Abstract
Valsa melanodiscus (anamorph Cytospora umbrina) has been associated with disease-related mortality of a riparian shrub, thinleaf alder (Alnus tenuifolia), across Alaska. The disease is characterized by diffuse, longitudinal stem cankers. Similar disease symptoms have also been observed on Siberian alder (Alnus fruticosa) in upland and alpine habitats. However, canker-related mortality on A. fruticosa appears to be much lower than on A. tenuifolia. We conducted a field inoculation study in upland, mixed stands to test whether the same pathogen species causing mortality in A. tenuifolia can incite disease on A. fruticosa. Because A. fruticosa can be subjected to water stress along roadsides, we also compared disease predisposition and susceptibility in roadside versus forested habitats. In July 2008, we inoculated A. fruticosa by wounding the stem and placing a colonized agar plug over the exposed inner bark and sapwood. After one year, V. melanodiscus was cultured from 91.5% of the inoculated stems. Fifty-five per cent of the inoculated stems developed sunken elliptical cankers, while wounded control stems produced callus that closed the wound. Percentage stem girdle was typically < 10%, and canker length was limited to 5 mm or less in the majority of inoculated stems. However, A. fruticosa stems inoculated along a south-facing roadside had more severe symptoms (cankers 10 to 13 mm in length). Our study suggests that disease-related mortality in upland, mixed stands is unlikely for A. fruticosa, but that site conditions associated with increased vulnerability to water stress may be related to disease susceptibility.
Résume
En Alaska, Valsa melanodisus (forme imparfaite de Cytospora umbrina) a été associé à la mort causée par la maladie d'un arbuste riverain, l'aulne à feuilles minces (Alnus tenuifolia). La maladie est caractérisée par des chancres diffus et longitudinaux de la tige. Des symptômes semblables ont également été observés sur l'aulne de Sibérie (Alnus fruticosa) dans les milieux secs et alpins. Toutefois, le taux de mortalité découlant du chancre chez A. fruticosa est beaucoup plus faible que chez A. tenuifolia. Nous avons mené une étude, basée sur des inoculations, dans les peuplements mixtes des milieux secs afin de vérifier si les mêmes espèces d'agents pathogènes causant la mort chez A. tenuifolia pouvaient provoquer la maladie chez A. fruticosa. Parce que A. fruticosa peut souffrir de stress hydrique le long des routes, nous avons également comparé la sensibilité et la prédisposition à la maladie le long des routes et en forêt. En juillet 2008, nous avons inoculé A. fruticosa en plaçant des disques d'agar colonisés sur l'écorce interne et l'aubier exposés de tiges blessées. Au bout d'un an, V. melanodisus a été cultivé à partir de 91,5 % des tiges inoculées. Cinquante-cinq pour cent des tiges inoculées avaient développé des chancres elliptiques creux, tandis que les tiges blessées témoins avaient produit des cals de cicatrisation. Chez la majorité des tiges inoculées, le pourcentage du cerne de la tige était typiquement de 10 % et la longueur du chancre, de 5 mm ou moins. Toutefois, les tiges d'A. fruticosa inoculées poussant le long de routes exposées au sud affichaient des symptômes plus graves (chancres de 10 à 13 mm de longueur). Notre étude suggère que la mort d'A. fruticosa causée par la maladie dans les peuplements mixtes des milieux secs est peu probable, mais que les conditions du site associées à une vulnérabilité accrue au stress hydrique peuvent être liées à la sensibilité à la maladie.
Introduction
A canker disease epidemic on Alnus incana (L.) Moench ssp. tenuifolia (Nutt.) Breitung (hereafter, Alnus tenuifolia), has been documented throughout the Rocky Mountain West since the late 1980s (Worrall, Citation2009) and has recently been documented in Alaska (USDA, Citation2009; USDA, Citation2010). Alaskan surveys indicate that thousands of hectares of dieback and mortality of A. tenuifolia are related to the occurrence of Cytospora canker disease. The canker disease is most severe on A. tenuifolia (Ruess et al., Citation2009; USDA, Citation2011), which colonizes floodplains and riparian areas, but similar disease symptoms have also been observed on the Siberian alder (Alnus viridis (Chaix) DC. ssp. fruticosa (Rupr.) Nyman; hereafter, Alnus fruticosa), which inhabits upland, later successional environments (USDA, Citation2011). Alder canker mortality on A. fruticosa has been detected along roadsides, in natural clearings, and alpine environments up to 457 m in elevation (USDA, Citation2011). Both Alnus (L.) species are symbiotic nitrogen fixers and are responsible for high N-inputs in Alaskan ecosystems (Uliassi & Ruess, Citation2002; Mitchell & Ruess, Citation2009).
The Cytospora canker disease of alder is characterized by slightly sunken cankers that extend longitudinally along the stem, resulting in girdling and dieback (Worrall et al., Citation2010; Stanosz et al., Citation2011). The disease has been frequently associated with Valsa melanodiscus G.H. Otth (but named Cytospora canker for its more commonly observed anamorph Cytospora umbrina Bornord. (Sacc.)) (Adams, Citation2008), which typically colonizes and kills the inner bark (bark periderm) and vascular tissues (Adams et al., Citation2009). Asexual Cytospora fruiting bodies consist of clusters of pycnidial conidiomata or conidiomata that contain labyrinthine chambers (Adams et al., Citation2006). The most obvious signs of Cytospora canker are abundantly produced conidiomata on canker faces, and, in moist conditions, exuding conidia in a gelatinous matrix of orange or cream coloured tendrils (cirrhi) (Adams et al., Citation2005, Citation2006). However, identification of a fungus as a particular Cytospora Ehrenb. or Valsa Fr. species based on the fruiting bodies is difficult because the morphology of the anamorph and teleomorph can differ according to characteristics of the host bark and cambium (Adams et al., Citation2005).
