Abstract
This study evaluates the enhancement of immune response of birds to Newcastle disease (ND) vaccine encapsulated in 1,2-dioleoyl-3-trimethylammonium propane (DOTAP)-based liposomes. The vesicles of the liposomal ND vaccine were physically characterized for shape, particle size and zeta potential. The results of the analyses showed that vesicles of the liposomal ND vaccine were spherical and tightly packed. The mean size distribution was below 100 nm. The mean zeta potential was 24 mV. Sixty experimental birds were then divided into an unvaccinated group, a liposomal ND vaccine group and a live La Sota® vaccine group. Both the liposomal ND vaccine and live La Sota® vaccine groups were vaccinated orally at 3 and 6 weeks of age. The mean antibody titres, total and differential white blood cell count, and blood chemistry, respectively, were assessed. Ten birds from each group were challenged by oral administration of 0.2 ml virulent Herts 33 strain at 9 weeks of age. The log2 mean antibody titre induced by the liposomal ND vaccine after secondary immunization of the birds was 9.60±0.95 while that of the live La Sota ® vaccine was 6.00±0.63. Nine of the 10 challenged birds in the unvaccinated group died while none died from the liposomal ND vaccine group or the live La Sota® vaccine group. After the boost vaccination, the chickens vaccinated with the liposomal ND vaccine had a higher mean antibody titre, indicating that encapsulating ND vaccine in DOTAP-based liposome induced significantly higher immunity than the live La Sota® vaccine.
Introduction
Newcastle disease (ND) is a highly infectious viral disease affecting both wild and domestic avian species (Seal et al., Citation2000; Alexander, Citation2003). Alexander (Citation2003) stated that the impact of ND is most notable in controlled poultry due to the high susceptibility of poultry. ND have been reported to represent a stronger drain on the world economy than any other animal viral disease, although the current epizootics of H5N1 avian influenza in Southeast Asia remains a major cause of high morbidity and mortality. Vaccination programmes are now designed to hyperimmunize chickens effectively to protect them from field/virulent strains of the ND virus, and for the vaccines to be effective it is important that they are delivered properly and effectively. Encapsulating ND vaccine in liposomes and administering them through the mucosal route have already been employed by some scientists to potentiate the immunogenicity of the vaccine (Tseng et al., Citation2009). Liposomes are small, spherical, self-closed vesicles of colloidal dimension that consist of amphiphilic lipids, enclosing an aqueous core. The lipids are predominantly phospholipids that form bilayers similar to those found in biomembranes. The major component is phosphatidylcholine. Liposomes are formed with one (unilamellar) or several concentric bilayers (multilamellar), depending on the method of preparation the chemical composition (Valiante et al., Citation2003). Liposomes have been employed in various applications of life sciences. (Lasic, Citation1992). Twenty-five years after the discovery of their immunological properties, liposomes now appear a major candidate adjuvant—with a liposome-based vaccine (against hepatitis A) being licensed for use in humans (Kirby, 1990; Gregoriadis, Citation1995). Vaccines based on novasomes (R) (non-phospholipid liposomes formed from single-chain amphiphiles, with or without other lipids) have been licensed for the immunization of fowl against ND virus and avian reovirus (Gupta et al., Citation1997). Inactivated hepatitis B virus and surface antigens of influenza virus have been licensed as liposomal vaccines and others such as tumour antigens encapsulated in liposomes, liposomal interleukin-2, muramyl peptides encapsualted within liposomes, polymerized liposomes and immunostimulatory CpG oligonucleotides encapsulated in liposomes are presently undergoing clinical trials. There are liposomal vaccines in pre-clinical development and clinical trials such as tumour antigens encapsulated in liposomes, liposomal interleukin-2, muramyl peptides encapsulated within liposomes, polymerized liposomes for oral vaccine delivery, and immunostimulatory CpG oligonucleotides encapsulated in liposomes (Maurer et al., Citation2001). Liposomes offer a number of advantages as carriers of vaccines because they are biodegradable and non-toxic, can elicit both humoral and cell-mediated immunity, and can be prepared entirely synthetically (Gregoriadis, Citation1990; Kersten & Crommelin, Citation1995). Cationic liposomes have a positive surface charge. They can improve the immune response against co-administered vaccine and protect antigen from clearance in the body and improve its immune response. They can fuse with negatively charged cell membranes and deliver the antigen endosomally. One of the most frequently used cationic lipids in formulating liposomes is 1,2-dioleoyl-3-trimethylammonium propane (DOTAP). In this study, DOTAP-based liposome was used to entrap a standard ND vaccine for possible enhancement of immunogenicity. The results suggest that the DOTAP-based liposome might be suitable as a potential immunoenhancer/adjuvant for oral vaccination against ND virus in chickens.
