Abstract
An earlier study on commercial chickens and turkeys with a history of respiratory disease established Mycoplasma gallisepticum infection rates on 164 poultry farms of the Russian Federation. Forty-seven (29%) of these poultry farms were M. gallisepticum-positive by polymerase chain reaction but isolation of the mycoplasma was successful only on 10 farms. Five field isolates from different farms were selected for pathogenicity studies in specific pathogen-free chicks. Clinical signs, seroconversion, culture rates, air sac and tracheal lesions and mean tracheal mucosal thickness were all assessed in comparison with the reference strain, S6. Of the five isolates, MG140905 and MG070607 appeared to be slightly more pathogenic than the other three, as indicated by clinical signs, culture-positive rates and lesions, but only isolate MG140905 differed statistically (P < 0.05) from them, thus proving to be the most pathogenic. However, none of the Russian field isolates was as pathogenic as the S6 strain by the parameters measured. Stress or other factors such as concurrent bacterial or viral infections may have served as exacerbating factors for the disease seen in the naturally affected flocks. Sequence analysis of the gapA and mgc2 genes showed that MG140905 clustered with M. gallisepticum Rlow and was more distant from the majority of the Russian isolates.
Introduction
The poultry industry suffers greatly from Mycoplasma gallisepticum infection (Levisohn & Kleven, Citation2000). The mycoplasma is known to cause chronic respiratory disease and downgrading of carcasses (Ley & Yoder, Citation1997; Kleven, Citation1998). It has tropism primarily for mucosal membranes of the respiratory tract, conjunctiva and sinuses (Levisohn & Kleven, Citation2000). Attachment of M. gallisepticum to host cells is considered important for colonization and pathogenesis (Ley & Yoder, Citation1997). The organism usually enters the host via the respiratory tract (except for in ovo infection), and the upper airways and trachea are the preferred sites of infection for most strains of M. gallisepticum. During the acute stage of infection, high numbers of organisms are found in the trachea and detection is most successful at this time (Levisohn & Dykstra, Citation1987; Hyman et al., Citation1989; Gaunson et al., Citation2006). However, lesions may develop in other parts of the respiratory tract, most notably the air sacs (Gaunson et al., Citation2006). Exacerbation of clinical signs and an increase in mortality in birds with M. gallisepticum infection caused by concurrent bacterial and/or viral infections have been widely reported (Bradbury, Citation2005; Raviv et al., Citation2009).
Russian isolates of M. gallisepticum have not so far been studied and the situation in Russia in terms of M. gallisepticum infection rates and virulence properties of strains remains uninvestigated. Therefore it was considered of high priority to assess the prevalence of M. gallisepticum both for research and for control of disease. Although the pathogenicity of M. gallisepticum is well documented (Kleven, Citation2003), no reports have been published concerning the biological and genetic properties of Russian isolates; moreover, no systematic investigations, including isolation of M. gallisepticum, have been carried out to date and the epidemiological features of the infection in poultry in Russia are still unknown.
Sprygin et al. (Citation2010a), Sprygin et al. (Citation2010b) attempted for the first time to detect, isolate and genetically characterize Russian M. gallisepticum-positive samples and isolates. It was shown that a majority of M. gallisepticum-positive samples formed a distinct cluster based on the pvpA target; however, there were some isolates that grouped to international strains such as the S6 strain (Sprygin et al., Citation2010a). The main goal of this study was to investigate the mdecular biological characteristics of selected M. gallisepticum isolates of Russian origin, to compare them with a well-known reference strain (S6) and to see whether there was any obvious correlation between genetic closeness and virulence.
Materials and Methods
Flocks
The study included commercial chickens and turkeys from 53 different regions of Russia from the Far East to the European part of Russia during the period of April 2006 to December 2008. The selection criterion for flocks in the study was strictly those suffering from slight to severe respiratory disease (coryza, râles, swelling of sinuses) that were submitted for testing. Sampling of birds and detection of M. gallisepticum by real-time polymerase chain reaction (PCR) and culture was performed as described earlier (Sprygin et al., Citation2010b). One hundred and sixty-four poultry farms were examined. The age of flocks ranged from 22 to 360 days and included broilers, layers and turkeys. The only history given at the time was that none of the flocks had ever been vaccinated against M. gallisepticum or Mycoplasma synoviae using live vaccines. On physical examination, any evidence of coryza, conjunctivitis, sneezing, and sinusitis, depression and weakness was recorded, as were any signs of tracheitis, airsacculitis, air sac lesions or exudates in the trachea at necropsy.
