Abstract
We compared the clinical signs, histopathological lesions and distribution of viral antigens among infected young (meat-type) and older (breeder) ducks that were naturally infected with the highly pathogenic avian influenza (HPAI) virus during the 2010–2011 Korean outbreak. The meat-type ducks had a high mortality rate (30%) and showed severe neurological signs such as head tremors and paresis. In contrast, HPAI-infected breeder ducks had minimal clinical signs but a decreased egg production rate. The histopathological characteristics of infected meat-type ducks included necrotic lesions of heart and brain, which may have primarily contributed to the high mortality rate. In contrast, the breeder ducks only presented necrotic splenitis, and viral antigens were only detected in the trachea, lungs and spleen. Younger ducks had a high viral titre in the organs, high levels of viral shedding and a high mortality rate after experimental HPAI virus infection. Compared to the breeder ducks, the meat-type ducks were raised in smaller farms that had poor quarantine and breeding facilities. It is therefore possible that better biosecurity in the breeder farms could have reduced the infection dose and subsequently the severity of the disease. Thus, age and management may be the influencing factors for HPAI susceptibility in ducks.
Introduction
Influenza virus, a segmented negative-sense RNA virus, is a member of the Orthomyxoviridae family that includes the genera influenza virus A, B and C. Among the three influenza virus types, the influenza type A virus is known to infect several avian and mammalian species (Webster et al., Citation1992; Sonnberg et al., Citation2013). The influenza type A virus genome consists of eight segments of linear negative-sense, single-stranded RNA that encode 11 viral proteins including the envelope glycoprotein, haemagglutinin (HA), which plays an important role in host invasion (Neumann et al., Citation2007).
Ducks serve as viral reservoirs for highly pathogenic avian influenza (HPAI) virus. Previously, both wild and domestic ducks were not typically associated with severe clinical signs (Alexander et al., Citation1986; Perkins & Swayne, Citation2002; Guan & Smith, Citation2013). However, H5N1 HPAI virus was isolated from waterfowl in Hong Kong and was shown to be pathogenic to ducks (Sturm-Ramirez et al., Citation2004).
In South Korea, there were four H5N1 HPAI outbreaks following the first incidence of viral infection that occurred during 2003–2004. During these outbreaks, increased mortality and severe clinical lesions associated with HPAI infection have been reported in ducks (Woo et al., Citation2011; Choi et al., Citation2013). The duration of the last HPAI outbreak was longer than that of the previously documented outbreak, and 2,788,388 ducks (546,526 breeder ducks and 2,241,862 meat-type ducks) were killed for stamping out infected areas and for disinfection. During the outbreak, carcasses from 27 HPAI-infected farms were submitted to the Animal and Plant Quarantine Agency (QIA) for diagnosis. These duck submissions included a history of distinct clinical signs that varied in severity observed by the veterinarians of the regional Veterinary Service Laboratory. The younger ducks (meat-type ducks) appeared to show more severe clinical signs and gross and histopathological lesions than the older ducks (breeder ducks). Therefore, we investigated the differences in HPAI susceptibility of the meat-type and breeder ducks by evaluating the clinical signs, gross findings, histopathological lesions and distribution of HPAI antigens in HPAI-infected meat-type and breeder ducks.
Materials and Methods
Birds
During the HPAI outbreak period from December 2010 to July 2011, a total of 588 duck submissions from 69 farms located in 6 provinces were examined for HPAI infections, and 220 duck submissions from 27 farms were found to be infected with HPAI. All submissions were identified as Pekin ducks, which are used for egg and meat production.
The farms were mainly located in the western part of South Korea and were in close proximity to the migratory bird habitats. The migratory birds routinely fly over the duck farms and the droppings may have been one of the major sources of HPAI during the Korean HPAI outbreak (Kim et al., Citation2012).