Materials and Methods
Materials
Cholesterol (Sigma grade, minimum purity 99%; Sigma Aldrich Chemie, St Louis, Missouri, USA), DOTAP (Sigma-Aldrich, donation from Prof. Godwin Nchinda, Rockefeller University, New York, USA), phospholipon 90H (donation from Phospholipid GmbH Nattermannallee, Cologne, Riedel-de Haen, Seelze Germany), methanol (extra pure; Scharlau Chemie S.A.), chloroform (Sigma-Aldrich GmbH, Germany), live La Sota® vaccine (National Veterinary Research Institute, Jos, Nigeria), and Herts 33 (National Veterinary Research Institute, Jos, Nigeria) were used.
Methods. Preparation of the liposome suspension
La Sota® vaccine containing 200 doses/vial was reconstituted with phosphate-buffered saline by dissolving a vial in 40 ml phosphate buffer solution. Then 196 mg phosphatidylcholine, 96.7 mg cholesterol and 50 µg DOTAP were weighed and dissolved in 3 ml chloroform/methanol system (2:1) in a round-bottomed flask. The solvent mixture was evaporated at room temperature and the flask rotated until a smooth, thin, dry film was obtained on the wall of the flask (Azmin et al., Citation1985). A 5-ml volume of the reconstituted vaccine was used to hydrate the dry film and agitated gently until multilamellar vesicles were formed.
Transmission electron microscopy
The prepared liposome-encapsulated ND vaccines were processed using copper grids to adsorb the particles from the suspension, staining in 2.5% uranyl acetate for 30 sec and drying. The specimens were observed under a JEM-1010 transmission electron microscope (JEOL, Tokyo, Japan) operated at 80 kV at×3400 and×10,500 magnifications (Chenatachan, Citation2008).
Vesicle size and zeta potential
The z-average vesicle diameter and zeta potential of the ND vaccine liposomes in phosphate buffer solution (pH 7.4) were determined by photon correlation spectroscopy using a nanosizer 3000 HS (Malvern Instruments, Malvern UK). The zeta potential was calculated from the mean of three runs. Each sample was diluted with bi-distilled water and the electrophoretic mobility determined at 25°C and a dispersant dielectric constant of 78.5 and pH 7. The obtained electrophoretic mobility values were used to calculate the zeta potentials using DTS software version 4.1 (Malvern Instruments) and applying Henry's equation (Zetasizer Nano Series, Citation2005):
where Z is the zeta potential, UE the electrophoretic mobility, ϵ the dielectric constant, η the viscosity of the medium and f(K) is Henry's function. The polydispersity index was determined as a measure of homogeneity.
