Abstract
Eimeria species parasites can cause the disease coccidiosis in all livestock species, most notably poultry. Traditional diagnostics such as faecal microscopy have now been supplemented by molecular assays including genus-specific and species-specific quantitative polymerase chain reaction (qPCR), although DNA extracted from faecal samples is commonly affected by PCR inhibition. This was confirmed when genomic DNA extracted from chicken faeces inhibited the threshold cycle value of internal positive control (IPC) DNA amplification by 15.33%. Hence, the objective of the present study was to use IPC qPCR to determine PCR inhibition in a series of experimental samples and use the increase in IPC qPCR threshold cycle value as an individual (sample-specific) correction factor for an established 5S rDNA qPCR used to estimate total Eimeria genome numbers. IPC-corrected genome counts were correlated with conventional oocyst per gram counts and compared with non-corrected counts, revealing a 0.1769 increase in correlation coefficient to outweigh underestimation of oocyst counts. Though the sample size used in this study is small, this limitation would be offset by the sample-specific correction factor determined using the IPC along with each sample.
Introduction
Coccidiosis is an economically important and commonly prevalent parasitic disease worldwide, caused by protozoa of the genus Eimeria (Apicomplexa). Seven distinct Eimeria species—Eimeria acervulina, Eimeria brunetti, Eimeria maxima, Eimeria mitis, Eimeria necatrix, Eimeria praecox and Eimeria tenella—affect chickens, causing intestinal lesions of variable extent and severity that reduce feed absorption leading to weight loss, diarrhoea, poor feed conversion and sometimes mortality. Conventionally, Eimeria oocysts are often counted microscopically using a McMaster chamber (Hodgson, Citation1970; Long et al., Citation1976) and expressed in terms of oocysts per gram (OPG) or oocysts excreted per unit time, although accurate identification of distinct species can be demanding. With the advent of molecular techniques such as quantitative polymerase chain reaction (qPCR), genome number estimations are replacing these microscopic calculations. The advantages of these molecular methods are their reduced requirement for specialist parasitological knowledge, speed, specificity, discriminatory power, objectivity, high throughput and automation potential. Total genome numbers can be estimated using the multicopy 5S rDNA as target (Blake et al., Citation2006; Vrba et al., Citation2010) and oocyst numbers calculated using standard or microscopically determined sporulation percentages (Morgan et al., Citation2009). One of the disadvantages of these techniques in addition to the costs involved is their susceptibility to the presence of PCR inhibitors (Hartman et al., Citation2005; Sarfo et al., Citation2011). Faecal samples are a potent source of PCR inhibition and extraction of DNA from these materials because application in qPCR may often lead to underestimation of the target genome copy numbers determined. The correlation between OPG and qPCR-based Eimeria genome number estimations are generally low, in the range of 0.50 (Morgan et al., Citation2009). If absolute numbers are needed to be estimated then the underestimation may compromise the results.
Materials and Methods
Sample collection and preparation
Fresh faecal droppings were collected from selected locations within poultry units of Tamil Nadu, India (n = 25) following a “W”-shaped sampling method. Briefly, one fresh faecal dropping was selected at random (caecal and/or intestinal) and collected every two strides along a notional “W” shape within a poultry pen and placed in a labelled plastic bag until approximately 5 g had been collected. Bags were then sealed and sent to the laboratory where they were stored under refrigeration conditions to minimize subsequent sporulation until tested. Large poultry units were represented by multiple bags. Oocysts from the faecal samples were purified using a saturated salt flotation method and counted using a standard McMaster protocol (Long et al., Citation1976). The OPG value was calculated for each sample after microscopic counting of the oocysts. DNA extraction was done from 600 mg of faecal material using a mini-bead beater to disrupt each sample with 0.5 mm glass beads in phosphate-buffered saline prior to purification using a DNA Stool kit (Qiagen, Valencia, California, USA) following the manufacturer's instructions. Similarly, DNA from chicken tissue (spleen), purified oocysts of E. tenella (after bead beater disruption) and white leghorn chicken feathers was purified using the tissue DNA extraction kit (Qiagen) following the manufacturer's recommendations.