M. gallisepticum field isolates
Five M. gallisepticum isolates examined in the study were from commercial poultry flocks exhibiting moderate to severe clinical signs of mycoplasma infection (airsacculitis, tracheal râles, nasal discharge) as described above. A brief history of the isolates is presented in . Their purity was verified by PCR and culture (Sprygin et al., Citation2010b). Once established as pure cultures, all underwent several in vitro passages (<10) and were stored at –80°C until needed.
Table 1. Details of the Russian M. gallisepticum isolates used.
M. gallisepticum strain S6 was used as a reference organism and was obtained from the American Type Culture Collection (Manassas, Virginia, USA). It underwent several in vitro passages before being used as a positive control.
Chickens and experimental design
One hundred and five 7-day-old specific pathogen free (SPF) embryonated eggs, known to be free from M. gallisepticum and M. synoviae infection, were obtained (Lohman Tierzucht GmbH, Germany). The newly-hatched chicks were divided randomly into seven experimental groups consisting of 15 chicks per group. The groups were housed in separate biosafety level 2 rooms. Municipal chlorinated water and feed were provided ad libitum. At 1 week of age (i.e. 1 week before exposure), all birds were bled for serum plate agglutination (SPA) test and enzyme-linked immunosorbent assay (ELISA). In addition, the palatine cleft of each bird was swabbed and cultured for M. gallisepticum and M. synoviae. The swabs were also tested for by real-time PCR as described below.
The experimental groups were: negative control (sham-inoculated), MG310807-inoculated, MG070306-inoculated, MG060705-inoculated, MG070607-inoculated, MG140905-inoculated and S6-inoculated. At 2 weeks of age the birds were infected by the intranasal route (50 µl dropped into the nares) and intratracheally (450 µl by gavage) with the appropriate M. gallisepticum culture. The titres of the inocula ranged from 1.0 × 105 to 1.0 × 106 colony-forming units/ml. The birds in the negative control group were sham-inoculated with 0.5 ml sterile mycoplasma medium. After inoculation all birds were observed daily for mortality and clinical signs of infection for 21 days. At 10 days, all birds in each inoculated group and 10 birds in the sham-inoculated group were bled for serology and palatine cleft swabs were taken for culture and PCR. At day 21, blood was collected from each bird along with palatine cleft swabs. The birds were then euthanized by carbon dioxide intoxication, examined for gross lesions and their tracheas were collected for histology.
Serology
SPA tests and ELISA were performed on all the serum samples collected pre-inoculation and post-inoculation. After inactivation and centrifugation M. gallisepticum SPA antigen was produced from the S6 strain. Known negative and positive sera for both M. gallisepticum and M. synoviae were used as controls. SPA reactions were graded from 0 (negative) to 4 (strong reaction). Agglutination scores ≥ 1 were considered positive.
ELISA was conducted using the ProFlock M. gallisepticum ELISA kit (Synbiotics, France) and results were interpreted following the manufacturer's recommendations. The ELISA S/P ratio was considered positive at ≥ 0.6.
Culture and identification of M. gallisepticum
Choanal cleft swabs from each experimental bird were agitated briefly in 2 ml Frey's broth, and then 1 ml was incubated at 37°C until the broth medium colour indicator changed. The duplex M. gallisepticum and M. synoviae (MGMS) PCR (Sprygin et al., Citation2010b) was performed using the 7500 Real Time PCR System (Applied Biosystems, USA). Reagents for the MGMS PCR with ROX reference dye (Syntol, Moscow) were used for the reaction. For all samples tested, any MGMS reaction that had a recorded threshold cycle number (Ct) value for 40 cycles was considered positive, and any MGMS reaction that had no recorded Ct value for 40 cycles was considered negative.
Pathological examination
The air sacs were examined for gross lesions and scores were given as follows: 0 = no lesions; 1 = clear but with small flecks of caseous exudate attached; 2 = cloudy and slightly thickened with moderate amount of caseous exudate attached; 3 = thickened, cloudy and opaque with moderate amount of caseous exudate attached; and 4 = thickened with surface covered with thick caseous exudate (Gaunson et al., Citation2006).