For virus isolation, tissues from trachea, caecal tonsil and kidney were used. Homogenized organs were centrifuged at 890 x g for 10 min. Filtered supernatants were inoculated into 9- to 11-day-old embryonated chicken eggs. Inoculated eggs were incubated at 37°C for 4 days, and the presence of avian influenza (AI) virus was tested by performing the haemagglutination assay. H5 of AI virus was subtyped by performing reverse transcription-polymerase chain reaction, and N1 of AI virus was subtyped by using neuraminidase inhibition test with reference antisera (Van Deusen et al., Citation1983; Munch et al., Citation2001). To confirm the HPAI diagnosis, H5 sequence analysis was used as previously described (Kim et al., Citation2010).
Each bird was examined by performing necropsy, and the organs (trachea, lung, heart, liver, spleen, kidney, pancreas, intestine and brain) were collected for histopathology and immunohistochemistry analyses.
Histopathology and influenza A immunohistochemistry
The collected tissues and organs were fixed in 10% buffered formalin for at least 24 h, processed routinely and embedded in paraffin. The paraffin-embedded tissues were sectioned into 2.5-µm-thick sections and stained with haematoxylin and eosin (H&E).
Immunohistochemistry was performed by staining the tissue sections with a monoclonal mouse antibody against influenza A virus nucleoprotein (Serotec, Kidlinton, UK, MCA 400) and the Discovery Red kit (Ventana Medical Systems, AZ, USA), and the stained samples were assessed by using a fully automatic immunohistochemical system (Ventana Discovery XT; Ventana Medical Systems), according to the manufacturer's instructions. Briefly, the paraffin sections were deparaffinized at 75°C for 8 min, antigens retrieved by Protease1 treatment (Ventana Medical Systems) at 37°C for 8 min and incubated for 32 min at room temperature (RT) with monoclonal anti-Influenza A antibody that was diluted 1:3000 using an antibody diluent (Dako North America, Inc., Carpinteria, CA, USA). The antigens–antibody complex was visualized by staining with a universal secondary antibody (Ventana Medical Systems) (at 37°C for 12 min at RT) and by using the Discovery Red kit (Ventana Medical Systems). Finally, the sections were counterstained with haematoxylin (4 min, RT) (Ventana Medical Systems, USA) and with a bluing solution (4 min, RT; Ventana Medical Systems, USA) and used for microscopic examination.
Statistical analysis
Gross and histopathological lesions, and immunohistochemical staining data were analysed using one-tailed Fisher's exact test to establish whether differences between meat-type duck group and breeder duck group were significant. P values of < 0.05 were considered statistically significant.
Results
Case history
The case history of meat-type and breeder ducks is summarized in . The case history was provided by veterinarians in the regional Veterinary Service Laboratory.
Table 1. Clinical signs of H5N1 HPAI virus-infected domestic ducks during the 2010–2011 Korean outbreak.
The mortality rates of the meat-type ducks reached 30%, and 2–3% of ducks died per day before diagnosis and culling. Five cases of meat-type ducks showed decreased feed consumption and water intake. Infected ducks from 10 farms died without exhibiting any specific clinical signs. However, half of the cases presented nervous, respiratory and digestive clinical signs. Neurological signs of paresis, tremor or unusual position of head and neck were observed in six out of the 18 cases. In addition, coughing (two cases) and diarrhoea (four cases) were also observed.
Analysis of clinical signs of the breeder ducks indicated that decreased egg production rate from 70% to 15% was the characteristic clinical sign of HPAI infection. However, there was no reported increase in mortality, or specific clinical signs including neurological and respiratory signs. Diarrhoea was reported in one case only. Four cases of breeder ducks showed decreased feed consumption rate and water intake.
