Abstract
Pathogenesis of Gallibacterium anatis was investigated in specific pathogen free cockerels. Birds aged 35 weeks were infected intranasally with G. anatis whereas negative controls were left uninfected. Following infection, necropsy, bacteriological and histopathological investigations were performed in birds killed at 3, 7, 10, 28 and 38 days post infection (d.p.i.). Additionally, semen samples were collected twice a week until 5 weeks post infection for quality assessment. No clinical signs and gross pathological lesions were seen throughout the experiment. Bacteriological investigation revealed that G. anatis colonized the upper respiratory tract at 3 d.p.i. and could be isolated from testis and epididymis at 7 d.p.i. onwards. Bacterial persistence was found in the respiratory tract, gut and testis until the termination of the study at 38 d.p.i. Furthermore, G. anatis was isolated from semen arguing for the possibility of vertical transmission. Histopathological examination showed infiltration of mononuclear cells in epididymal tissue, indicating an inflammation. Density, total motility, progressive motility and membrane integrity of sperms were significantly decreased in infected birds as compared with control chickens. Along with these findings, an increase in spermatozoa with morphological defects was observed at different time points. In conclusion, the present study provides novel data on the impact of a G. anatis infection in cockerels in a natural infection model, thus helping to elucidate bacterial distribution, pathological lesions as well as influences on semen quality.
Introduction
Gallibacterium anatis, belonging to the family Pasteurellaceae, is a Gram-negative, non-motile, rod-shaped or pleomorphic bacterium (Christensen et al., Citation2003). In the last decade, the organism has been widely reported from chickens mainly associated with salpingitis, peritonitis and drop in egg production (Bojesen et al., Citation2007). Co-infections with other pathogens, increasing antibiotic resistance, a higher prevalence in farms with low biosecurity level and single clonal lineages of isolates in an individual chicken flock have been shown to exist as features of a natural infection (Shaw et al., Citation1990; Bojesen et al., Citation2003, Citation2011; Neubauer et al., Citation2009; Alispahic et al., Citation2012). Clinical and pathological manifestations of an experimental infection with G. anatis have so far focused on layers (Bojesen et al., Citation2004; Paudel et al., Citation2014). However, Zepeda et al. (Citation2010) and Paudel et al. (Citation2013) demonstrated that these bacteria could also be re-isolated from the reproductive tract of young male chickens but the impact of an infection with G. anatis in cockerels still remains unknown. It has been shown for other bacteria that they can reduce the semen quality; for example, sperm motility (Haines et al., Citation2013). Furthermore, orchitis, epididymitis and epididymo-orchitis in birds have been reported in cases of infections with Salmonella species, Escherichia coli, Staphylococcus aureus, Pasteurella multocida and Chlamydophila (Gauger, Citation1934; Kokosharov et al., Citation1984; Gerlach, Citation1994; Shalaby et al., Citation1994; Monleon et al., Citation2008). Such conditions can not only exert influence on the health status of cockerels but can also affect breeding performance. The aim of the present study was therefore to evaluate the progression of an infection with G. anatis in adult male birds focusing on clinical signs, bacterial distribution and pathological lesions. Additionally, the influence of G. anatis on semen quality was evaluated based on different parameters such as pH, differential motility characteristics, viability and pathomorphology.
Materials and Methods
Bacterial isolate and inoculum preparation
The stock culture of G. anatis biovar haemolytica strain 07990 was grown in Lennox L Broth (Invitrogen, Vienna, Austria) at 37°C for 24 h under microaerobic condition (GENbox microaer; BioMérieux, Vienna, Austria). The concentration of the original suspension was determined by counting colony-forming units on Columbia agar supplemented with 5% sheep blood (COS agar plates; BioMérieux) in serial dilutions in duplicates. The bacterial culture was mixed with glycerol and stored at −20°C. On the day of infection, the suspension was thawed, washed and resuspended with phosphate-buffered saline for preparing inoculation material. Viability of the bacteria in the inoculum was controlled by direct plating before and after infection.
Birds, housing and experimental design
The trial was approved by the institutional ethics committee and the national authority according to § 8ff of the law for Animal Experiments, Tierversuchsgesetz-TVG (License number: BMWF-68.205/0237-II/3b/2012).