The upper part (just below the larynx) of the trachea was fixed in 10% formalin, embedded in paraffin, stained with haematoxylin and eosin, and sections prepared from each bird were examined microscopically for mucosal lesions. These were evaluated by the following scoring system: 0 = no significant changes; 0.5 = very small aggregates (one to two foci) or very slight, diffuse infiltration of lymphocytes; l = small aggregates (more than several foci) of lymphocytes or slight thickening of the mucosa due to diffuse lymphocytic infiltration; 2 = moderate thickening of the mucosa due to heterophil and lymphocyte infiltration and oedema accompanied by epithelial degeneration, with or without luminal exudation; 3 = extensive thickening due to heterophil and lymphocyte infiltration and oedema with squamous metaplasia or degeneration of epithelia with luminal exudation. Tracheal mucosal thickness was determined for each bird at each tracheal location by averaging measurements of four points transected by vertical and horizontal lines (Nunoya et al., Citation1987). Tracheal mucosal thickness was measured using the software-aided measurement system Olympus Bx61 (Olympus, Japan).
DNA sequence analysis
Nucleic acid sequencing of two gene fragments (gapA and mgc2) and the 16-23S rRNA intergenic spacer region (ISR) was carried out on the five M. gallisepticum isolates used in the study and on 19 M. gallisepticum-positive samples detected previously. The sequences were compared with those of M. gallisepticum strains retrieved from GenBank. Analysis of nucleotide sequences of the pvpA gene of the field isolates was described earlier (Liu et al., Citation2001, Sprygin et al., Citation2010a).
The nucleotide sequences from a PCR product from a surface protein gene mgc2 of the field isolates were obtained using primers mgc2 2F (CGCAATTTGGTCCTAATCCCCAACA) and mgc2 2R (TAAACCCACCTCCAGCTTTATTTCC) as described previously (Garcia et al., Citation2005). Nucleotide sequences of the gapA gene of the field isolates were obtained using primers gapA 3F (TTCTAGCGCTTTARCCCTAAACCC) and gapA 4R (CTTGTGGAACAGCAACGTATTCGC) as described previously (Garcia et al., Citation2005), and sequences for the ISR were obtained using primers MG IGSR F (GTAGGGCCGGTGATTGGAGTTA) and MG IGSR R (CCCGTAGCATTTCGCAGGTTTG) as described by Raviv et al. (Citation2007).
The purified PCR products of the 26 M. gallisepticum field samples were sequenced in an automatic sequencer (ABI Prism 3130; Applied Biosystems, USA) using the respective primers. The sequences were aligned and compared using the Clustal W program. The sequences were submitted to GenBank with the following accession numbers assigned: FJ965750 to FJ965767, FJ965769 to FJ965783, FJ965785 to FJ965815, FJ965817 to FJ965827, FJ972630 to FJ972632, and GQ847777 to GQ847804. The ISR sequence for MG140307 was not determined.
Dendrograms were constructed using the neighbour-joining method with 1000-bootstrap replicates using MEGA 3.1 (http:// www.megasoftware.net).
Statistical analysis
Serology results, air sac lesion scores and trachea mucosal thickness scores were analysed by the Mann–Whitney test (STATISTICA version 6). Values were considered significantly different at P < 0.05.
Results
Mortality and clinical manifestations
No birds died due to mycoplasma infection during the experiment. No clinical signs were observed in the groups infected with MG310807, MG070306 or MG060705 and these birds could not be distinguished from the control group by appearance. At necropsy neither tracheitis nor sinusitis was observed.
In the MG140905-inoculated group conjunctivitis and sinusitis was seen in 11 birds and tracheitis in 13 birds. Conjunctivitis and sinusitis were seen in seven birds in the MG070607 group while tracheitis was rarely observed. All the chickens in the S6 group developed conjunctivitis, and tracheitis was found in 80% of birds.
Serology, culture and PCR
Prior to inoculation all of the chickens were negative for M. gallisepticum by serology, culture and real-time PCR. The sham-inoculated control group tested negative by all these methods throughout the experiment.