Gross findings
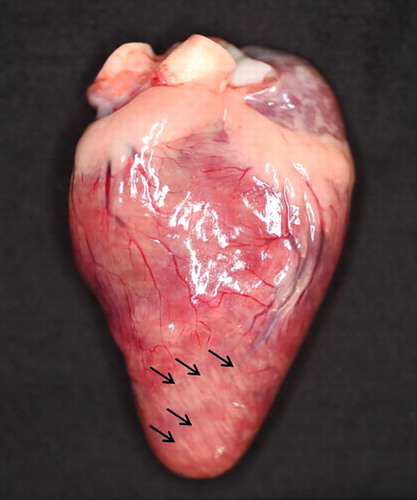
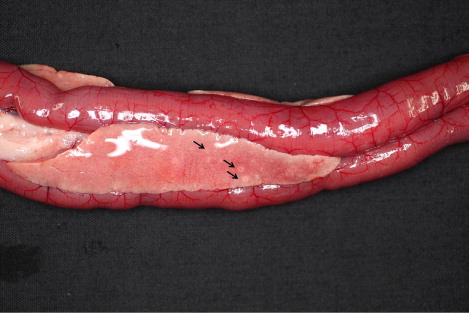
The gross findings of meat-type and breeder ducks are summarized in . Necropsy analysis of the organs of HPAI-infected meat-type ducks showed severe haemorrhagic lesions and various sized white to grey foci. Petechial haemorrhages in the epicardium (five cases), friable and enlarged or ruptured liver (eight cases) and bilateral congested lung (five cases) were recorded. Five cases showed uric acid depositions on the epicardium, and two cases showed white striations on the myocardium (). A flaccid heart with increased pericardial fluid (three cases) was also observed. Some HPAI-infected meat-type ducks showed variable sized white or grey foci on the pancreas (seven cases; ) and an enlarged spleen (seven cases).
Table 2. Gross findings of HPAI virus-infected domestic ducks in the 2010–2011 outbreak.
In breeder ducks, ovarian follicle lesions including haemorrhages, abnormal shapes or ruptures (five cases) were observed. In addition, enlargement of the liver (five cases) and spleen (seven cases) was noted. Petechial haemorrhage in the epicardium (three cases) and white foci on spleen (three cases) were also present.
The analysis of the gross findings revealed a significant difference of the number of white or grey foci of pancreas between the meat-type and breeder ducks (P = 0.0358).
Histopathological findings
The histological findings of meat-type and breeder ducks are summarized in . The histopathology scores were assigned according to the severity as mild (+), moderate (++) or severe (+++).
Table 3. Histopathological findings of HPAI virus-infected domestic ducks in the 2010–2011 outbreak.
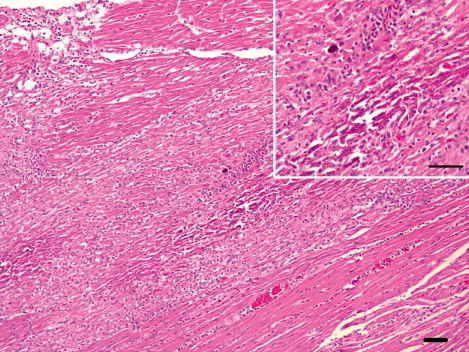
In the meat-type ducks, the brain showed moderate-to-severe lymphocytic meningoencephalitis that was characterized by perivascular cuffs (13 cases), neuronal degeneration and multifocal gliosis, especially in the cerebrum. In nine cases, there was multifocal encephalomalacia (). These lesions were also observed in the cerebellum and brain stem. The heart showed moderate-to-severe lymphohistiocytic myocarditis (10 cases) with haemorrhages and myocardial necrosis (four cases). Myocardial calcification was observed in two severe heart lesions (). The pancreas had multifocal necrosis (10 cases) with vacuolation of acinar epithelium and infiltration of the mononuclear cells in the parenchyma. In addition, mild infiltration of lymphocytes and macrophages in the periphery of portal triades, and multifocal hepatic necrosis were observed (six cases; ). In the spleen, there was mild-to-moderate lymphoid depletion in the periellipsoid region and necrosis in the red pulp (nine cases). Interstitial pneumonia with severe congestion and haemorrhages was also observed.
