Abstract
Effective terminal cleaning and disinfection (C&D) is regarded as a necessary step for the elimination of Salmonella spp. from laying houses. A total of 60 commercial laying houses that had housed laying flocks infected with Salmonella enterica serovar Enteritidis or Salmonella enterica serovar Typhimurium that were representative of all production systems (cage, barn, free-range) were intensively sampled immediately after C&D as well as in the follow-on flock. The procedures investigated were: (1) a compound disinfectant consisting of a mixture of formaldehyde, glutaraldehyde and quarternary ammonium applied at the recommended concentration; (2) a 10% (vol/vol) dilution of the standard 37% commercial formalin, applied by a contractor; and (3) other disinfection procedures selected and applied by the farmer. The recovery of Salmonella in the cleaned and disinfected houses was variable, with samples from floor and dropping boards/belts (cage houses) and scratching areas (non-cage houses) being the most likely to remain contaminated. In cage houses, the use of the 10% formalin dilution led to a statistically greater reduction in the sample prevalence than using any of the other C&D methods. A negative post-C&D result predicted clearance of Salmonella in 52% of cases, although the isolation of Salmonella from the houses immediately after C&D was not a perfect predictor of carry-over of infection.
Introduction
Salmonella infection is one of the most common causes of infectious gastroenteritis in humans worldwide (World Health Organization, Citation2005). In the European Union (EU), Salmonella enterica serovar Enteritidis is the agent most frequently involved in human salmonellosis and it is also the serovar most widespread in egg production, followed at a distance by S. enterica serovar Typhimurium (Anonymous, Citation2006).
Eggs are regarded as the main source of infection of Salmonella Enteritidis for humans (Gillespie et al., Citation2005; de Jong & Ekdahl, Citation2006). Over the past few years, the egg industry has stepped up its efforts to control Salmonella in laying houses. These have mostly involved an upgrade of biosecurity and hygiene procedures. In the UK and other countries, vaccination with attenuated, live or killed vaccines is also now widespread among commercial laying flocks.
Despite this, effective interventions to control Salmonella infection during the life of the flocks are limited, and effective terminal cleaning and disinfection (C&D) is regarded as a crucial step to reduce the risk of infection to new flocks placed in houses that have held infected birds (van de Giessen et al., Citation1994).
In the UK, carry-over of S. Enteritidis between consecutive laying flocks has been shown to be a common occurrence (Carrique-Mas et al., Citation2008b, Citation2009; Wales et al., Citation2006b), which was concomitant with a generally low standard of C&D, although the presence of rodents has also been described to be a major factor contributing to carry-over (Carrique-Mas et al., Citation2009). C&D is a costly and laborious task, and its success in eliminating Salmonella from the houses depends on the attention to detail, as well as on the choice and correct application of disinfectants (Davies & Wray, Citation1995; Wales et al., 2006a). There is a wide range of disinfectant formulations available on the market, but there are marked differences in their efficacy (McDonnell & Russell, Citation1999). Furthermore the efficacy of a disinfectant in the field is highly dependent on the level of residual organic matter remaining on the house surfaces. Because of their design, C&D of cage laying houses is known to be particularly problematic (Davies & Breslin, Citation2003; Wales et al., Citation2006a). Aldehydes are generally more effective than other disinfectants in poultry houses (Rose et al., Citation2000; Wales et al., Citation2006a), and where formaldehyde has been investigated it has performed better than any other product, both in laboratory models using artificially inoculated surfaces (Gradel et al., Citation2004) and in field conditions (Davies & Wray, 1995). Historically C&D has typically been carried out in the same manner over subsequent production cycles, irrespective of the Salmonella status of the laying houses. However the introduction in 2009 of Salmonella legislation across the EU (enacted by National Control Programmes) banning the sale of fresh eggs from S. Enteritidis-positive or S. Typhimurium-positive flocks (EC No. 1237/2007) is likely to alter this situation, and more effective disinfection procedures will be sought by farmers with Salmonella-positive flocks.
The aim of the present study was to determine the comparative effectiveness of disinfection programmes in Salmonella-positive cage and non-cage (i.e. free-range, barn) houses in the field; in particular, an intensive method using 10% formalin applied with a pressure washer by a specialist contractor.