The results obtained at day 10 and 21 post inoculation (p.i.) are given in . There was no seroconversion by day 10 p.i. as shown by SPA or by ELISA, except for the S6 group where nine SPA-positive reactions were observed (). At day 21 p.i. there were SPA-positive reactions in all 15 birds in the S6 group, 10 in the group inoculated with MG060705, with lower numbers of positives in the other groups and only one positive in the MG070607 group. However, no statistical difference was shown among the SPA-positives from different treatment groups except for MG070607, which had a very weak SPA result (P < 0.05).
Table 2. Serology, culture and real-time PCR results in chickens inoculated with Russian M. gallisepticum (MG) isolates or S6 strain.
Weak ELISA-positive titres were detected only in two specimens from group MG060705. Moderately strong ELISA-positives were observed in samples from the MG140905 group and the S6 group, being statistically significant (P < 0.05). Birds from the other inoculated groups remained ELISA-negative.
Birds inoculated with the Russian isolates were culture-negative at 10 days p.i. but 12 of the 15 inoculated with S6 were culture-positive. By 21 days p.i. culture was positive in two samples from the MG060705 group, three samples from the MG070607 group, 10 from the MG140905 group and 14 from the S6 group. There was no positive sample at 21 days p.i. from the MG310807 or the MG070306 group. At 10 and 21 days p.i. the PCR was positive for M. gallisepticum in all of the palatine cleft swab samples collected from the infected groups.
M. gallisepticum samples that were positive by culture and real-time PCR were amplified and sequenced using the aforementioned primers in order to confirm their identity as the isolate inoculated. No cross-contamination was observed and the sequences of the M. gallisepticum field isolates were submitted to Genbank with the accession numbers assigned ().
Air sac lesion scores, tracheal lesion scores and mean tracheal mucosal thickness
The lesion scores and the mean tracheal mucosal thickness at day 21 p.i. are given in . Sham-inoculated birds and groups inoculated with isolates MG310807, MG070306 and MG060705 had no air sac lesions at necropsy. The MG070607-inoculated and MG140905-inoculated groups had significantly higher air sac lesion scores than the above groups (P < 0.05) but there was no statistical significance between these two groups (P > 0.05). Air sac lesion scores for the S6 group were numerically and statistically different from all other groups.
Table 3. Air sac lesion scores, tracheal lesion scores and mean tracheal mucosal thickness in chickens inoculated with Russian M. gallisepticum isolates or S6 strain.
Compared with the sham-inoculated chickens, the mean tracheal mucosal thickness of the MG310807 group was numerically different but not significantly so (P > 0.05) (); nor was there any statistical significance among the groups MG070607, MG070306 and MG060705 (P > 0.05), although the groups inoculated with MG140905 and S6 were statistically different (P < 0.05).
Sequence analysis of M. gallisepticum isolates
MG140905 proved to be genetically closer to the international reference strains than many of the other Russian isolates; that is, it clustered with Rlow based on the gapA and mgc2 targets (). Supplementary analysis showed that it shared 100% and 98% identity with Rlow strain based on the gapA (247 bp fragment) and mgc2 (184 to 252 bp fragment) targets, respectively. Despite the low discriminatory index of 0.713 calculated previously by Ferguson et al. (Citation2005) for gapA, both isolates MG140905 and MG310807 fell outside the main Russian group of isolates in the gapA dendrogram with the latter grouping with some USA and Australian isolates, including USA house finch isolates. The mgc2 target placed MG310807 within the group of Russian isolates.
Figure 1. Dendrograms constructed with Russian M. gallisepticum sequences from 26 M. gallisepticum samples using the neighbour-joining (NJ) method with 1000-bootstrap replicates using MEGA 3.1 (http:// www.megasoftware.net). Numbers denote the confidence interval percentage for a certain branch to occur. The Russian M. gallisepticum samples are in bold and italicized. Rlow was chosen as an outgroup.
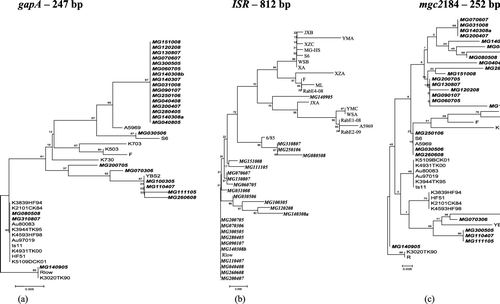
Due to the absence of ISR sequences of major M. gallisepticum strains in GenBank, a comprehensive analysis based on the ISR sequences (812 bp fragment) could not be accomplished. However, an analysis was performed that included some isolates from China and Egypt. In this analysis MG310807 was close to the vaccine strain 6/85 (that was sequenced in our laboratory) while MG140905 was closer to the group containing YMC and JXA from China and Egyptian strains, with bootstrap support of 70% (Figure 1b).