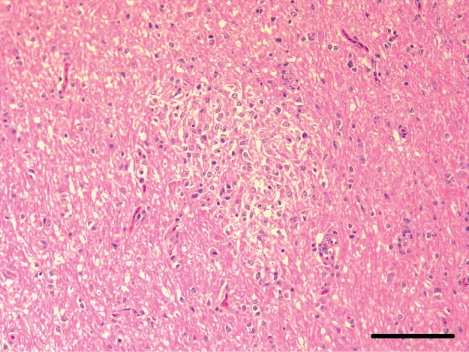
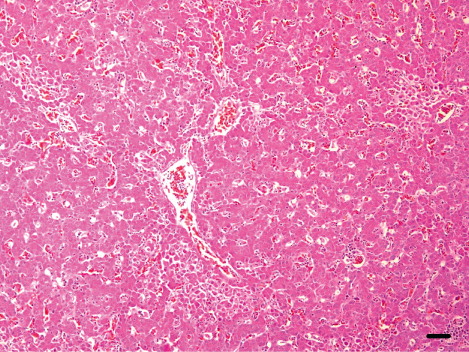
In the breeder ducks, the organs showed minor non-specific lesions. Moderate lymphoid depletion in the periellipsoid region and necrotic splenitis in the red pulp was detected in four cases (), and histopathological lesions including multifocal necrotic lesions in the brain, heart, liver and pancreas were seen in one case.
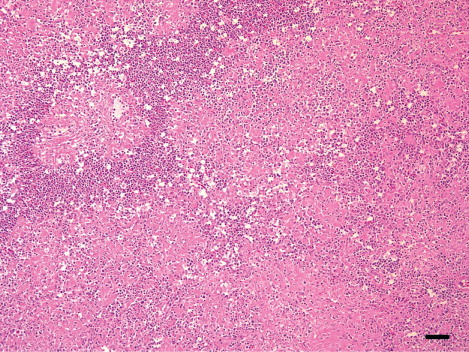
There were significant differences in the scores for necrotic lesions found in cerebrum (P = 0.0040), cerebellum (P = 0.0024), brain stem (P = 0.0175), heart (P = 0.0326), pancreas (P = 0.0326) and interstitial pneumonia (P = 0.0194) between the meat-type and breeder ducks.
Distribution of viral antigens
The distribution of AI antigens in various tissues of the infected ducks is summarized in . Immunostaining was scored based on the number of positive staining as few (+), moderate numbers (++) or numerous (+++). The viral antigens were detected in association with lesions, but in a few cases, the antigens were seen in organs without a detectable lesion.
Table 4. Distribution of immunohistochemically assessed influenza viral antigens in samples obtained from HPAI virus-infected domestic ducks in the 2010–2011 outbreak.
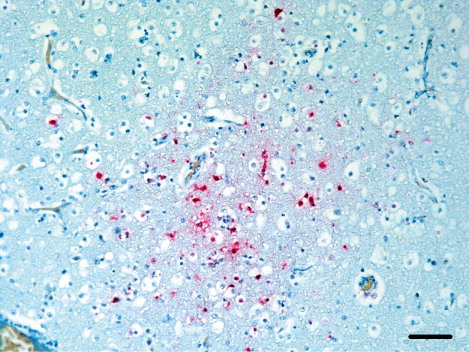
In the meat-type ducks, the viral antigens were predominantly found in the brain tissue. In 14 cases diffuse influenza antigens were detected in various brain cells including neurons, glial cells and Purkinje cells (). Choroid plexus and ependymal cells associated with the cerebral ventricle circling the cerebrospinal fluid showed strong staining. Lesions associated with influenza viral antigens were abundant in association with meningitis and focal malacia. The epithelial cells and mononuclear cells in the lamina propria of the trachea (seven cases), bronchial epithelial cells, type II pneumocytes and histiocytes in lung (14 cases) were frequently stained with the viral antigens detection antibody. Kupffer cells (four cases), pulmonary macrophages and mononuclear cells of red pulp, but not the lymphocytes of the white pulp in the spleen (12 cases), were also stained. Viral antigens were distributed diffusely in myocardial cells and lymphocytes (five cases) that were located around the region with myocardial necrosis or myocarditis () and in acinar epithelium of pancreas (six cases). Hepatocytes, renal tubular epithelium and vascular endothelial cells in various organs showed poor staining for viral antigens.
