Pathobiol Rep. 6:1–13.
- Quigley E. 2018. The gut-brain axis and the microbiome: clues to pathophysiology and opportunities for novel management strategies in irritable bowel syndrome (IBS). Journal of Clinical Medicine. 7(1):6. doi:10.3390/jcm7010006.
- Rej A, Avery A, Ford AC, Holdoway A, Kurien M, McKenzie Y, Thompson J, Trott N, Whelan K, Williams MJ, et al. 2018. Clinical application of dietary therapies in irritable bowel syndrome. J Gastrointestin Liver Dis. 27(3):307–316.
- Ren YJ, Zhang L, Bai T, Yu HL, Li Y, Qian W, Jin S, Xiong ZF, Wang H, Hou XH. 2017. Transfer of CD11c+ lamina propria mononuclear phagocytes from post-infectious irritable bowel syndrome causes mucosal barrier dysfunction and visceral hypersensitivity in recipient mice. International Journal of Molecular Medicine. 39(6):1555–1563.
- Rettger LF, Cheplin HA. 1921. A treatise on the transformation of the intestinal flora: with special reference to the implantation of bacillus acidophilus. Vol. 13. New Haven, CT: Yale University Press.
- Rhee SH, Keates AC, Moyer MP, Pothoulakis C. 2004. MEK is a key modulator for TLR5-induced interleukin-8 and MIP3α gene expression in non-transformed human colonic epithelial cells. Journal of Biological Chemistry. 279(24):25179–25188.
- Ringel-Kulka T, Choi CH, Temas D, Kim A, Maier DM, Scott KP, Galanka JA, Ringel Y. 2015. Altered colonic bacterial fermentation as a potential pathophysiological factor in irritable bowel syndrome. American Journal of Gastroenterology. 110(9):1339–1346.
- Ritchie J. 1973. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut. 14(2):125–132.
- Rossi M, Aggio R, Staudacher HM, Lomer MC, Lindsay JO, Irving P, Probert C, Whelan K. 2018. Volatile organic compounds in feces associate with response to dietary intervention in patients with irritable bowel syndrome. Clinical Gastroenterology and Hepatology. 16(3):385–391.e381.
- Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, et al. 2018. Environment dominates over host genetics in shaping human gut microbiota. Nature. 555:210–215.
- Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M. 2007. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nature Medicine. 13(1):35.
- Ryle JA. 1928. An address on chronic spasmodic affections of the colon and the diseases which they stimulate. The Lancet. 212(5492):1115–1119.
- Saggioro A. 2004. Probiotics in the treatment of irritable bowel syndrome. Journal of Clinical Gastroenterology. 38:S104–S106.
- Sánchez B, Delgado S, Blanco-Míguez A, Lourenço A, Gueimonde M, Margolles A. 2017. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. 61(1):1600240.
- Selye H. 1950. The physiology and pathology of exposure to stress. 6th ed. Montreal: Acta, Inc.
- Shapiro J, Bernica J, Hernaez R. 2019. Risk of bias analysis of systematic reviews of probiotics for treatment of Irritable bowel syndrome. Clinical Gastroenterology and Hepatology. 17(4):784.
- Sikander A, Rana SV, Prasad KK. 2009. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clinica Chimica Acta. 403(1-2):47–55.
- Silverman DHS, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. 1997. Regional Cerebral activity in normal and Pathological perception of visceral pain. Gastroenterology. 112(1):64–72.
- Simrén M, Castedal M, Svedlund J, Abrahamsson H, Bjõrnsson E. 2000. Abnormal propagation pattern of duodenal pressure waves in the irritable bowel syndrome (IBS). Digestive Diseases and Sciences. 45(11):2151–2161.
- Sinn DH, Song JH, Kim HJ, Lee JH, Son HJ, Chang DK, Kim Y-H, Kim JJ, Rhee JC, Rhee P-L. 2008. Therapeutic effect of lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Digestive Diseases and Sciences. 53(10):2714–2718.
- Skodje GI, Sarna VK, Minelle IH, Rolfsen KL, Muir JG, Gibson PR, Veierød MB, Henriksen C, Lundin KE. 2018. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. 154(3):529–539.e522.
- Snape Jr WJ, Carlson GM, Matarazzo SA, Cohen S. 1977. Evidence that abnormal Myoelectrical activity produces colonic motor dysfunction in the irritable bowel syndrome. Gastroenterology. 72(3):383–387.
- Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. 2010. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motiliy in rats. Gastroenterology. 138(5):1772–1782.
- Sperber AD, Dumitrascu D, Fukudo S, Gerson C, Ghoshal UC, Gwee KA, Hungin APS, Kang J-Y, Minhu C, Schmulson M. 2017. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. 66(6):1075–1082.
- Spiller R, Lam C. 2012. An update on post-infectious irritable bowel syndrome: role of genetics, immune activation, serotonin and altered microbiome. Journal of Neurogastroenterology and Motility. 18(3):258.
- Staudacher HM, Lomer MC, Anderson JL, Barrett JS, Muir JG, Irving PM, Whelan K. 2012. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. The Journal of Nutrition. 142(8):1510–1518.