The other three isolates (MG070306, MG060705 and MG070607) clustered with other “Russian type” isolates with all three targets.
Discussion
A total of 164 poultry farms (originating from 53 Russian regions) with a history of respiratory disease were previously tested, of which 47 (29%) farms were established to be M. gallisepticum-positive (Sprygin et al., Citation2010b). Isolation procedures for M. gallisepticum were successful in samples from 10 farms. The widespread occurrence of this pathogen might be explained by the fact that Russian farmers cannot afford to send seropositive flocks for slaughter to maintain flocks free of infection as is the case in Europe or the USA (Kleven, Citation2008). Moreover, the introduction of M. gallisepticum live vaccine strains is not favoured. Mostly killed vaccine strains are currently accepted on Russian poultry farms (V. Irza, personal communication 2008). Despite this, some farmers in Russia are starting to administer live M. gallisepticum vaccine strains, but such flocks were not used in our study, therefore our data suggest a field exposure was responsible for infection of these flocks.
The pvpA sequence analysis revealed previously that most Russian M. gallisepticum samples clustered separately from the major strains and isolates from USA, Australia and China (Sprygin et al., Citation2010b). Therefore, we focused our research on the potential pathogenicity of Russian M. gallisepticum isolates on three isolates in the “Russian type” group (MG070306, MG060705, MG070607) and two isolates in the “non-Russian type” group (MG310807, MG140905). For the evaluation of biological characteristics, various commonly used diagnostic procedures were performed (culture, serology, histopathology), but it is important to note that all five of the isolates used in the study were from flocks with concurrent infections () and therefore assessment of M. gallisepticum virulence based on the clinical manifestation of submitted chickens was difficult. Thus SPF chicks were used in an attempt to determine the true pathogenicity of M. gallisepticum isolates in uncomplicated infection.
Daily observations of clinical manifestations during the 21-day p.i. period showed no deaths but it is possible that additional time could be needed for the M. gallisepticum isolates to develop more severe disease, although it is more likely that other concurrent infections need to be present to exacerbate disease (Chu & Uppal, Citation1975; Raviv et al., Citation2009) or other complicating factors (e.g. environmental insults and stress) that are also known to increase disease in M. gallisepticum-infected flocks (Stipkovits & Kempf, Citation1996). In addition, the route of infection could be a possible reason for the low pathogenicity exhibited by most of the Russian isolates. The turkey isolate, MG070607, and MG140905 were slightly more virulent than the other Russian isolates based on clinical signs but the Russian isolates were of low virulence in comparison with the S6 strain.
Following infection some birds seroconverted by 21 day p.i. as shown by SPA tests and ELISA but only the groups infected with MG140905 and S6 showed a consistently high proportion of birds positive to both tests, with these groups showing significantly different results in the ELISA (P < 0.05).
Although palatine cleft swabs were negative for culture at 10 days p.i., with the exception of the S6 group, real-time PCR revealed the presence of M. gallisepticum DNA in 100% of palatine swab samples. The sham-inoculated birds remained PCR-negative, culture-negative and serologically negative throughout the experiment.
The tracheas of inoculated birds may not have been colonized effectively by the Russian M. gallisepticum isolate as suggested by very low numbers of culture positives obtained from the palatine clefts. There were only two positive culture samples from the MG060705 group and three from the MG070607 group. Only the MG140905 group produced 10 positive samples.
Air sacs were probably not colonized extensively either, with lesion scores being 0 for the MG310807, MG070306 and MG060705 groups. Only the MG070607 and MG140905 groups developed slight air sac lesions (P < 0.05). The mean tracheal mucosal thickness measured at 21 days p.i. established no statistical significance between groups MG070306, MG060705 and MG070607 but a significant difference between these groups and MG140905, which in turn was significantly different to the S6 group (P < 0.05). Values for the MG310807 and sham-inoculated groups were numerically different but without any statistical significance (P > 0.05).