Materials and Methods
Farms, laying houses and disinfection procedures
A total of 60 post-C&D visits to houses where either S. Enteritidis or S. Typhimurium had been isolated were carried out on 47 different laying houses in 23 different farms (sites) between February 2003 and September 2008. Prior to disinfection, houses were washed using a pressure washer and allowed to dry. The disinfection procedures investigated were: (1) a 10% (vol/vol) formalin dilution of the standard 37% commercial dilution, applied by a specialist contractor (Elebert Pestforce, Lymm, UK) using a high-pressure washer to run-off point; (2) a formaldehyde, glutaraldehyde quaternary ammonium (FGQ) compound (Superkill®; AFS Animal Care, Thetford, UK) applied at the “General Orders” (GO) rate (1:22) (Department for Environment, Food and Rural Affairs [DEFRA], Citation2009) (the GO rate is the minimum concentration recommended by DEFRA, based on an in vitro suspension approval test), with the disinfectant applied by the farmer by power or pressure washer to run-off point; and (3) “in house” procedures, other disinfection procedures that were routinely used by the producers (). In all cases the disinfectants were applied to all surfaces of the house and anteroom (where present). Houses were restocked 1 to 3 days after the disinfectants had dried out from the house surfaces.
Table 1. Number of laying houses investigated by type of production and disinfection procedure
Sampling for Salmonella to assess the effectiveness of C&D
The effectiveness of the C&D procedure was assessed by the culture of hand-held gauze swabs impregnated with buffered peptone water (BPW) that were used to wipe a range of surfaces in the house, before they were placed back into jars containing 225 ml BPW (Davies & Breslin, 2003; Wales et al., Citation2006a; Carrique-Mas et al., Citation2008b). For cage houses, 10 samples from each of the following areas were collected: cage interiors (eight cages per swab); drinker cups/troughs (eight per swab); feed troughs (0.5 m2 per swab); droppings boards/belts/flaps (1 m2 per swab); house floor (0.5 m2 per swab); and egg belts (0.5 m2 per swab). From non-cage houses, samples were collected from: drinkers (four bell drinkers per swab); feeders (0.5 m2 per swab); scratching area (if present) (1 m2 per swab); slats (0.5 m2 per swab); and nest box interiors (five per swab). In addition, in free-range houses the soil from the paddocks was sampled by scraping off 25 g topsoil, which was added to the 225 ml BPW jars (Davies & Breslin, 2003). For each sampling visit and sample type, a prevalence of positive samples was calculated.
Sampling of flocks in lay
For each house investigated, laying flocks before and after terminal C&D were sampled in an identical way. Dust and faeces were collected from the occupied houses from flocks in lay placed before disinfection and from the first flock after restocking. For this, 10 pooled faeces (25 g) and 10 dust samples (15 g) were collected from each flock directly into 225 ml BPW pots using large gauze swabs. This is regarded as a very sensitive sampling method (Carrique-Mas et al., Citation2008a).
Bacteriological methods
Samples in BPW were cultured following a simplified protocol of ISO 6579:2002 (Annex D), consisting of pre-enrichment in BPW followed by enrichment in modified semi-solid Rappaport Vassiliadis medium and plating onto Rambach agar (Wales et al., Citation2006a). Suspect Salmonella colonies were confirmed by serotyping using the Kauffmann − White typing scheme.
Statistical analyses
The level of contamination of flocks before and after C&D was estimated by calculating an average (weighted) percentage of faeces and dust samples that were positive. Chi-square tests were used to assess differences in proportions and the (non-parametric) Wilcoxon test statistic was used to compare the sample prevalence before and after the disinfection. All analyses were carried out using S-Plus (Insight, USA).