Twenty-eight specific pathogen free White Leghorn chickens (VALO; Lohmann, Cuxhaven, Germany) were hatched in our facilities and reared on a deep litter system in closed rooms under negative air pressure. Daily inspection was done in order to ensure ad libitum feed and water supply, adequate temperature, proper functioning of the air flow channel as well as normal health status of the birds. Ten randomly selected birds were tested for G. anatis at 3-week to 4-week intervals using swab samples from the choana, trachea and cloaca. At week 30, birds were assigned to two different groups (). Four cockerels used for semen collection from each group were labelled with an individual number tag. In week 35, 12 cockerels in group 1 were left uninfected as negative controls whereas 16 birds from group 2 were infected intranasally with 0.4 ml of 1.42 × 108 colony-forming units/ml G. anatis 07990. Following experimental infection, the clinical status of the birds was observed daily. In addition, two control birds and three infected birds from groups 1 and 2, respectively, were killed at 3, 7, 10 and 28 days post infection (d.p.i.) for necropsy and sampling. Semen samples were always collected from the same four birds from each group at 3-day to 4-day intervals until the end of the study. At 38 d.p.i., these birds were killed and necropsied. Samplings for bacteriological and histopathological examinations were performed as in the preceding killing points.
Table 1. Experimental design of the trial.
Bacteriology
During necropsy, swabs from individual organs (choana, trachea, lung, liver, spleen, heart, testis, epididymis, duodenum and cloaca) from two control birds and three infected birds were streaked aseptically on COS agar plates for bacterial re-isolation. Semen collected from all the cockerels at all time points () was investigated in the same way. The plates were incubated at 37°C for 24 h under microaerobic condition. G. anatis colonies were identified by morphological characteristics (Christensen et al., Citation2003), oxidase test and matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS). Details of sample preparation, parameters and analytical procedures of MALDI-TOF MS were followed as described previously (Alispahic et al., Citation2011).
Table 2. Schedule for semen collection.
Histopathology
The trachea, testis and epididymis from necropsied birds were fixed in 4% neutral buffered formalin for at least 24 h and embedded in paraffin. Each tissue specimen was then sectioned into 5 µm slices and stained with haematoxylin and eosin.
Semen collection
Semen was collected twice a week from eight cockerels (four each from groups 1 and 2; ) until 5 weeks post infection (w.p.i.) by abdominal massage. After stimulating the cockerels, ejaculates were collected in an individual sterilized glass cup and kept in a metal chamber containing pre-warmed glass beads to avoid temperature shock. The semen samples were immediately processed for quality assessment.
Assessment of semen quality
pH and mass motility in raw semen
The pH of the semen was measured with pH indicator strips (Merck, Darmstadt, Germany). Mass motility of sperm was viewed under a Nikon Optiphot 2 microscope (40× magnifications; Nikon GmbH, Vienna, Austria) connected with an external screen.
Semen concentration
The semen concentration in individual samples was analysed with the NucleoCounter® SP-100™ (Chemometec, Allerød, Denmark). For analysis, raw semen was mixed with Reagent S100 (Chemometec) with a dilution factor of 401 and the concentration was calculated by the system as given in the manufacturer’s instruction.
Computer-assisted sperm analysis
The motility and membrane integrity of spermatozoa were evaluated with computer-assisted technology (Sperm Vision™; Minitüb GmbH, Tiefenbach, Germany) as described earlier (Schäfer-Somi & Aurich, Citation2007) with slight modifications.
Raw 8semen was diluted with modified Ringer’s solution prepared according to Olsen & Neher (Citation1948). For motility assessment, 3 µl diluted semen was loaded on pre-warmed standard count chamber slides (Leja B. V., Nieuw Vennep, the Netherlands) and placed on the Nikon Optiphot 2 microscope (200× magnification). All motility parameters of sperm cells were recorded with Sperm Vision™. The following parameters were evaluated: total motility, progressive motility, distance curved line (µm), distance average path (µm), distance straight line (µm), velocity curved line (VCL, µm/sec), velocity average path (VAP, µm/sec), velocity straight line (VSL, µm/sec), linearity (LIN%, VSL/VCL × 100), straightness (STR%, VSL/VAP x 100), wobble (%, VAP/VCL × 100), beat cross frequency (Hertz) and amplitude of lateral head displacement (µm). The software setting was adjusted to count either eight fields or 4000 cells. Other settings used to define various motility characteristics in this study were: average orientation change of the head (degrees) < 3 means sperm cells are immotile; distance straight line < 5 denotes local motility of the cells; VCL > 80, LIN < 0.65 and amplitude of lateral head displacement > 6.5 indicate spermatozoa with hypermotility; STR > 0.9 and LIN > 0.5 defines linear progressive motility; STR < 0.9 and LIN < 0.5 indicate non-linear progressive motility.