Quantitative polymerase chain reaction
The extracted DNA was subjected to qPCR for Eimeria genome quantification using the 5S rDNA as target (Blake et al., Citation2006). qPCR was performed in a Realplex 4S cycler (Eppendorf, Hamburg, Germany) using 500 nM primer concentration, 250 nM probe (labelled with FAM and TAMRA) concentration (sequences as described by Blake et al., Citation2006), and 2 µl undiluted extracted DNA with the TaqMan® Universal PCR Master Mix (Applied Biosystems, Foster City, California, USA). The qPCR was performed in triplicate with the following cycle conditions: 50°C for 2 min, 10 min at 95°C and 40 cycles of 95°C for 15 sec and 60°C for 1 min. No template control and positive controls were performed for all assays.
The 5S rDNA PCR product of 119 base pairs was cloned in a TA cloning vector (RBC TA cloning kit; Taipei City, Taiwan). A standard curve was developed for plasmid DNA using 109 to 104 copies of the gene target. Amplification plots and standard curves were generated and used to estimate genome numbers from unknown samples (Morgan et al., Citation2009).
Internal positive control quantitative polymerase chain reaction
An internal positive control (IPC) kit containing IPC DNA, primer, probe labelled with VIC–TAMRA and Exo Block DNA (Applied Biosystems) was used in qPCR following the universal qPCR cycling conditions described above to determine the presence of PCR inhibitors. IPC generally is included in samples to be tested for target genes as a means to monitor non-specific inhibition of nucleic acid amplification. IPC qPCR was performed in the presence of equal concentrations (200 ng each) of DNA isolated from different sources to establish whether DNA extracted from faecal samples inhibited IPC qPCR amplification.
The effect of DNA extracted from individual faecal samples on IPC qPCR was analysed as follows: qPCR was performed with the IPC kit and the mean threshold cycle (Ct) values determined (A); qPCR was carried out for 5S rDNA target with DNA extracted from faecal samples and the mean Ct value determined (B); and qPCR was performed for IPC along with the addition of that particular faecal sample DNA in the same tube and the mean Ct value determined (C).
Importantly, if A and C were found to be similar this would indicate the relative absence of PCR inhibition. If the extracted DNA from a particular faecal sample was inhibitory, then it would increase the Ct value of the associated IPC qPCR. (C-A) would thus be a measure of the PCR inhibition. If the difference between (C-A) could be used to correct the mean Ct value of B, then this would offset the possible inhibition-induced increase of Ct (B) values of qPCR for the 5S rDNA gene target of that particular sample. Hence the objective of the present study was to use IPC qPCR to determine qPCR inhibition for each particular sample and apply the increase in Ct value of IPC in the presence of DNA from particular samples as an individual (sample-specific) correction factor. This was correlated with genome numbers estimated from OPG values.
Eimeria species identification using standard polymerase chain reaction
Samples found to contain Eimeria using microscopy and/or qPCR were subsequently subjected to standard PCR to identify the species present, as described previously (Aarthi et al., Citation2010).
Results
Comparison of oocyst microscopy and standard quantitative polymerase chain reaction
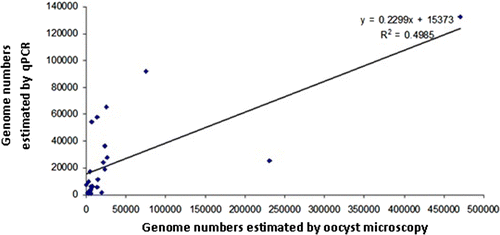
The coefficient of determination (r 2) for genome numbers estimated from direct microscopy of oocysts (OPG) or by 5S rDNA qPCR-based genome quantification was found to be 0.4985 (correlation coefficient 0.706; ).