Results
Post C&D sampling visit
All houses sampled after C&D had previously contained flocks positive for S. Enteritidis, except one case where S. Typhimurium was present. A total of 1080, 1140, and 660 samples (combined data) were collected for houses treated with the “in house”, FGQ (G.O rate), and 10% formalin C&D programmes, respectively. The total prevalence of positive samples was 31.5%, 9.0%, and 3.3%, respectively. For cage houses, the sample prevalence was (from highest to lowest): floors (24.8%), dropping boards/belts/flaps (22.7%), drinkers (15.2%), feeders (13.8%), cage interiors (11.4%) and egg belts (9.4%). A comparable proportion of positive samples were recovered from floor and dropping board samples (χ2=0.35; P=0.55), and the proportion of positive samples from both were significantly greater than that obtained from any other type of sample (P < 0.05). In non-cage houses, the highest sample prevalence was in scratching areas (32.6%), drinkers (7.5%), feeders (5.7%), nest boxes (3.3%) and slats (2.7%). The proportion of positive samples from scratching areas was greater than any other type of sample (P < 0.01). A total of 35.7% soil samples from the paddocks tested positive. The mean sample prevalence by disinfection type is presented in , and the median prevalence of positive samples by sample type and disinfection method is shown in and . Results for non-cage houses treated using the formalin 10% method are not shown given that only two houses were examined.
Figure 1. Results of the C&D procedure of cage houses using three disinfection methods: an “in-house” method (n=18), formaldehyde/glutaraldehyde/quarternary ammonium (FGQ) at the GO rate (n=19) and using 10% formalin dilution (n=11).
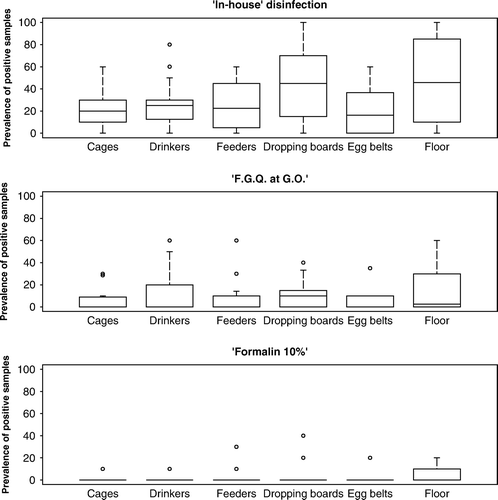
Figure 2. Results of the cleaning and disinfection of non-cage houses using an “in-house” disinfection method (n=6), and formaldehyde/glutaraldehyde/quarternary ammonium (FGQ) at the GO rate (n=4).
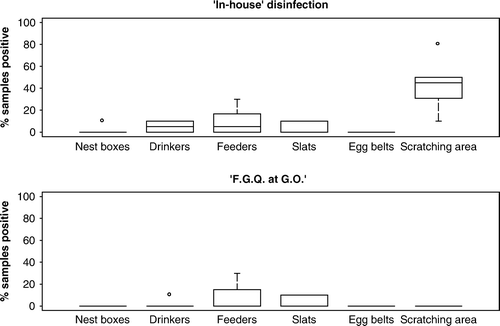
Table 2. Salmonella-positive sample prevalence by sample type
Level of infection in flocks before treatment
Flocks in cage houses sampled in lay before the C&D had a median (dust and faeces weighted) sample prevalence of 39.7% (interquartile range, 15.3 to 65.2%), and in non-cage houses the median (dust and faeces weighted) sample prevalence was 48.4% (interquartile range, 28.6 to 71.2%). These differences were not statistically significant (Wilcoxon Z= − 0.75; P=0.454). Flocks in houses that were later treated using the “in-house” method had a significantly higher level of infection (63.2%; interquartile range, 41.4 to 81.2%) than those treated with formalin (44.3%; interquartile range, 20.7 to 65.9%) (Wilcoxon Z=2.0; P = 0.045) or FGQ (GO) (25.9%; interquartile range, 13.7 to 43.9) (Wilcoxon Z=3.54; P < 0.001). The percentage of cases where the follow-on flock was Salmonella free (i.e. Salmonella was eliminated from the building) was 7/13 (54%), 8/23 (35%) and 5/24 (21%) for houses treated with formalin 10%, FGQ (GO) and the in-house method, respectively.