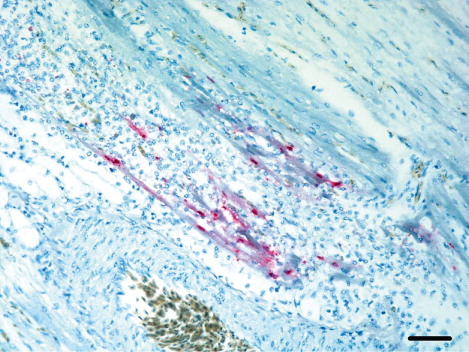
In breeder ducks, the influenza antigens were not present as extensively in tissues as in the meat-type ducks. Six breeder duck cases had viral antigens in mononuclear cells of the red pulp in spleen, and two cases, in the lymphocytes of the white pulp.
Statistical analysis showed that the occurrence of immunostained cells in brain were significantly different between the meat-type and breeder ducks (P = 0.0341).
Discussion
HPAI reservoirs are commonly present in ducks and are not typically associated with disease development in the birds. However, the recent increase in duck fatalities due to HPAI infections indicate that ducks do not merely serve as transmission vectors for HPAI (Capua & Mutinelli, Citation2001; Sturm-Ramirez et al., Citation2004). Several experimental infection studies were performed to investigate the effects of HPAI infection on ducks. The studies showed that Asian HPAI virus strains were associated with severe clinical signs in ducks (Vascellari et al., Citation2007; Yamamoto et al., Citation2007; Bingham et al., Citation2009). Previous studies also have shown that younger ducks had a high virus titre in the organs, high levels of viral shedding and a high mortality rate after HPAI infection (Kwon et al., Citation2005; Pantin-Jackwood et al., Citation2007; Aiello et al., Citation2013). Pantin-Jackwood suggested that the age-related differences in disease susceptibility may be due to the age-related differences in innate immune responses induced by HPAI virus infection (Pantin-Jackwood et al., Citation2012). However, in the 2008 HPAI outbreak in Korea, the duck mortality rate was not associated with age, with the mortality rate being 50% in adult ducks (Woo et al., Citation2011).
In 2010–2011 HPAI outbreaks, carcasses obtained from 27 duck farms, which included nine breeder duck farms and 18 meat-type duck farms, tested positive for HPAI. The meat-type ducks had a moderate mortality rate (up to 30%) and presented severe neurological signs such as head tremors and paresis. Furthermore, multifocal to diffuse necrotic lesions were presented in various parenchymal organs such as brain, heart, spleen, liver and pancreas of the meat-type duck. In contrast, breeder ducks did not exhibit any severe clinical signs for the infection and only presented decreased egg production and necrotic splenitis. Statistical analysis showed that necrotic lesions of the brain, heart and pancreas were significantly different between the meat-type and breeder ducks (P value < 0.05).
The duck submissions during the HPAI outbreak were used to isolate and sequence 27 representative H5N1 HPAI viruses including five isolates from meat-type and breeder ducks. All isolated H5N1 viruses during the 2010–2011 HPAI outbreak showed high sequence homology and had a high haemagglutinin gene homology (>99.5%; Kim et al., Citation2012). Although there have been several publications in the literature on the effects of age, they were all in experimentally infected birds. In contrast, the present study investigated the influence of age and management on disease outcome in the natural habitat.
The genetic background of the ducks and the management practice of duck farms may be contributing factors for the duck disease susceptibility. We assessed whether either or both these factors influenced the duck disease susceptibility in our study.