- Staudacher HM, Lomer M, Louis P. 2016. 1133 the low FODMAP diet reduces symptoms in irritable bowel syndrome compared with placebo diet and the microbiota alterations may be prevented by probiotic co-administration: a 2×2 factorial randomized controlled trial. Gastroenterology. 150(4):S230. doi:10.1016/S0016-5085(16)30842-3.
- Staudacher HM, Whelan K. 2017. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut. 66(8):1517–1527.
- Stewart G. 1950. Post-dysenteric colitis. British Medical Journal. 1(4650):405.
- Suau A, Bonnet R, Sutren M, Godon J-J, Gibson GR, Collins MD, Doré J. 1999. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 65(11):4799–4807.
- Sundin J, Öhman L, Simrén M. 2017. Understanding the gut microbiota in inflammatory and functional gastrointestinal diseases. Psychosom Med. 79(8):857–867.
- Tack J, Drossman D. 2017. What’s new in Rome IV? Neurogastroenterology & Motility. 29:e13053.
- Taylor I, Darby C, Hammond P, Basu P. 1978. Is there a myoelectrical abnormality in the irritable colon syndrome? Gut. 19:391–395.
- Thompson WG. 1974. The irritable colon. Canadian Medical Association Journal. 111(11):1236.
- Törnblom H, Lindberg G, Nyberg B, Veress B. 2002. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 123(6):1972–1979.
- Triantafyllou K, Chang C, Pimentel M. 2014. Methanogens, methane and gastrointestinal motility. Journal of Neurogastroenterology and Motility. 20(1):31–40.
- Uribe A, Alam M, Johansson O, Midtvedt T, Theodorsson E. 1994. Microflora modulates endocrine cells in the gastrointestinal mucosa of the rat. Gastroenterology. 107(5):1259–1269.
- Valdez-Morales EE, Overington J, Guerrero-Alba R, Ochoa-Cortes F, Ibeakanma CO, Spreadbury I, Bunnett NW, Beyak M, Vanner SJ. 2013. Sensitization of peripheral sensory nerves by mediators from colonic biopsies of diarrhea-predominant irritable bowel syndrome patients: a role for PAR2. The American Journal of Gastroenterology. 108(10):1634.
- Verdu EF, Bercik P, Verma-Gandhu M, Huang X-X, Blennerhassett P, Jackson W, Mao Y, Wang L, Rochat F, Collins SM. 2006. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 55(2):182–190.
- Vrees MD, Pricolo VE, Potenti FM, Cao W. 2002. Abnormal motility in patients with ulcerative colitis: the role of inflammatory cytokines. Archives of Surgery. 137(4):439–446.
- Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. 1996. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. The Journal of Neuroscience. 16(7):2352–2364.
- Wang H-B, Wang P-Y, Wang X, Wan Y-L, Liu Y-C. 2012. Butyrate enhances intestinal epithelial barrier function via Up-regulation of tight junction protein claudin-1 transcription. Digestive Diseases and Sciences. 57:3126–3135.
- Wannemuehler M, Kiyono H, Babb J, Michalek S, McGhee J. 1982. Lipopolysaccharide (LPS) regulation of the immune response: LPS converts germfree mice to sensitivity to oral tolerance induction. The Journal of Immunology. 129(3):959–965.
- White BV, Jones CM. 1940. Mucous colitis: a delineation of the syndrome with certain observations on its mechanism and on the role of emotional tension as a precipitating factor. Annals of Internal Medicine. 14(5):854–872.
- White D, Savas L, Daci K, Elserag R, Graham D, Fitzgerald S, Smith SL, Tan G, El-Serag H. 2010. Trauma history and risk of the irritable bowel syndrome in women veterans. Alimentary Pharmacology & Therapeutics. 32(4):551–561.
- Whitehead WE, Holtkotter B, Enck P, Hoelzl R, Holmes KD, Anthony J, Shabsin HS, Schuster MM. 1990. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 98(5):1187–1192.
- Whorton J. 2000. Civilisation and the colon: constipation as the “disease of diseases”. BMJ. 321(7276):1586–1589.
- Williams CL, Villar RG, Peterson JM, Burks TF. 1988. Stress-induced changes in intestinal transit in the rat: a model for irritable bowel syndrome. Gastroenterology. 94(3):611–621.
- Wong M, Inserra A, Lewis M, Mastronardi CA, Leong L, Choo J, Kentish S, Xie P, Morrison M, Wesselingh S. 2016. Inflammasome signaling affects anxiety-and depressive-like behavior and gut microbiome composition. Molecular Psychiatry. 21(6):797.
- Wouters MM, Balemans D, Van Wanrooy S, Dooley J, Cibert-Goton V, Alpizar YA, Valdez-Morales EE, Nasser Y, Van Veldhoven PP, Vanbrabant W. 2016. Histamine receptor H1–mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology. 150(4):875–887.e879.
- Zhou S-Y, Gillilland M, Wu X, Leelasinjaroen P, Zhang G, Zhou H, Ye B, Lu Y, Owyang C. 2018. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. The Journal of Clinical Investigation. 128(1):267–280.
- Zhou Q, Zhang B, Verne GN. 2009. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 146(1-2):41–46.
- Zhuang X, Xiong L, Li L, Li M, Chen M. 2017. Alterations of gut microbiota in patients with irritable bowel syndrome: a systematic review and meta-analysis. J Gastroenterol Hepatol. 32(1):28–38.