Internal positive control quantitative polymerase chain reaction
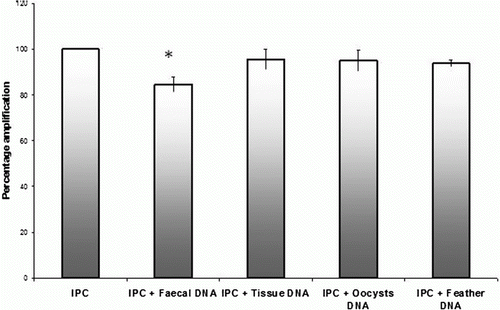
When IPC qPCR was carried out in the presence of DNA extracted from chicken tissue, faeces, feathers or purified Eimeria oocysts, it was found that faecal sample DNA exerted a maximum mean inhibition of 15.33%±3.11 of the IPC Ct values (). DNA from other sources exerted inhibitions of 4.55%±4.16 (tissue DNA), 4.93%±4.412 (oocyst DNA) and 6.14%±1.52 for feather DNA. Only the Ct values of IPC with faecal DNA were significantly less sensitive than those with IPC alone (P < 0.05, one-way analysis of variance), while those with other DNA did not vary significantly.
Figure 2. PCR inhibition of IPC amplification in the presence of extraneous DNA obtained from various sources. The percentage change in IPC Ct value induced by the addition of DNA from various sources compared with the unsupplemented IPC (i.e. in the absence of extraneous DNA, taken as 100%) is depicted. *Group significantly different from the control unsupplemented IPC (P < 0.05).
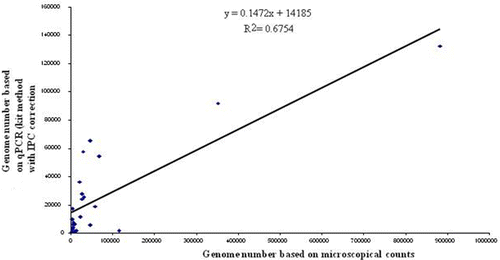
Comparison of oocyst microscopy and internal positive control-normalized quantitative polymerase chain reaction
The coefficient of determination for parasite genome numbers estimated from direct faecal microscopy (OPG) and by 5S rDNA qPCR-based genome quantification using the sample-specific IPC-based correction factor was found to be 0.6754 (~35% higher than standard non-normalized qPCR, correlation coefficient 0.822; ).
Identification of Eimeria species tested
Comparison of the increase (change) in the correlation coefficients due to the inclusion of the IPC correction factor with respect to each species of Eimeria identified is presented in . All seven Eimeria species known to infect the chicken were found in at least one of the samples tested here, although co-infection was common and E. maxima, E. mitis, E. praecox and E. brunetti were present in less than five samples tested. Application of the IPC correction factor increased the correlation by 0.16 (E. tenella) and 0.70 (E. necatrix), did not change correlation for E. acervulina, and decreased the correlation by 0.18 for E. maxima. When one outgroup value was removed from E. necatrix, the increase was only 0.37. The change in correlation coefficients for other species could not be assessed due to the small sample size.
Table 1. Influence of Eimeria species on inhibition of IPC DNA amplification by chicken faecal samples collected in Tamil Nadu.
Discussion
It is well documented that faecal material can be a source of PCR inhibition in conventional PCR and qPCR reactions (Hartman et al., Citation2005; Sarfo et al., Citation2011). This was confirmed in the current study when, even using a small sample set, DNA extracted from chicken faeces significantly inhibited an IPC qPCR. DNA extracted from chicken tissues, feathers or oocysts caused minimal inhibition. Thus, in addition to complications resulting from variable rates of oocyst sporulation, the use of DNA extracted from faecal samples in qPCR reactions for quantitative estimations is fraught with the possibility of underestimating the biological entity (Morgan et al., Citation2009). This could lead to false negative qualitative results or inaccurate quantitative results where absolute parasite numbers are important given the differing pathogenicity associated with distinct species within the Eimeria genus. This underestimation is of crucial significance when inferring risk to a flock based upon absolute parasite numbers (compare microscopic OPG and qPCR genome counts). One of the methods used to overcome this type of problem is the inclusion of parallel IPC qPCR assays to confirm the presence/absence of PCR inhibition and to introduce a correction factor (Hartman et al., Citation2005). In this study, the same principle was used to outweigh the possible underestimation of Eimeria species genome numbers due to the presence of PCR inhibitors.