For analysis of membrane integrity, 100 µl diluted semen was stained with SYBR-14/propidium iodide (LIVE/DEAD® Sperm Viability Kit; Life Technologies, Grass Island, NY, USA) and incubated for at least 7 min. A drop of stained solution was placed on a pre-warmed glass slide and analysed with the Olympus AX70 florescence microscope (Olympus Austria GmbH, Vienna, Austria). Dead (red stained) and live (green stained) spermatozoa were automatically recognized and counted with Sperm Vision™ software adjusted with the setting to quantify either 1000 cells or 15 observation fields.
Pathomorphology
One microlitre of raw semen was mixed well with 200 µl buffered formol-saline solution for fixation. Afterwards, 200 sperm cells from each sample were evaluated for defects of acrosome, head, mid piece and tail with phase contrast microscopy (1000× magnification, Nikon Optiphot 2).
Statistical analysis
Average differences of sperm variables in weekly semen samples between control and infected groups were evaluated with Student’s t test for independent samples (P ≤ 0.05). Values are expressed as mean ± standard error of mean.
Results
Clinical signs and necropsy findings
No clinical signs and gross pathological lesions were observed in birds during the experiment.
Bacteriology
All of the samples examined before infection were free from G. anatis. Also, G. anatis could not be isolated from semen or any of the organs sampled during necropsy of control birds throughout the trial. In specimens from infected birds, G. anatis was identified based on the growth of translucent colonies with a strong β-haemolytic zone. All of the isolates were oxidase-positive and further confirmed as G. anatis by MALDI-TOF MS analysis (data not shown). The results from direct plating (re-isolation) from infected birds are presented in . G. anatis was re-isolated from choana of all three birds killed at 3 d.p.i. In addition, the bacteria were found in the lungs, testis, epididymis and cloaca at 7 d.p.i. and additionally in the trachea, liver, heart and duodenum at 10 d.p.i. Persistency of G. anatis was noted until 38 d.p.i. in the choana, trachea, lungs, testis and duodenum.
Table 3. Results of direct plating (re-isolation) following intranasal infection with G. anatis in specific pathogen free cockerels.
In semen, G. anatis was found at 18 d.p.i. (in 4/4 birds examined), at 22 d.p.i. (3/4 birds), at 25 d.p.i. (1/4 birds) and at 29 d.p.i. (1/4 birds).
Histopathology
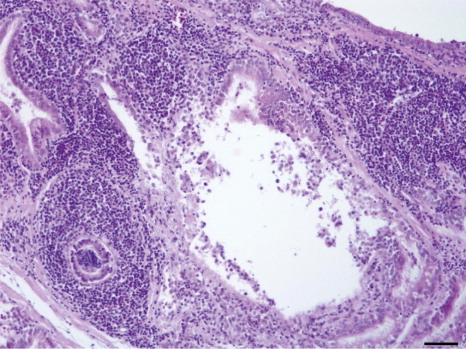
No microscopic lesions were observed in tissue samples collected from control birds. In the infected group, one bird had thickened tracheal mucosa with infiltration of lymphocytic cells at 10 d.p.i. Testes were found normal whereas multifocal aggregation of mononuclear cells were noted in interstitial regions of epididymis in 2/3, 1/3 and 2/3 birds at 7, 10 and 28 d.p.i. respectively ().
Semen collection
A sufficient volume of semen required for the laboratory examinations was obtained from all cockerels except for one bird at 5 w.p.i. (first sampling) in the control group and one bird at 1 week prior to (second sampling) and after infection (first and second samplings) in the infected group, respectively (). At these dates, only three samples were included for further analysis.