Comparison of the efficacy of each treatment on the levels of infection of follow-on flock
The level of infection of the follow-on flocks, as judged from the percentage of samples that were positive, increased in 6/18 (33.3%), 3/19 (15.8%) and 0/11 (0%) of cases using the “in-house” method, FGQ (GO), and formalin 10%, respectively. In all remaining cases there was a reduction of the levels of infection of the follow-on flock. This included all non-cage houses regardless of the treatment (). The variation in the prevalence of positive samples from the laying flocks before and after C&D is presented in . The median observed reductions were −71.0% (interquartile range, −95.9 to −14.2) and −97.3% (interquartile range, −100 to −79.4) for cage and non-cage houses, respectively (Z=2.10, P=0.035). Overall, a significantly greater reduction was observed only with formalin 10%, compared with the in-house programme (Z= − 2.64, P=0.008). In cage houses, the 10% formalin dilution method performed better than the FGQ (Z= − 2.13, P = 0.033) and the “in-house” method (Z= − 3.41, P < 0.001). In non-cage houses, the FGQ method performed better than the “in-house” method, but the difference was only borderline significant (Z= − 1.80, P = 0.07). No statistical comparisons with the 10% formalin dilution method were attempted in non-cage houses due to the small number of houses treated with formalin (n=2).
Figure 3. Change in the percentage of positive samples collected from the flocks before and after disinfection using each of the three methods.
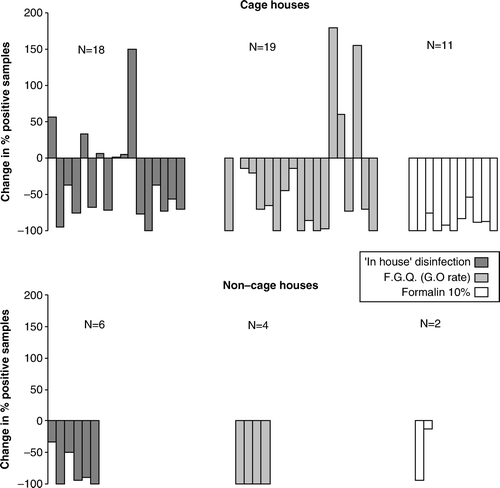
Table 3. Change in the sample prevalence between Flock 1 (prior to treatment) and Flock 2 (after treatment)
Significance of post-C&D sampling results
A negative post-C&D sampling result resulted in a Salmonella-negative follow-on flock in 57% of cases (). There was a linear trend towards an increased risk in carry-over for houses with higher levels of Salmonella after cleaning and disinfection (χ2 for linear trend 8.57; P = 0.0031), although houses with a negative C&D result still had a 43% probability of carry-over.
Table 4. Relationship between post C&D sampling results and follow-on flock
In the case of free-range houses, there was evidence of contamination in the soil of the paddocks at the time of the post C&D visit in 6/9 cases. None of the follow-on flocks placed in the three houses with negative paddocks tested positive, compared with 4/6 of those with evidence of contamination of the paddocks.
Discussion
The perpetuation of S. Enteritidis infection in laying flocks is commonly related to the presence of a contaminated house environment (van de Giessen et al., Citation1994; Wales et al., Citation2007; Carrique-Mas et al., Citation2008b). Therefore adequate C&D is regarded as a crucial step in eliminating infection from the houses. The present study demonstrated the variable impact of different C&D regimes on Salmonella-positive laying houses. An ideal study design to investigate this in the field would be a randomized controlled trial with assessment of compliance but this was not possible because of cost issues. Therefore, we need to accept the limitations and possible biases of this descriptive study.
The best results were achieved using 10% formalin applied by a specialist contractor, and the second best results were obtained using a blended disinfectant that also contained formaldehyde, in combination with glutaraldehyde and quarternary ammonium compounds. Other disinfection methods did not perform as well, although the diversity of treatments does not permit conclusions regarding any particular product to be made. The efficacy of the application of the 10% formalin treatment by spray was reflected not only in the lower recovery of Salmonella from the treated surfaces in the house, but in lower rate of infected follow-on flocks and a reduction in the levels of infection in houses where an infected flock was present. It is important to emphasize that this is different from the application of formalin by fogging, a less intensive treatment that is common in the poultry industry. Fogging is regarded as not very efficient on its own due to the considerable shadowing effect associated with the complicated structures of the house. For health and safety reasons, it is better that formalin is applied by a specialist contractor. This is likely to represent an additional expense, which may largely be compensated by the improved performance of this disinfection method and improved health and production in the subsequent flocks.