qPCR of the eimerian 5S rDNA has been used for total genome estimation of Eimeria, irrespective of species (Blake et al., Citation2006), and a variety of additional qPCR assays have been developed for species-specific quantitative identification of different Eimeria species (Blake et al., Citation2006; Morgan et al., Citation2009; Vrba et al., Citation2010). However, the advantage of this method is fully exploited only if truly quantitative estimations (or relative abundance) of each species are obtained (Morgan et al., Citation2009). Importantly, direct comparison of microscopic faecal oocyst counts versus qPCR assays has not always been concordant (r 2=0.5059 [Morgan et al., Citation2009] and r 2=0.4985 [present study]). One major reason for this is PCR inhibition exerted by the faecal samples used for DNA extraction. In this study, 15 of 25 samples (60%) showed an underestimation of qPCR genome numbers compared with the IPC-corrected values, despite use of an inhibitor-reducing stool kit DNA extraction protocol. Though the sample size used in this study is small, this limitation would be offset by the sample-specific correction factor determined using the IPC along with each sample.
An interesting observation was that the faecal samples investigated exerted an unequal qPCR inhibition, suggesting that a common correction factor is unlikely to work. Thus, while 15 samples exhibited IPC qPCR inhibition, the remaining 10 exerted either no apparent effect or appeared to improve qPCR efficiency. Overall, the sample-specific IPC correction factor r 2 value increased by ~35% from 0.4985 to 0.6754, a difference of 0.1769. Despite this apparent improvement, the derivation of a relatively low r 2 value after incorporation of the correction factor suggests that qPCR inhibition was not the only factor contributing to the genome underestimation. The use of pooled samples, including caecal and intestinal-type faecal samples, suggests that faecal type is not responsible for the variation observed between the samples tested here. Nonetheless, it is possible that inhibitor levels may vary between faecal types. Similarly, no link could be made between the Eimeria species present and the occurrence of inhibition, although the lack of single species samples and overall small sample size limited statistical power. Variable sporulation has previously been identified as a major potential source of variation with a maximum four-fold difference possible in comparison of diploid unsporulated and haploid sporulated oocysts (Morgan et al., Citation2009). In this study, fresh faecal samples were preferentially selected to minimize sporulation-associated variation and improve inter-sample standardization. Faecal samples quickly lose form in poultry accommodation under the action of bird movement. Intact samples were taken to represent faeces less than 24 h old, although it is important to note that sporulation could still have made significant progress under favourable conditions (Novaes et al., Citation2012). Other possible factors include the PCR efficiency of the 5S rDNA and IPC qPCRs. The 5S rDNA qPCR had an efficiency of 0.970 during these studies, while that of the IPC qPCR could not be calculated as this was supplied as a kit containing IPC DNA, primer probes and Exo block DNA. Any dilutions done on this would also dilute the other reagents. Nonetheless, the use of IPC qPCR to offset the underestimation of genome numbers due to PCR inhibition is recommended due to a higher correlation that may be useful during absolute quantification of Eimeria genome numbers by qPCR. The addition of IPC is likely to be of value with any sample capable of influencing qPCR efficiency. In this context, the modification should be applied in all assays working with faecal, litter or other environmental samples in commercial or experimental settings. Work with purified oocysts or tissue samples containing intracellular Eimeria lifecycle stages may not require IPC, although the importance of inhibition warrants preliminary testing to establish its absence.