Thus no Russian M. gallisepticum isolate proved as virulent as the S6 strain by any of the parameters measured (i.e. seroconversion, culture-positive rates, lesion scores, or tracheal mucosal thickness). The closest was MG140905 but it did not equal S6.
We employed single locus typing using gapA, mgc2 and ISR targets that reportedly have good discriminatory indices for studying M. gallisepticum isolates (Ferguson et al., Citation2005; Raviv et al., Citation2007). In terms of the sequences, it was interesting that the MG140905 isolate was close to Rlow, sharing 100% and 98% nucleotide identity based on the gapA and mgc2 genes, respectively. However, the earlier pvpA sequence analysis did not confirm that close genetic relatedness but did place MG140905 outside the Russian group (Sprygin et al., Citation2010a).
MG310807 also grouped outside the Russian isolates with the gapA target, being closer to some US and Australian strains and the Australian-origin ts-11 vaccine strain. The ISR dendrogram grouped MG310807 with the 6/85 vaccine strain with bootstrap values of 95%. In the light of the fact that ISR sequences are only available for a limited number of isolates, the congruence of the dendrograms in could not evaluated in detail, but building on the present ISR tree we established the relatedness of MG140905 to strains from China and Egypt (e.g. JXA, RabE1-08).
The gapA is least preferred as a genotypic tool due to its lower discriminatory index and its inability to distinguish between 6/85 and ts11 vaccine strains (Ferguson et al., Citation2005). Overall, sequence analyses showed an obvious difference between MG140905, MG310807 and most of the Russian strains. However, these loci display different evolutionary patterns, which is an expected trend, and it is likely that MG140905 and MG310807 isolates are not related to international M. gallisepticum strains originally.
Some correlation between phylogenetic and biological properties might be interpreted from this study, where MG140905 and MG310807 also appeared to differ biologically. However, this issue requires more in-depth genetic analysis.
Overall, our study characterized biological properties of M. gallisepticum isolates of Russian origin. The M. gallisepticum isolates in SPF chicks caused neither mortality, nor severe clinical signs. They did not cause severe air sac lesions or strong ELISA responses. Therefore the more severe signs that had been observed under field conditions were probably the result of exacerbation of the condition by concurrent bacterial and viral infections, stress or other factors.
The high infection rates among the poultry farms tested show that infected poultry needs more attention in terms of control measures, including management factors such as good hygiene and disinfection procedures, and antibiotic treatment. Consideration should also be given to the possibility of M. gallisepticum transmission by wild birds, which has already set a precedent (Luttrell et al., Citation2001). However, further extensive studies are required to clarify the issue on Russian poultry farms.
Acknowledgements
The present research was supported by the ISTC grant 3017 from the US Department of Agriculture Agricultural Research Service. The authors gratefully acknowledge E. Ovchinnikova and Z. Nikonova for assistance with diagnosis of concurrent infections. They also thank V. Reshetnikova for excellent assistance with serology.
References
- Bradbury , J.M. 2005 . Poultry mycoplasmas: sophisticated pathogens in simple guise . British Poultry Science , 46 : 125 – 136 .
- Chu , H.P. and Uppal , P.K. 1975 . Single and mixed infections of avian infectious bronchitis virus and Mycoplasma gallisepticum . Developments in Biological Standardization , 28 : 101 – 114 .
- Ferguson , N.M. , Hepp , D. , Sun , S. , Ikuta , N. , Levisohn , S. , Kleven , S.H. and Garcia , M. 2005 . Use of molecular diversity of Mycoplasma gallisepticum by gene-targeted sequencing (GTS) and random amplified polymorphic DNA (RAPD) analysis for epidemiological studies . Microbiology , 151 : 1883 – 1893 .
- Garcia , M. , Ikuta , N. , Levisohn , S. and Kleven , S.H. 2005 . Evaluation and comparison of various PCR methods for detection of Mycoplasma gallisepticum infection in chickens . Avian Diseases , 49 : 125 – 132 .
- Gaunson , J.E. , Philip , C.J. , Whithear , K.G. Browning , G.F. 2006 . The cellular immune response in the tracheal mucosa to Mycoplasma gallisepticum in vaccinated and unvaccinated chickens in the acute and chronic stages of disease . Vaccine , 24 , 2627 2633 .