In 2010, no grand parent stocks of ducks existed in South Korea. Therefore most of the parent stocks (Pekin ducks) used in farms were imported from Cherry Valley Farms Ltd, UK, and Grimaud Farms, France. Both the breeder and meat-type ducks were determined to have the same breed and therefore had similar genetic background. However, as compared to the breeder ducks, the meat-type ducks were raised in smaller farms that had poor quarantine and breeding facilities. Although the duck housing conditions of farms varied, many meat-type duck farms were built of vinyl plastic and with non-automatic facilities. Poor conditions and management systems likely resulted in higher stress in meat-type ducks than in breeder ducks. In the 2010–2011 Korean HPAI outbreak, droppings of the migratory birds were a major sources of HPAI (Kim et al., Citation2012), and the movement of vehicles or feedstuffs containing the AI virus may have spread the disease. Based on these results, better biosecurity and less stressful environment in breeder ducks may reduce the opportunity for infection, thereby reducing the infection dose and subsequently the severity of the disease.
Additionally, better facilities may enable farmers to notice AI infection earlier. Diseased ducks including AI infection often show decreased feed consumption and water intake. Modernized facilities can measure feed consumption quantity easily, and a sudden decrease in feeding may serve as a marker of infection. In our case, a higher proportion of breeder duck farms noticed decreased feed consumption and water intake compared to meat-type duck farms. Naturally, HPAI infection can result in the death of ducks without any other clinical signs. Considering the increased pathogenicity of HPAI in ducks, measurement of the quantity of food or water consumption is an important way to predict disease. Therefore, early detection and reporting could likely have decreased the lesion severity in breeder ducks during the 2010–2011 HPAI outbreak.
A comparative analysis of the histopathology data from the previous outbreaks in meat-type ducks indicates that the HPAI virus may have gradually adapted to the host (duck). In the third HPAI outbreak in 2008, the ducks had lesions that were more advanced and broadly distributed than those in ducks that were infected in the two previous Korean outbreaks (Woo et al., Citation2011). In the 2010 HPAI outbreak, there was an increased development of histopathological lesions in the heart and the brain, respectively. Extensive necrotic myocarditis with calcification, which was a major contributing factor for the increased mortality in 2008 HPAI cases, was found frequently in the present study. However, encephalomalacia was not reported in the 2008 outbreak and was observed in several cases in the 2010 HPAI outbreak. These data indicate that duck fatality may be related to nervous or circulatory system failure with virus-associated necrotic lesions.
In a previous study, Swayne showed that AI virus is carried by vascular endothelial cells in chicken, which leads to altered permeability of blood vessels. This can lead to haemorrhage, oedema, and microthrombosis, which can result in death by multi-organ failure as a result of endothelial tropism (Swayne, Citation2007). However, there were different views for HPAI pathogenesis in ducks because lesions of vascular damage were rarely observed in previous HPAI experimental infection studies in ducks (Kuiken et al., Citation2010; Pantin-Jackwood et al., Citation2012). Multiple virus-associated necrotic lesions in one or more organs (pancreas, heart, brain) were responsible for death, and tissue tropism has also been observed in avian species such as mute swans, ruddy shelducks and mandarin ducks (Kwon et al., Citation2010). The data from this study also showed negligible vascular damage lesions and absence of viral antigens in the vascular endothelial cells in 220 ducks.
In the 2010–2011 HPAI outbreak, meat-type ducks showed more severe clinical signs and pathological lesions than breeder ducks. In our study, the age of ducks was associated with HPAI susceptibility, and the disease susceptibility of the younger ducks was higher than that of older ducks. Additionally, farm management practices could influence the transmission and disease susceptibility of the ducks. Further studies are required to investigate the HPAI infection mechanisms in breeder ducks, and modernized facilities should be equipped to decrease the damage associated with AI infection.
Acknowledgements
We thank Mr Jung-Won Park and Mr Jong-Hyeong Lee, Laboratory of Pathology, Animal Disease Diagnostic Division, QIA, for their technical assistance.