The work reported here describes an attempt to improve correlation between microscopic and molecular determination of Eimeria species presence in faecal samples. Molecular approaches to identify and quantify Eimeria are attractive as they reduce the requirement for user expertise, can be adapted to medium/high-throughput systems and be applied to old or preserved samples. While factors including laboratory cost and a requirement for specialist equipment remain limiting, advances in reproducibility and reliability promote the use of qPCR as a routine veterinary diagnostic. This method may also be useful for quantifying DNA from other enteric pathogens such as Mycobacterium avium subsp. or Paratuberculosis in ruminants.
Acknowledgements
The work carried out in this study was supported in part by the Department for International Development and the Biotechnology and Biological Sciences Research Council, UK (grant number BB/H009337/2).
References
- Aarthi , S. , Dhinakar Raj , G. , Raman , M. , Gomathinayagam , S. and Kumanan , K. 2010 . Molecular prevalence and preponderance of Eimeria spp. among chickens in Tamil Nadu, India . Parasitology Research , 107 : 1013 – 1017 . doi: 10.1007/s00436-010-1971-2
- Blake , D.P. , Hesketh , P. , Archer , A. , Shirley , M.W. and Smith , A.L. 2006 . Eimeria maxima: the influence of host genotype on parasite reproduction as revealed by quantitative real-time PCR . International Journal for Parasitology , 36 : 97 – 105 . doi: 10.1016/j.ijpara.2005.09.011
- Hartman , L.J. , Coyne , S.R. and Norwood , D.A. 2005 . Development of a novel internal positive control for Taqman based assays . Molecular and Cellular probes , 19 : 51 – 59 . doi: 10.1016/j.mcp.2004.07.006
- Hodgson , J.N. 1970 . Coccidiosis: oocyst counting technique for coccidiostat evaluation . Experimental Parasitology , 28 : 99 – 102 . doi: 10.1016/0014-4894(70)90073-1
- Long , P.L. , Joyner , L.P. , Millard , B.J. and Norton , C.C. 1976 . A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis . Folia Veterinaria Latina , 6 : 201 – 217 .
- Morgan , J.A.T. , Morris , G.M. , Wlodek , B.M. , Byrnes , R. , Jenner , M. , Constantinoiu , C.C. , Anderson , G.R. , Lew-Tabor , A.E. , Molloy , J.B. , Gasser , R.B. and Jorgensen , W.K. 2009 . Real-time polymerase chain reaction (PCR) assays for the specific detection and quantification of seven Eimeria species that cause coccidiosis in chickens . Molecular and Cellular Probes , 23 : 83 – 89 . doi: 10.1016/j.mcp.2008.12.005
- Novaes , J.L. , Rangel , T. , Ferro , M. , Abe , R.Y. , Manha , A.P. , de Mello , J.C. , Varuzza , L. , Durham , A.M. , Madeira , A.M. and Gruber , A. 2012 . A comparative transcriptome analysis reveals expression profiles conserved across three Eimeria spp. of domestic fowl and associated with multiple developmental stages . International Journal for Parasitology , 42 : 39 – 48 . doi: 10.1016/j.ijpara.2011.10.008
- Sarfo , F.S. , Lavender , C.J. , Fyfe , J.A.M. , Johnson , P.D.R. , Stinear , T.P. and Phillips , T.O. 2011 . Mycobacterium ulcerans DNA not detected in faecal samples from Buruli ulcer patients: results of a pilot study . PLoS ONE , 6 : e19611 doi: 10.1371/journal.pone.0019611
- Vrba , V. , Blake , D.P. and Poplstein , M. 2010 . Quantitative real-time PCR assays for detection and quantification of all seven Eimeria species that infect the chicken . Veterinary Parasitology , 174 : 183 – 190 . doi: 10.1016/j.vetpar.2010.09.006