Vaccination schedule of the birds
The chicks were raised from 1 day old until termination of the experiment. All bird handling and experiments were conducted following the guidelines stipulated by University of Nigeria Research Ethics Committee on animal handling and use. At 2 weeks of age, all of the birds were tested for the absence of residual haemagglutination inhibiting (HI) antibody. Sixty birds were divided into three groups of 20 birds; the unvaccinated group, the liposomal ND vaccine group, and the La Sota® vaccine group. The different groups were kept in separate rooms before vaccination. At 3 weeks of age, the liposomal ND vaccine group were given 0.2 ml/bird (a dose) of the liposomal ND vaccine orally. The La Sota® vaccine group were given 0.2 ml/bird (a dose) of the reconstituted La Sota® vaccine orally. The unvaccinated group served as the control. Two weeks after primary vaccination, all birds were bled from the jugular vein and serum samples assessed for antibody to ND virus by the HI technique (OIE, Citation2004). Booster vaccination was done at 6 weeks of age. Two weeks after secondary vaccination, all birds were bled and serum samples assessed for antibody to ND virus. Results of the study are presented as the mean with standard deviation for each of the parameters.
Haematology and blood biochemistry profile of the birds
Blood was also collected after primary vaccination in ethylenediamine tetraacetic acid bottles for the following analyses.
Determination of total white blood cell count
A 0.02-ml volume of blood was pipetted into a small test tube containing 0.38 ml avian white blood cell diluting fluid to make a 1:20 dilution of the blood sample. The diluted sample was loaded onto the Neubauer counting chamber and all cells on the four corner squares were counted using a light microscope at×10 objective. The number of cells counted for each blood sample was multiplied by 50 to obtain the total white blood cell count per microlitre of blood (Schalm, 1975a).
Determination of differential white blood cell count
The blood samples were shaken gently and a drop of blood was placed on a clean grease-free slide. The drop of blood was carefully smeared on the slide using a coverslip to make a thin smear. The smear was air-dried and thereafter stained by the Leishman technique using Leishman stain. The stained slides were later examined with an immersion objective using a light microscope. Two hundred cells were counted by the longitudinal counting method and each cell type was identified and scored using the differential cell counter. Results for the heterophils, lymphocytes, monocytes, eosinophils and basophils were expressed as a percentage of the total count and converted to the absolute value per microlitre of blood (Schalm, 1975b).
Determination of alanine aminotransferases
A 0.5-ml volume of alanine aminotransferase substrate was incubated for 5 min at 37°C. A 0.1-ml volume of the test samples were added and incubated at 37°C for 30 min. Then 0.5 ml colour developer was added and left to stand at room temperature for another 20 min, after which 5 ml of 0.4 N NaOH was added and left to stand for 15 min at room temperature. The readings were taken at a wavelength of 520 nm using a colorimeter (Reitman & Frankel, Citation1957).
Determination of total protein
A 0.02-ml volume of the samples was added to 1 ml Biuret reagent, mixed and allowed to stand for 10 min at room temperature. The readings were taken at a wavelength of 540 nm. Albumin and globulin were calculated.
Determination of uric acid
A 0.02-ml volume of the test samples was mixed with 1 ml working reagent (made ready to use). This was mixed and allowed to stand for 10 min at 35°C. The readings were taken at a wavelength of 505 nm.
Challenge experiment
The challenge experiment was carried out at 9 weeks of age in 10 birds from each group. The challenge was performed by administering 0.2 ml of 105.5 median embryo lethal dose/ml Herts 33 strain to each bird by the oral route. After challenge, the birds were monitored for 1 week for clinical signs of disease and mortality. Chickens without clinical signs of ND were considered protected; those that showed the typical neurological signs or died within this period were considered not protected. Dead birds were subjected to post-mortem examination and histopathology.
Data analysis
Data were fed into the SPSS statistics program (version 16.0; SPSS Inc.) applying a one-way analysis of variance test with least-squared difference multiple comparisons at P < 0.05.