Additional information
Funding
References
- Aiello, R., Beato, M.S., Mancin, M., Rigoni, M., Tejeda, A.R., Maniero, S., Capua, I. & Terregino, C. (2013). Differences in the detection of highly pathogenic avian influenza H5N1 virus in feather samples from 4-week-old and 24-week-old infected Pekin ducks (Anas platyrhynchos var. Domestica). Veterinary Microbiology, 165, 443–447. 10.1016/j.vetmic.2013.03.019
- Alexander, D.J., Parsons, G. & Manvell, R.J. (1986). Experimental assessment of the pathogenicity of eight avian influenza a viruses of H5 subtype for chickens, turkeys, ducks and quail. Avian Pathology, 15, 647–662. 10.1080/03079458608436328
- Bingham, J., Green, D.J., Lowther, S., Klippel, J., Burggraaf, S., Anderson, D.E., Wibawa, H., Hoa, D.M., Long, N.T., Vu, P.P., Middleton, D.J. & Daniels, P.W. (2009). Infection studies with two highly pathogenic avian influenza strains (Vietnamese and Indonesian) in Pekin ducks (Anas platyrhynchos), with particular reference to clinical disease, tissue tropism and viral shedding. Avian Pathology, 38, 267–278. 10.1080/03079450903055371
- Capua, I. & Mutinelli, F. (2001). Mortality in Muscovy ducks (Cairina moschata) and domestic geese (Anser anser var. Domestica) associated with natural infection with a highly pathogenic avian influenza virus of H7N1 subtype. Avian Pathology, 30, 179–183. 10.1080/03079450120044597
- Choi, J.-G., Kang, H.-M., Jeon, W.-J., Choi, K.-S., Kim, K.-I., Song, B.M., Lee, H.-S., Kim, J.-H. & Lee, Y.-J. (2013). Characterization of clade 2.3.2.1 H5N1 highly pathogenic avian influenza viruses isolated from wild birds (mandarin duck and Eurasian eagle owl) in 2010 in Korea. Viruses, 5, 1153–1174. 10.3390/v5041153
- Guan, Y. & Smith, G.J.D. (2013). The emergence and diversification of panzootic H5N1 influenza viruses. Virus Research, 178, 35–43. 10.1016/j.virusres.2013.05.012
- Kim, H.-R., Lee, Y.-J., Park, C.-K., Oem, J.-K., Lee, O.-S., Kang, H.-M., Choi, J.-G. & Bae, Y.-C. (2012). Highly pathogenic avian influenza (H5N1) outbreaks in wild birds and poultry, South Korea. Emerging Infectious Diseases, 18, 480–483. 10.3201/1803.111490
- Kim, H.-R., Park, C.-K., Lee, Y.-J., Woo, G.-H., Lee, K.-K., Oem, J.-K., Kim, S.-H., Jean, Y.-H., Bae, Y.-C., Yoon, S.-S., Roh, I.-S., Jeong, O.-M., Kim, H.-Y., Choi, J.-S., Byun, J.-W., Song, Y.-K., Kwon, J.-H. & Joo, Y.-S. (2010). An outbreak of highly pathogenic H5N1 avian influenza in Korea, 2008. Veterinary Microbiology, 141, 362–366. 10.1016/j.vetmic.2009.09.011
- Kuiken, T., van den Brand, J., van Riel, D., Pantin-Jackwood, M. & Swayne, D.E. (2010). Comparative pathology of select agent influenza A virus infections. Veterinary Pathology, 47, 893–914. 10.1177/0300985810378651
- Kwon, Y.-K., Joh, S.-J., Kim, M.-C., Sung, H.-W., Lee, Y.-J., Choi, J.-G., Lee, E.-K. & Kim, J.-H. (2005). Highly pathogenic avian influenza (H5N1) in the commercial domestic ducks of South Korea. Avian Pathology, 34, 367–370. 10.1080/03079450500181257
- Kwon, Y.K., Thomas, C. & Swayne, D.E. (2010). Variability in pathobiology of South Korean H5N1 high-pathogenicity avian influenza virus infection for 5 species of migratory waterfowl. Veterinary Pathology, 47, 495–506. 10.1177/0300985809359602
- Munch, M., Nielsen, L.P., Handberg, K.J. & Jørgensen, P.H. (2001). Detection and subtyping (H5 and H7) of avian type A influenza virus by reverse transcription-PCR and PCR-ELISA. Archives of Virology, 146, 87–97. 10.1007/s007050170193
- Neumann, G., Shinya, K. & Kawaoka, Y. (2007). Molecular pathogenesis of H5N1 influenza virus infections. Antiviral Therapy, 12, 617–626.