- Hyman , H.C. , Levisohn , S. , Yogev , D. and Razin , S. 1989 . DNA probes for Mycoplasma gallisepticum and Mycoplasma synoviae: application in experimentally infected chickens . Veterinary Microbiology , 20 : 323 – 337 .
- Kleven , S.H. 1998 . “ Mycoplasmosis ” . In A Laboratory Manual for the Isolation and Identification of Avian Pathogens 4th edn , Edited by: Swayne , D.E. , John , R. , Jackwood , M.W. , Pearson , J.E. and Reed , W.M. 100 – 105 . Kennett Square, PA : University of Pennsylvania, New Bolton Center .
- Kleven , S.H. 2003 . “ Mycoplasmosis. Introduction ” . In Diseases of Poultry 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 719 – 766 . Ames, IA : Iowa State University Press .
- Kleven , S.H. 2008 . Control of avian mycoplasma infections in commercial poultry . Avian Diseases , 52 : 367 – 374 .
- Levisohn , S. and Dykstra , M.J. 1987 . A quantitative study of single and mixed infection of the chicken trachea by Mycoplasma gallisepticum . Avian Diseases , 31 : 1 – 12 .
- Levisohn , S. and Kleven , S.H. 2000 . “ Avian mycoplasmosis (Mycoplasma gallisepticum) ” . In Diseases of Poultry: World Trade and Public Health Implications , Edited by: Beard , C.W. and McNulty , S. Vol. 19 , 425 – 442 . Paris, , France : Office International des Epizooties .
- Ley , D.H. and Yoder , H.W. Jr. 1997 . “ Mycoplasma gallisepticum infection ” . In Diseases of Poultry 10th edn , Edited by: Calnek , B.W. , Barnes , H.J. , Beard , C.W. , McDougald , L.R. and Saif , Y.M. 194 – 207 . Ames : Iowa State Press .
- Liu , T. , Garcia , M. , Levisohn , S. , Yogev , D. and Kleven , S.H. 2001 . Molecular variability of the adhesin-encoding gene pvpA among Mycoplasma gallisepticum strains and its application in diagnosis . Journal of Clinical Microbiology , 39 : 1882 – 1888 .
- Luttrell , M.P. , Stallknecht , D.E. , Kleven , S.H. , Kavanaugh , D.M. , Corn , J.L. and Fischer , J.R. 2001 . Mycoplasma gallisepticum in house finches (Carpodacus mexicanus) and other wild birds associated with poultry production facilities . Avian Diseases , 45 : 321 – 329 .
- Nunoya , T. , Tajima , M. , Yagihashi , T. and Sannai , S. 1987 . Evaluation of respiratory lesions in chickens induced by Mycoplasma gallisepticum . The Japanese Journal of Veterinary Science , 49 : 621 – 629 .
- Raviv , Z. , Callison , S. , Ferguson-Noel , N. , Laibinis , V. , Wooten , R. and Kleven , S.H. 2007 . The Mycoplasma gallisepticum 16S–23S rRNA intergenic spacer region sequence as a novel tool for epizootiological studies . Avian Diseases , 51 : 555 – 560 .
- Raviv , Z. , Ferguson-Noel , N. , Laibinis , V. , Wooten , R. and Kleven , S.H. 2009 . Role of Mycoplasma synoviae in commercial layer Escherichia coli peritonitis syndrome . Avian Diseases , 51 : 685 – 690 .
- Sprygin , A.V. , Andreychuk , D.B. , Elatkin , N.P. , Kolosov , S.N. , Zinyakov , N.G. Mudrak , N.S. 2010a . Genetic diversity of Mycoplasma gallisepticum field isolates using partial sequencing of the pvpA gene fragment in Russia . Avian Diseases , 54 : 899 – 904 .
- Sprygin , A.V. , Andreychuk , D.B. , Kolotilov , A.N. , Volkov , M.S. , Runina , I.A. , Mudrak , N.S. , et al. 2010b . Development of a duplex real-time Taqman PCR assay with internal control for the detection of Mycoplasma gallisepticum and Mycoplasma synoviae in clinical samples from commercial and backyard poultry . Avian Pathology , 39 , 99 109 .
- Stipkovits , L. and Kempf , I. 1996 . Mycoplasmoses in poultry . Revue Scientifique et Technique , 15 : 1495 – 1525 .