Semen quality parameters
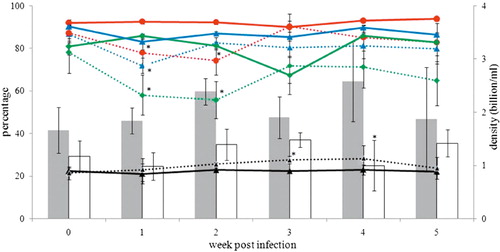
The pH of seminal fluid did not differ between control and infected birds (data not shown). Semen concentration as well as total motility, progressive motility, membrane integrity and pathomorphology of spermatozoa are presented in (absolute values of sperm quality parameters are given in supplementary data, Table S1). Statistically significant differences were seen in the following parameters between control and infected birds: average concentration of semen at 4 w.p.i. (2.6 ± 0.42 vs. 1.0 ± 0.47 billion/ml), total motility of spermatozoa at 1 and 2 w.p.i. (92.4 ± 0.87% vs. 77.9 ± 6.50% and 92.3 ± 1.13% vs. 74.1 ± 6.67% respectively), progressive motility at 1 and 2 w.p.i. (85.8 ± 0.90% vs. 58.0 ± 9.39% and 81.2 ± 1.69% vs. 55.7 ± 8.62% respectively), percentage of membrane-intact spermatozoa at 1 w.p.i. (83.1 ± 3.78% vs. 71.9 ± 3.52%), and percentage of spermatozoa with impaired morphology at 3 w.p.i. (22.3 ± 0.00% vs. 27.5 ± 1.75%). Among the other motility characteristics, differences were significant for the amplitude of lateral head displacement (control: 2.1 ± 0.12 µm vs. infected: 2.4 ± 0.11 µm) and beat cross frequency (control: 41.0 ± 0.79 Hertz vs. infected: 38.3 ± 0.69 Hertz) at 3 and 4 w.p.i. respectively. Semen samples collected from all eight cockerels at 10 d.p.i. are shown in . Abnormal consistency of semen was visibly noticed in the first three samples from infected cockerels.

Discussion
Bacterial and viral infections of male reproductive organs have shown to be associated with poor reproductive performance of cockerels (Villarreal et al., Citation2007; Monleon et al., Citation2008). This underlines the necessity for a comprehensive knowledge on pathogenicity of disease-causing agents in order to maintain a healthy and productive breeding flock. With regard to G. anatis, an infection has been well acknowledged as a cause of severe problems in layers in natural cases as well as in experimental trials (Bojesen et al., Citation2007; Paudel et al., Citation2014). However, until now all studies were concentrated on layers and pullets. The present investigation is therefore the first of its kind, reporting clinical signs, bacterial distribution, pathological lesions as well as the influence of a G. anatis infection on semen quality parameters in cockerels.
Following G. anatis infection, birds did not show any clinical abnormalities or gross pathological lesions. Similar findings were previously reported also in non-mature male birds (Zepeda et al., Citation2010; Paudel et al., Citation2013). G. anatis could be re-isolated already at 3 d.p.i. from the choana. At 7 and 10 d.p.i., the highest re-isolation rates of the bacteria were found in the testis and epididymis, comparing all tested organs. G. anatis invaded the gonads until the end of the trial (38 d.p.i.). The nature of tenacity of the bacteria for long-lasting invasion of reproductive organs in older males is thus in agreement with data from young chicks (Paudel et al., Citation2013). In another study, Zepeda et al. (Citation2010) re-isolated G. anatis anatis and Gallibacterium genomospecies 2 from testis after experimental infection in 12-week-old birds. Thus, it might be a common tendency of the genus Gallibacterium to invade the reproductive organs in male chickens. Besides the reproductive tract, G. anatis was regularly isolated from the respiratory organs but very scantly from liver and heart and never from spleen. These findings are in agreement with earlier experimental studies (Zepeda et al., Citation2010; Paudel et al., Citation2013), thus it seems that the liver, spleen and heart are sites less targeted by G. anatis. Such differences in the preference of the bacteria to colonize local and systemic organs should be considered during sampling for diagnostics or in future experimental trials, so as not to miss the important sites of localization.