There is potentially a source of bias in the study resulting from the uneven distribution of the level of contamination in the houses investigated by type of treatment, since the allocation of C&D treatments was not under our control. The houses that were treated with the “in-house” method had also contained flocks with the overall highest levels of infection. However, flocks that were treated with formalin had a greater initial level of infection than those treated with the FGQ product, and, even so, the disinfection effect was greater using the 10% formalin dilution. In one of the two non-cage houses (a barn), the formalin treatment did not succeed. This was the first house to be treated in the study. The reason for this failure was a very poor standard of cleaning before the application of the disinfectant, being particularly bad in nest boxes and drinker cups, which where largely missed by the cleaning team. In addition the house was quickly re-contaminated by mouse faeces.
Laying houses are notoriously difficult to clean thoroughly because of their intrinsically complicated structures, which are even more complex in the case of cage laying houses (Wales et al., Citation2006a). Access to cage interiors, feeders, egg belts, and so forth is very difficult unless a great deal of effort and time is invested. It seems that in these circumstances a large amount of residual organic matter is expected after a standard disinfection procedure. Our results are consistent with previous results where formalin has also been shown to perform better than any other disinfectant. This has been confirmed using laboratory models using Salmonella-spiked surfaces (Berchieri & Barrow, Citation1996; Gradel et al., Citation2004) but also in field studies (Davies & Wray, 1995; Rose et al., Citation2000).
The general trend in the houses investigated was a reduction of the levels of infection of the follow-on flocks regardless of the treatment used, although there was a poor predictive value of a negative C&D result. Only in 42% of cases where a negative post-C&D result was obtained was there evidence of clearance of infection of the follow-on flock. This is likely to be a consequence of the presence of other major sources of infection to the flocks, notably rodents (Carrique-Mas et al., Citation2008b). Likewise, a positive result after C&D, particularly at a low level, does not necessarily lead to infection of the new flock, as has been reported previously (Davies & Wray, Citation1996). It is possible in some cases that our involvement in the study may also have lead to a more pro-active attitude by the farmer in terms of upgraded rodent control and biosecurity measures. In houses where relatively small numbers of rodents were present, an upgrade of baiting procedures may have been sufficient to reduce the challenge to the newly placed flocks.
An important observation in the present study is the widespread lack of knowledge by the farmers of the appropriate concentrations of disinfectants. In the UK, activity against Salmonella is evaluated and the efficacy reported in the GO concentration. The main confusion arising from the use of disinfectants appears to be related to their reconstitution at a lower than optimal concentration, as is recommended for other uses. It would be preferable if a suitable concentration for disinfection of Salmonella in biofilms on soiled surfaces could be defined for all products so that operators could obtain clear guidance on appropriate disinfection.
Paddocks adjacent to free-range houses are rarely included in the C&D programme. Although in many cases paddocks have been reported to carry Salmonella, they are considered to be less of a risk for birds than residual contamination in the house, since carry-over has been rare provided that the house has been correctly disinfected (Davies & Breslin, 2003).
The current results are particularly relevant in the context of the enforcement of restriction on the sale of fresh eggs from flocks infected with S. Enteritidis and/or S. Typhimurium. The present study provides evidence that the use of the 10% formalin dilution is particularly useful in the decontamination of infected cage laying flocks, although in most cases this intervention has to be concomitant with rodent control to reduce the chances of carry-over of infection.
Acknowledgements
The authors wish to thank all participating farmers for their support. This work was funded through DEFRA of the Government of the United Kingdom (Project OZ0325). The authors are also grateful to Dr Sarah Evans for comments and improvements to the original manuscript.
References
- Anonymous 2006 Reducing Salmonella in European egg-laying hens: EU targets now set . Eurosurveillance , 11 , E060810 3 .
- Berchieri , A. Jr. & Barrow , P.A. 1996 The antibacterial effects for Salmonella Enteritidis phage type 4 of different chemical disinfectants and cleaning agents tested under different conditions . Avian Pathology , 25 , 663 – 673 .