Results
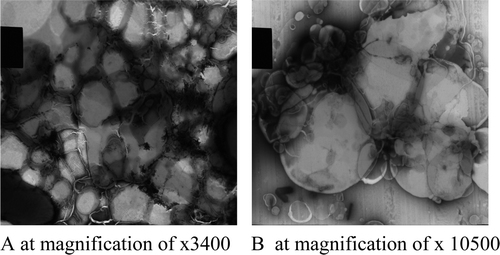
Transmission electron microscope images of the liposomal ND vaccine
The technique used for the preparation of the liposomes was the lipid film hydration technique, which formed films on the walls of the flasks and on hydration produced a thick, gel-like, milky colloidal dispersion. The images of the vesicles were taken at magnifications of×3600 and ×10,500 as shown in a,b. The vesicles at magnifications of ×3400 were closely packed, with each vesicle pressing on each other and conferring a polygonal shape. The images show vesicles of varying shapes and sizes. The vesicles at magnifications of ×10,500 were more spherical, appearing as self-closed vesicles, and were also closely packed with little space between them.
Particle size and zeta potential of the ND vaccine-encapsulated liposomes
Using a nanosizer, the mean particle size and zeta potential were determined. The mean size distribution and zeta potential were <100 nm and + 24 mV as shown in a,b.
Immune response of the birds
All birds screened prior to administration of vaccine were negative for HI antibody. All control (unvaccinated) birds had no antibody throughout the experiment. The immune response of the birds is shown in . After primary vaccination, the La Sota® vaccine group had a higher mean antibody titre (log2) and standard deviation of 5.50±0.67 while the liposomal ND vacine group had an antibody titre of 5.30±0.56. After secondary vaccination the chickens further seroconverted, and the liposomal ND vaccine had a higher mean antibody titre (log2) of 9.60±0.95, which was significantly higher than that for the La Sota® vaccine group with (log2) 6.00±0.63.
Table 1. Immune response of birds after primary and secondary immunization.
Haematological profile
The haematological profile of the birds is shown in . The mean number of white blood cells (×1000/µl±standard deviation) for the unvaccinated group was 13.85×103±1.31 cells/µl blood. The La Sota® vaccine group had 26.69×103±2.69 cells/µl blood while the liposomal ND vaccine group had the highest value of 31.23×103±2.51 cells/µl blood. The lymphocyte count for the unvaccinated group was 9.76×103±1.26 cells/µl blood and for the La Sota® vaccine group was 16.89×103±1.67 cells/µl blood, and the liposomal ND vaccine group had the highest value of 26.24×103±2.21 cells/µl blood. The value of the lymphocyte count for the liposomal ND vaccine group was significantly higher at P < 0.05 than for the La Sota® vaccinated group or the unvaccinated group.
Table 2. Haematological profile of the birds after primary immunization.
Blood chemistry profile
The blood chemistry of the birds is shown in . The alanine aminotransferase and uric acid of the unvaccinated group, liposomal ND vaccine group and La Sota® vaccinated group, respectively, were within normal limits. The alanine aminotransferase for the unvaccinated group was 6.18±1.51 IU/l, for the ND vaccine-encapsulated group was 5.09±1.23 IU/l and for the La Sota® vaccine group was 3.68±1.31 IU/l. The normal values for alanine aminotransferase are between 5 and 30 IU/l. The blood uric acid for the unvaccinated group was 2.50±0.82 mg/dl, for the liposomal ND vaccine group was 2.75±0.90 mg/dl and for the La Sota® vaccine group was 2.00±0.37 mg/dl. The normal blood uric acid value for most birds is 2 to 15 mg/dl. The total protein for the unvaccinated control group was 2.88±0.07 g/dl, for the liposomal ND vaccine group was 3.52±0.12 g/dl and for the La Sota® vaccine group was 2.87 + ±.25 g/dl. The normal range of the total protein level in most birds is from 3 to 5 g/dl, which is in agreement with the results of the present study (Coles & Campbell, Citation1986; Coleman et al., Citation1988; Kaneko et al., Citation1997; Khazraiinia et al., Citation2006). The globulin titre of the unvaccinated group was 1.66±0.14 g/dl, of the liposomal ND vaccine group was 2.40±0.10 g/dl and that of the La Sota® vaccine group was 1.61±0.06 g/dl. The value of globulin for the liposomal ND vaccine group was significantly higher at P < 0.05 than the La Sota® vaccine group or the unvaccinated group.