Histopathological lesions were noted in epididymis from 7 to 28 d.p.i. but not in testis. Studies in mammals showed that immune responses within the testis and epididymis are entirely different in several aspects and the risk of infection in the epididymis is greater than that in the testis. The testis is therefore considered an immunoprivileged site (Hedger, Citation2011). In field cases, the incidence of orchitis in cockerels is rarely reported and mostly associated with epididymal lesions as well (Villarreal et al., Citation2007; Monleon et al., Citation2008). In a previous study, we noted degenerative changes in the seminiferous tubules in young chicks; thus the age of the host seems to have an influence on occurrence of the lesions in testis after G. anatis infection (Paudel et al., Citation2013).
Unlike in mammals, spermatozoa are stored in female birds for a couple of days after mating in typical sperm storage tubules before entering the oviduct (Stepinska & Bakst, Citation2007). Afterwards, within a very short lapse of time, spermatozoa reach the infundibulum and penetrate the inner perivitalline membrane of an ovum for successful fertilization (Bakst, Citation1993). During this process, the fate of sperm to penetrate the ova is dependent on their concentration, motility and membrane function (McDaniel et al., Citation1998). These parameters are largely correlated with the fertilizing ability of spermatozoa (Wishart, Citation2009). In the present study, G. anatis had detrimental effects on all these parameters at different time points after experimental infection. Additionally, an increased number of morphologically defect spermatozoa was noticed. These findings highlight the negative impact of G. anatis on the breeding performance of cockerels. Haines et al. (Citation2013) demonstrated the role of six different bacteria on sperm motility of broiler breeders in vitro, with or without influence on pH. Similarly, we could find influences of G. anatis infection on semen quality without any alteration in pH of seminal fluid. Furthermore, presence of G. anatis and infiltration of inflammatory cells in epididymis should most probably be the cause of such deterioration in semen parameters. In humans, bacterial infection of the male genital tract results in immuno-inflammatory mechanisms that ultimately impair spermatozoal function (Comhaire et al. Citation1999). Such mechanisms need further elucidation in cockerels infected with G. anatis. In the present study, G. anatis was isolated from semen collected from infected cockerels. Previously, several other bacterial pathogens were also reported to be present in avian semen (Reiber et al., Citation1995; Cox et al., Citation2002; Cariou et al., Citation2013). Furthermore, the ability of Campylobacter and Salmonella spp. to attach to different segments of spermatozoa was demonstrated applying electron microscopy (Vizzier-Thaxton et al., Citation2006). Similar mechanisms can also be expected to exist in the case of G. anatis for bacterial contamination of sperms. Furthermore, it is also possible that sterile semen comes into contact with the bacteria while passing through the cloaca supported by the presence of the bacteria in the gut until termination of the study. However, colonization of testis and epididymis provides ample evidence for the presence of G. anatis in seminal fluid already in the reproductive tract. In breeding flocks, contamination of semen with G. anatis can lead to the transfer of the pathogen into hens during natural mating or artificial insemination and subsequently to the hatchlings as well.
In conclusion, the present investigation presents, for the first time, insights into the pathogenesis of an infection with G. anatis in mature male birds. It could be shown that G. anatis persistently invades the gonads causing epididymitis. Furthermore, the infection not only leads to decreased sperm quality but also has the potential for venereal transmission through infected semen.
Supplemental data
Supplemental data for this article can be accessed here.
Sperm_quality_parameters_from_control_and_infected_SPF_cockerels.docx
Download MS Word (16.8 KB)Acknowledgements
The authors would like to thank Boehringer Ingelheim for funding the project. The support from Dr Hermann Dietrich, Central Laboratory Animal Facilities, Innsbruck Medical University to become familiar with collecting semen is highly appreciated. The authors also thank Silvia Kluger from the Center for Artificial Insemination and Embryo Transfer, University of Veterinary Medicine Vienna for her excellent technical assistance in analysing semen.
References
- Alispahic, M., Christensen, H., Hess, C., Razzazi-Fazeli, E., Bisgaard, M. & Hess, M. (2011). Identification of Gallibacterium species by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry evaluated by multilocus sequence analysis. International Journal of Medical Microbiology, 301, 513–522. 10.1016/j.ijmm.2011.03.001
- Alispahic, M., Christensen, H., Hess, C., Razzazi-Fazeli, E., Bisgaard, M. & Hess, M. (2012). MALDI-TOF mass spectrometry confirms clonal lineages of Gallibacterium anatis between chicken flocks. Veterinary Microbiology, 160, 269–273. 10.1016/j.vetmic.2012.05.032
- Bakst, M. (1993). Oviducal sperm storage in poultry: a review. Reproduction, Fertility and Development, 5, 595–599. 10.1071/RD9930595
- Bojesen, A.M., Christensen, J.P. & Bisgaard, M. (2007). Gallibacterium infections and other avian Pasteurellaceae. In M. Pattison, P.F. McMullin, J.M. Bradbury & D.J. Alexander (Eds.). Poultry Diseases 6th edn (pp.160–163). Edinburgh: Saunders Elsevier.