- Carrique-Mas , J.J. , Breslin , M. , Sayers , A.R. , McLaren , I. , Arnold , M. and Davies , R. 2008a . Comparison of environmental sampling methods for detecting Salmonella in commercial laying flocks in the UK . Letters in Applied Microbiology , 47 : 514 – 519 .
- Carrique-Mas , J.J. , Breslin , M. , Snow , L. , Arnold , M.E. , Wales , A. , McLaren , I. and Davies , R.H. 2008b . Observations related to the Salmonella EU layer baseline survey in the UK: Follow-up of positive flocks and sensitivity issues . Epidemiology and Infection , 47 : 514 – 519 .
- Carrique-Mas , J.J. , Breslin , M. , Snow , L. , McLaren , I. , Sayers , A.R. and Davies , R.H. 2009 . Persistence and clearance of different Salmonella serovars in buildings housing laying hens . Epidemiology and Infection , 137 : 837 – 846 .
- Davies , R. and Breslin , M. 2003 . Observations on Salmonella contamination of commercial laying farms before and after cleaning and disinfection . Veterinary Record , 152 : 283 – 287 .
- Davies , R.H. and Wray , C. 1995 . Observations on disinfection regimens used on Salmonella enteritidis infected poultry units . Poultry Science , 74 : 638 – 647 .
- Davies , R.H. and Wray , C. 1996 . Persistence of Salmonella enteritidis in poultry units and poultry food . British Poultry Science , 37 : 589 – 596 .
- de Jong , B. & Ekdahl , K. 2006 Human salmonellosis in travellers is highly correlated to the prevalence of Salmonella in laying hen flocks . Eurosurveillance , 11 , E060706 1 .
- Department for Environment, Food and Rural Affairs 2009 Disinfectants Approved for use in England and Wales . Available online at http://disinfectants.defra.gov.uk/Default.aspx?Location=None&module=ApprovalsList_SI (accessed 9 October 2008)
- Gillespie , I.A. , O'Brien , S.J. , Adak , G.K. , Ward , L.R. and Smith , H.R. 2005 . Foodborne general outbreaks of Salmonella Enteritidis phage type 4 infection, England and Wales, 1992–2002: where are the risks? . Epidemiology and Infection , 133 : 795 – 801 .
- Gradel , K.O. , Sayers , A.R. and Davies , R.H. 2004 . Surface disinfection tests with Salmonella and a putative indicator bacterium, mimicking worst-case scenarios in poultry houses . Poultry Science , 83 : 1636 – 1643 .
- McDonnell , G. and Russell , A.D. 1999 . Antiseptics and disinfectants: activity, action, and resistance . Clinical Microbiology Reviews , 12 : 147 – 179 .
- Rose , N. , Beaudeau , F. , Drouin , P. , Toux , J.Y. , Rose , V. and Colin , P. 2000 . Risk factors for Salmonella persistence after cleansing and disinfection in French broiler-chicken houses . Preventive Veterinary Medicine , 44 : 9 – 20 .
- van de Giessen , A.W. , Ament , A.J. and Notermans , S.H. 1994 . Intervention strategies for Salmonella enteritidis in poultry flocks: a basic approach . International Journal of Food Microbiology , 21 : 145 – 154 .
- Wales , A. , Breslin , M. and Davies , R. 2006a . Assessment of cleaning and disinfection in Salmonella-contaminated poultry layer houses using qualitative and semi-quantitative culture techniques . Veterinary Microbiology , 116 : 283 – 293 .
- Wales , A. , Breslin , M. and Davies , R. 2006b . Semiquantitative assessment of the distribution of Salmonella in the environment of caged layer flocks . Journal of Applied Microbiology , 101 : 309 – 318 .
- Wales , A. , Breslin , M. , Carter , B. , Sayers , R. and Davies , R. 2007 . A longitudinal study of environmental Salmonella contamination in caged and free-range layer flocks . Avian Pathology , 36 : 187 – 197 .
- World Health Organization 2005 Drug-resistant Salmonella Factsheet No. 139 . Available online at http://www.who.int/mediacentre/factsheets/fs139/en/ accessed 5 November 2008