Table 3. Blood biochemistry of birds after immunization.
Challenge experiment
The pattern of morbidity and mortality showed that the unvaccinated group succumbed to the challenge with Herts 33 strain before the seventh day, with all birds showing clinical signs of disease; loss of appetite, torticollis, excess salivation paralysis, greenish and foul-smelling droppings, and so forth. By the seventh day, nine of the 10 unvaccinated birds had died (90% mortality). The birds of the liposomal ND vaccine group and of the La Sota® vaccine group showed no visible signs of disease. There was no mortality in these groups (100% survival).
Discussion
The vaccines used for ND control must be able to protect the susceptible poultry against velogenic strains of the virus. Antibody titres of 23 are protective, but current poultry practices are now designed to hyperimmunize chickens to protect them more effectively from field/virulent strains of the ND virus. This could be achieved by administering live plus inactivated vaccination to boost the antibody titres to as high as 27. van Eck (2003) stated that Broiler breeders have also been hyperimmunized against ND by subcutaneous injection of an experimental high-potency inactivated oil emulsion vaccine in order to achieve high and long-lasting passive protection in broiler progeny. In our study, the liposomal ND vaccine was evaluated for its ability to hyperimmunize the birds. This would be useful in low-biosecurity areas with high ND prevalence (Hutchinson, Citation1975).
A look at the liposomes showed vesicles that were mostly spherical in appearance, entrapping the vaccine within their core. The vesicles appeared tightly packed under the transmission electron microscope, indicating that the phosphatidylcholine, cholesterol and DOTAP, which are amphipathic lipids, thermodynamically organized themselves to form stable self-closed vesicles. The tight packing of the vesicles may also have reduced the binding/insertion of proteins, which normally destabilize membranes and mark liposomes for removal by phagocytic cells. Generally, the more ordered and hence tightly packed the membrane of a liposome, the less permeable it is (de Gier, Citation1968). Particle size is one of the determining factors for macrophage clearance a technique that measures time-dependent fluctuations in the intensity of scattered light was employed to measure size distribution of the particles. Large liposomes are rapidly removed from circulation. If the size of the liposome is below 200 nm, as obtained in the experiment, it would escape phagocytosis and the circulation time will be prolonged (Maurer et al., Citation2001). If the circulation time is prolonged, there will be more contact time of the liposomes with the immune cells, resulting in higher immunity. Encapsulation of the ND vaccine in the liposomes may also have reduced the volume of distribution of the vaccine and increased targeting to the immune cells (Maurer et al., Citation2001).
Knowledge of the zeta potential of the liposomal ND vaccine would help to predict its fate in vivo. Positively charged liposomes will normally fuse with the negatively charged cells. Once internalized, the particles are offered to the continuous lymphoid tissue (Eldridge et al., 1989). The cationic liposome would also protect the antigen against degradation on mucosal surfaces, and enhance its uptake in mucosa-associated lymphoid tissue.
The liposomal ND vaccine had a significant effect on the immune cells, inducing antibody titres as high as 29. Association of antigen with liposomes also allows the antigen to gain access to both the MHC class I pathway as well as the MHC class II pathway in antigen-presenting cells (APCs). As a result, liposomal antigens can stimulate antibody production as well as cellular immune responses (Zhou & Huang, Citation1994; Rao & Alving, Citation2000). Experiments to demonstrate the intervention of cell-mediated immunity were not attempted in this work but it is believed that mucosal immunity and some level of cell-mediated immunity may have been responsible for the protection of the vaccinated chickens against the velogenic Herts 33 strain. Post-challenge signs observed among the unvaccinated birds and the lesions observed at post-mortem of deceased chickens were identical with those described for ND (Gordon & Jordan, Citation1988; Echeonwu et al., Citation2008).