- Pantin-Jackwood, M.J., Smith, D.M., Wasilenko, J.L., Cagle, C., Shepherd, E., Sarmento, L., Kapczynski, D.R. & Afonso, C. L. (2012). Effect of age on the pathogenesis and innate immune responses in Pekin ducks infected with different H5N1 highly pathogenic avian influenza viruses. Virus Research, 167, 196–206. 10.1016/j.virusres.2012.04.015
- Pantin-Jackwood, M.J., Suarez, D.L., Spackman, E. & Swayne, D.E. (2007). Age at infection affects the pathogenicity of Asian highly pathogenic avian influenza H5N1 viruses in ducks. Virus Research, 130, 151–161. 10.1016/j.virusres.2007.06.006
- Perkins, L.E. & Swayne, D.E. (2002). Pathogenicity of a Hong Kong–origin H5N1 highly pathogenic avian influenza virus for emus, geese, ducks, and pigeons. Avian Diseases, 46, 53–63. 10.1637/0005-2086(2002)046[0053:POAHKO]2.0.CO;2
- Sonnberg, S., Webby, R.J. & Webster, R.G. (2013). Natural history of highly pathogenic avian influenza H5N1. Virus Research, 178, 63–77. 10.1016/j.virusres.2013.05.009
- Sturm-Ramirez, K.M., Ellis, T., Bousfield, B., Bissett, L., Dyrting, K., Rehg, J.E., Poon, L., Guan, Y., Peiris, M. & Webster, R.G. (2004). Reemerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks. Journal of Virology, 78, 4892–4901. 10.1128/JVI.78.9.4892-4901.2004
- Swayne, D.E. (2007). Understanding the complex pathobiology of high pathogenicity avian influenza viruses in birds. Avian Diseases, 51, 242–249. 10.1637/7763-110706-REGR.1
- Van Deusen, R.A., Hinshaw, V.S., Senne, D.A. & Pellacani, D. (1983). Micro neuraminidase-inhibition assay for classification of influenza A virus neuramidases. Avian Diseases, 27, 745–750. 10.2307/1590317
- Vascellari, M., Granato, A., Trevisan, L., Basilicata, L., Toffan, A., Milani, A. & Mutinelli, F. (2007). Pathologic findings of highly pathogenic avian influenza virus A/Duck/Vietnam/12/05 (H5N1) in experimentally infected Pekin ducks, based on immunohistochemistry and in situ hybridization. Veterinary Pathology, 44, 635–642. 10.1354/vp.44-5-635
- Webster, R.G., Bean, W.J., Gorman, O.T., Chambers, T.M. & Kawaoka, Y. (1992). Evolution and ecology of influenza a viruses. Microbiology and Molecular Biology Reviews, 56, 152–179.
- Woo, G.H., Kim, H.Y., Bae, Y.C., Jean, Y.H., Bak, E.J., Kim, M.J., Hwang, E.K. & Joo, Y.S. (2011). Comparative histopathological characteristics of highly pathogenic avian influenza (HPAI) in chickens and domestic ducks in 2008 Korea. Histology and Histopathology, 26, 167–175.
- Yamamoto, Y., Nakamura, K., Kitagawa, K., Ikenaga, N., Yamada, M., Mase, M. & Narita, M. (2007). Severe nonpurulent encephalitis with mortality and feather lesions in call ducks (Anas platyrhyncha var. Domestica) inoculated intravenously with H5N1 highly pathogenic avian influenza virus. Avian Diseases, 51, 52–57. 10.1637/0005-2086(2007)051[0052:SNEWMA]2.0.CO;2