- Bojesen, A.M., Nielsen, O.L., Christensen, J.P. & Bisgaard, M. (2004). In vivo studies of Gallibacterium anatis infection in chickens. Avian Pathology, 33, 145–152. 10.1080/03079450310001652059
- Bojesen, A.M., Nielsen, S.S. & Bisgaard, M. (2003). Prevalence and transmission of haemolytic Gallibacterium species in chicken production systems with different biosecurity levels. Avian Pathology, 32, 503–510. 10.1080/0307945031000154107
- Bojesen, A.M., Vazquez, M.E., Bager, R.J., Ifrah, D., Gonzalez, C., & Aarestrup, F.M. (2011). Antimicrobial susceptibility and tetracycline resistance determinant genotyping of Gallibacterium anatis. Veterinary Microbiology, 148, 105–110. 10.1016/j.vetmic.2010.08.011
- Cariou, N., Christensen, H., Salandre, O., Albaric, O., Bisgaard, M. & Malher, X. (2013). Genital form of pasteurellosis in breeding turkeys infected during artificial insemination and isolation of an unusual strain of Pasteurella multocida. Avian Diseases, 57, 693–697. 10.1637/10471-121812-Case.1
- Christensen, H., Bisgaard, M., Bojesen, A. M., Mutters, R. & Olsen, J.E. (2003). Genetic relationships among avian isolates classified as Pasteurella haemolytica, ‘Actinobacillus salpingitidis’ or Pasteurella anatis with proposal of Gallibacterium anatis gen. nov., comb. nov. and description of additional genomospecies within Gallibacterium gen. nov. International Journal of Systemic and Evolutionary Microbiology, 53, 275–287. 10.1099/ijs.0.02330-0
- Comhaire, F.H., Mahmoud, A.M., Depuydt, C.E., Zalata, A.A. & Christophe, A.B. (1999). Mechanisms and effects of male genital tract infection on sperm quality and fertilizing potential: the andrologist’s viewpoint. Human Reproduction Update, 5, 393–398. 10.1093/humupd/5.5.393
- Cox, N.A., Stern, N.J., Wilson, J.L., Musgrove, M.T., Buhr, R.J. & Hiett, K.L. (2002). Isolation of Campylobacter spp. from semen samples of commercial broiler breeder roosters. Avian Diseases, 46, 717–720. 10.1637/0005-2086(2002)046[0717:IOCSFS]2.0.CO;2
- Gauger, H.C. (1934). A chronic carrier of fowl typhoid with testicular focalization. Journal of American Veterinary Medical Association, 84, 248–251.
- Gerlach, H. (1994). Chlamydia. In B.W. Ritchie, G.J. Harrison, & L.R. Harrison (Eds.). Avian Medicine: Principles and Applications (pp.984–996). Lake Worth, FL: Wingers.
- Haines, M.D., Parker, H.M., McDaniel, C.D. & Kiess, A.S. (2013). Impact of 6 different intestinal bacteria on broiler breeder sperm motility in vitro. Poultry Science, 92, 2174–2181. 10.3382/ps.2013-03109
- Hedger, M.P. (2011). Immunophysiology and pathology of inflammation in the testis and epididymis. Journal of Andrology, 32, 625–640. 10.2164/jandrol.111.012989
- Kokosharov, T., Petkov, I. & Dzhurova, I. (1984). Cocks with experimentally induced acute typhoid. Veterinarno-meditsinski nauki, 21, 18–26.