The results of the blood chemistry confirm that the liposomal ND vaccine was not injurious to the organs of the birds and did not cause toxic side effects in healthy tissues. Total leukocyte counts, differential leukocyte counts and blood chemistry reflect the systemic status of an animal in relation to its response and adjustment to injurious agents, stress and/or deprivation; the indices are of value in confirming or eliminating a tentative diagnosis, in making a prognosis and in guiding therapy (Coles, Citation1986; Meyer & Harvey, Citation1998). The blood chemistry profile is also an indicator of hepatocellular integrity and function. The components of the liposome were biocompatible and biodegradable.
In conclusion, ND virus is an important pathogen for chickens, causing significant economic losses in the commercial and village poultry. Improving the vaccine delivery could promote animal health and consequently improve food security. Formulating a ND vaccine as an oral cationic liposome holds good potential for improved vaccine delivery. From the results, the ND vaccine encapsulated into a liposome elicited significantly higher antibodies, lymphocytes and globulin titres, respectively. The liposome did not affect the organs of the birds or cause toxic side effects. Further field trials are to be carried out to confirm this potential.
Acknowledgements
The authors wish to thank Nattermann Köln, Germany for the gift of phospholipin 90 H and Professor Godwin Nchinda for donating DOTAP. The authors also want to thank the academic staff of the Faculty of Veterinary Medicine, University of Nigeria, Nsukka for assisting in the research work.
References
- Alexander , D.J. 2003 . “ Newcastle disease, other avian paramyxoviruses, and pneumovirus infections ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D. 63 – 99 . Ames : Iowa State University Press .
- Azmin , M.N. , Florence , A.T. , Handjani-Vila , R.M , Stuart , J.F.B. , Vanlerberghe , G. and Whittaker , J.S. 1985 . The effect of non-ionic surfactant vesicle (niosome) entrapment on the absorption and distribution of methotrexate in mice . Journal of Pharmacy & Pharmacology , 37 : 237 – 242 .
- Chenatachan , P. , Akarachalanon , P. , Worawirunwory , D. , Dararutana , P. , Bangtrakulnonth , A. , Bunjop , M. and Kongmuang , S. 2008 . Ultrastructural characterisation of liposomes using transmission electron microscope . Advanced Materials Research , 55–57 : 709 – 711 .
- Coles , E.H. 1986 . “ Erythrocytes, leukocytes and the bone marrow ” . In Veterinary Clinical Pathology , 4th edn , Edited by: Coles , E.H. 10 – 97 . Philadelphia : W.B. Saunders Company .
- Coleman , J.S. , Fraser , J.D. and Scanlon , P.F. 1988 . Hematocrit and protein concentration of black vulture and turkey vulture blood . Condor , 90 : 937 – 938 .
- Coles , E.H. and Campbell , T.W. 1986 . Veterinary Clinical Pathology , 4th edn , 279 – 291 . Philadelphia : Saunders .
- De Gier , J. , Mandersloot , J.G. and Van Deenen , L.L.M. 1968 . Lipid composition and permeability of liposomes . Biochimica et Biophysica Acta , 150 : 666 – 675 .
- Echeonwu , B. C. l. , Ngele , M. B. , Echeonwu , G. O. N. , Joannis , T. M. , Onovoh , E. M. and Paul , G. 2008 . Response of chickens to oral vaccination with Newcastle disease virus vaccine strain I2 coated on maize offal . African Journal of Biotechnology , 7 ( 10 ) : 1594 – 1599 .
- Gordon , R.F. & Jordan , F.T.W. 1988 . Newcastle disease . In: R.F. Gordon , Poultry Disease . Bailliere Tindal , London , pp. 65 – 79 .
- Gregoriadis , G. 1990 . Immunological adjuvants: a role for liposomes . Immunology Today , 11 : 89 – 97 .
- Gregoriadis , G. 1995 . Engineering liposomes for drug delivery: progress and problems . Trends in Biotechnology , 13 : 527 – 537 .
- Gupta , R.K. , Varanelli , C.L. , Griffin , P. , Wallach , D.F. and Sibber , G.R. 1997 . Adjuvant properties of non-phospholipid liposomes (Novasomes) in experimental animals for human vaccine antigens . Vaccine , 14 : 219 – 225 .
- Hutchinson , H.L. 1975 . The control and eradication of Newcastle disease in Northern Ireland . The Veterinary Record , 96 : 213 – 217 .
- Kaneko , J.J. , Harvey , J.W. and Bruss , M.L. 1997 . Clinical Biochemistry of Domestic Animals , 5th edn , San Diego : Academic Press .
- Kersten , G.F.A. and Crommelin , D.J.A. 1995 . Liposomes and ISCOMS as vaccine formulations . Biochimica et Biophysica Acta , 1241 : 117 – 138 .
- Khazraiinia , P. , Saei , S. , Mohri , M. , Haddadzadeh , H.R. , Darvisihha , H.R. and Khaki , Z. 2006 . Serum biochemistry of ostrich (Striuthio camelus) in Iran . Comparative Clininical Pathology , 15 : 87 – 89 .
- Lasic , D.D. 1992 . Liposomes . American Scientist , 80 : 20 – 31 .
- Maurer , N. , Fenske , D.B. and Cullis , P.R. 2001 . Developments in liposomal drug delivery systems . Expert Opinion on Biological Therapy , 1 ( 6 ) : 1 – 25 .
- Meyer , D.J. and Harvey , J.W. 1998 . Veterinary Laboratory Medicine and Diagnosis , Philadelphia : W.B. Saunders Company .
- Office International des Epizooties (OIE) (World Organization of Animal Health) . 2004 . Manual of Standards of Diagnostic Tests and Vaccines .
- Rao , M. and Alving , C.R. 2000 . Delivery of lipids and liposomal proteins to the cytoplasm and Golgi of antigen-presenting cells . Advanced Drug Delivery Reviews , 41 : 171 – 188 .
- Reitman , S. and Frankel , S.A. 1957 . Colorimetric method for the determination of serum oxaloacetic and glutamic pyruvate transaminase . American Journal of Clinical Pathology , 28 : 56 – 63 .
- Schalm , O.W. , Jain , N.C. and Carrol , E.J. 1975 . Veterinary Haematology , 3rd edn , 129 – 225 . Philadelphia : Lea and Febiger .
- Schalm , OW , Jain , NC and Carrol , EJ. 1975 . Veterinary Haematology , 3rd edn , 19 – 25 . Philadelphia : Lea and Febiger .
- Seal , B.S. , King , D.J. and Sellers , H.S. 2000 . The avian response to Newcastle disease virus . Developmental and Comparative Immunology , 24 : 257 – 268 .
- Tseng , L.P. , Chiou , C.J. , Chen , C.C. , Deng , M.C. , Cheng , T.W. , Huang , Y.Y. and Liu , D.Z. 2009 . Effect of lipopolysaccharide on intranasal administration of liposomal Newcastle disease virus vaccine to SPF chickens . Veterinary Immunology and Immunopathology , 131 : 285 – 289 .
- Valiante , N.M. , O'Hagan , D.T. and Ulmer , J.B. 2003 . Innate immunity and biodefence vaccines . Cellular Microbiology , 5 : 755 – 760 .
- van Eck , J.H.H. 1990 . Protection of broilers against Newcastle disease by hyperimmunization of the dams . Veterinary Quarterly , 12 : 139 – 145 .
- Zetasizer Nano Series . 2005 . Malvern Instruments , England, User Manual Issue 2.2 .
- Zhou , F and Huang , L. 1994 . Liposome-mediated cytoplasmic delivery of proteins: an effective means of accessing the MHC class Irestricted antigen presentation pathway . Immunomethods , 4 : 229 – 235 .