- McDaniel, C., Hannah, J., Parker, H., Smith, T., Schultz, C. & Zumwalt, C. (1998). Use of a sperm analyzer for evaluating broiler breeder males. 1. Effects of altering sperm quality and quantity on the sperm motility index. Poultry Science, 77, 888–893. 10.1093/ps/77.6.888
- Monleon, R., Martin, M.P. & John Barnes, H. (2008). Bacterial orchitis and epididymo-orchitis in broiler breeders. Avian Pathology, 37, 613–617. 10.1080/03079450802499134
- Neubauer, C., De Souza-Pilz, M., Bojesen, A.M., Bisgaard, M. & Hess, M. (2009). Tissue distribution of haemolytic Gallibacterium anatis isolates in laying birds with reproductive disorders. Avian Pathology, 38, 1–7. 10.1080/03079450802577848
- Olsen, M.W. & Neher, B.H. (1948). The site of fertilization in the domestic fowl. Journal of Experimental Zoology, 109, 355–366. 10.1002/jez.1401090303
- Paudel, S., Alispahic, M., Liebhart, D., Hess, M. & Hess, C. (2013). Assessing pathogenicity of Gallibacterium anatis in a natural infection model: the respiratory and reproductive tracts of chickens are targets for bacterial colonization. Avian Pathology, 42, 527–535. 10.1080/03079457.2013.843160
- Paudel, S., Liebhart, D., Hess, M. & Hess, C. (2014). Pathogenesis of Gallibacterium anatis in a natural infection model fulfils Koch’s postulates: 1. Folliculitis and drop in egg production are the predominant effects in specific pathogen free layers. Avian Pathology, 43, 1–7.
- Reiber, M.A., McInroy, J.A. & Conner, D.E. (1995). Enumeration and identification of bacteria in chicken semen. Poultry Science, 74, 795–799. 10.3382/ps.0740795
- Schäfer-Somi, S. & Aurich, C. (2007). Use of a new computer-assisted sperm analyzer for the assessment of motility and viability of dog spermatozoa and evaluation of four different semen extenders for predilution. Animal Reproduction Science, 102, 1–13. 10.1016/j.anireprosci.2005.03.019
- Shalaby, A.A., Deeb, S., Sokkar, S.M., EI Begawi, M.B. & EI Menoufy, A.A. (1994). Pathological changes in the genitalia of cockerels experimentally infected with Salmonella. Egyptian Journal of Comparative Pathology and Clinial Pathology, 7, 443–452.
- Shaw, D.P., Cook, D.B., Maheswaran, S.K., Lindeman, C.J. & Halvorson, D.A. (1990). Pasteurella haemolytica as a co-pathogen in pullets and laying hens. Avian Diseases, 34, 1005–1008. 10.2307/1591397
- Stepinska, U. & Bakst, M.R. (2007). Fertilization. In B.G.M. Jamieson (Ed.). Reproductive Biology and Phylogeny of Birds: Phylogeny, Morphology, Hormones, Fertilization (Volume 6 A, pp. 553–587). Enfield: Science.
- Villarreal, L.Y.B., Brandão, P.E., Chacón, J.L., Assayag, M.S., Maiorka, P.C., Raffi, P., Saidenberg, A.B.S., Jones, R.C. & Ferreira, A.J.P. (2007). Orchitis in roosters with reduced fertility associated with avian infectious bronchitis virus and avian metapneumovirus infections. Avian Diseases, 51, 900–904. 10.1637/7815-121306-REGR4.1
- Vizzier-Thaxton, Y., Cox, N.A., Richardson, L.J., Buhr, R.J., McDaniel, C.D., Cosby, D.E., Wilson, J.L., Bourassa, D.V. & Ard, M.B. (2006). Apparent attachment of Campylobacter and Salmonella to broiler breeder rooster spermatozoa. Poultry Science, 85, 619–624. 10.1093/ps/85.4.619
- Wishart, G.J. (2009). Semen quality and semen storage. In P.M. Hocking (Ed.). Biology of Breeding Poultry, Poultry Science Symposium Series (Volume 29, pp. 151–161). Wallingford: CAB International.
- Zepeda, V.A., Calderón-Apodaca, N.L., Paasch, M.L., Martín, P.G., Paredes, D.A., Ramírez-Apolinar, S. & Soriano-Vargas, E. (2010). Histopathologic findings in chickens experimentally infected with Gallibacterium anatis by nasal instillation. Avian Diseases, 54, 1306–1309. 10.1637/9423-061410-ResNote.1