Despite their prevalence as canker fungi on over 85 species of woody hosts (Sinclair et al., Citation1987), most Cytospora species have not been tested for pathogenicity and the parasitic nature of some members of the genus is unclear (Adams et al., Citation2005). Cytospora species can be very destructive pathogens on certain groups of plants, including cultivated species of Prunus L. (Adams et al., Citation2006) and Populus L. (Kepley & Jacobi, Citation2000). However, Cytospora species can also inhabit asymptomatic living plants (Chapela, Citation1989; Adams et al., Citation2005), be strictly saprophytic on dying trees (Christensen, Citation1940), or become facultative parasites on plants weakened by extensive wounding or stress (Christensen, Citation1940; Kamiri & Laemmlen, Citation1981; Filip et al., Citation1992; Adams et al., Citation2005).
Given the variation in the parasitic nature of Cytospora species, only responses to inoculation can be used to determine if a certain Cytospora species is able to incite and cause disease on a particular host plant. Following the Cytospora canker epidemic in Alaska, V. melanodiscus was confirmed to be pathogenic on A. tenuifolia (Stanosz et al., Citation2011). However, whether A. fruticosa is included among hosts of this pathogen has yet to be confirmed. Therefore, the first objective of this study was to conduct an inoculation trial to determine whether the same pathogen (V. melanodiscus) associated with canker disease and extensive mortality in A. tenuifolia can also incite disease in A. fruticosa.
The second objective of this study was to evaluate whether site location, along roadsides or within wooded areas, could be related to predisposition and susceptibility of A. fruticosa to the canker disease. Alnus fruticosa commonly colonizes roadsides, which have low canopy cover, receive high solar radiation, and have poor soil structure. Previous studies (within the same forest type) have found that A. fruticosa is more susceptible to water stress in open canopy sites (≤ 50 % canopy cover) that receive high solar radiation (up to 1000 μmol m−2 s−1) and have high fluctuations in daily temperature (Rohrs-Richey, Citation2011). Furthermore, poor soil conditions along roadsides, such as high bulk density, low organic matter, and high soil compaction (Karim & Mallik, Citation2008), are likely to limit water availability to roadside plants and increase susceptibility to Cytospora canker disease (Adams et al., Citation2005). As water stress has been implicated as a factor that can increase host predisposition or susceptibility to Cytospora canker diseases (Bier, Citation1953; Bloomberg, Citation1962; Kamiri & Laemmlen, Citation1981; Guyon et al., Citation1996; Adams et al., Citation2005), we expected that if V. melanodiscus is pathogenic on A. fruticosa, inoculated roadside alders would be more susceptible to the canker disease compared with similarly inoculated alders on forested transects.
Methods
Site selection
This study took place in the interior of Alaska, which has a cold, semi-arid climate. We conducted our study at sites that were part of a larger, long-term study (2001–2008), which evaluated cumulative herbivore and pathogen damage on A. fruticosa across a local climate gradient (Mulder et al., Citation2008). Three replicate sites (BNZ-South, East, West) at the Bonanza Creek Experimental Forest LTER (64°44′80′′N, 148°19′20′′W), 20 km south of Fairbanks, Alaska were located in mixed stands of predominantly white spruce (Picea glauca (Moench) Voss), resin birch (Betula neoalaskana Sarg.) or aspen (Populus tremuloides Michx.). These upland, secondary successional stands had similar stand characteristics: dense canopy cover (76–79%), low coverage by mosses and lichens (0–2% ground cover) and low litter accumulation (0–5.0 cm depth). Although chosen for similarity of forest type and stand characteristics, the sites differed in elevation and aspect ().
Table 1. Environmental parameters at the Bonanza Creek sites
Forest and roadside conditions
The Bonanza Creek Experimental Forest Road is a dirt road that is lightly maintained and approximately 5 m wide. Roadside alders were growing on the bank of a road cut, where the soil was frequently disturbed and eroded with heavy rains. The organic layer of soil had been stripped away, leaving the mineral layer exposed, with pockets of light organic, eroded debris in areas of the road cut. The roadside was heavily colonized by A. fruticosa, but other understorey shrubs (Salix L. spp.), several herbs (e.g. Chamerion angustifolium (L.) Holub), and grasses were also present. In contrast to the roadside, alder density was lower along the wooded transect and the overstorey canopy was relatively closed by white spruce (Picea glauca), birch (Betula papyrifera Marsh.) or quaking aspen (Populus tremuloides Michx.). The forested transects were also characterized by a duff layer several cm deep, a well-accumulated organic soil layer, and patches of moss and grass on the forest floor. One of the most common understorey species along the wooded transects was bog cranberry (Vaccinium vitis-idaea L.).
Environmental conditions
Air temperature and relative humidity have been monitored at the three Bonanza Creek sites since 2004. Soil moisture, canopy density, moss depth, litter accumulation and per cent cover of different vegetation layers were also characterized in 2004. To quantify any additional environmental differences between the roadside and forested transects, data loggers (HOBO H8 Pro Series, Onset Comp. Corp., Bourne, MA) recording air temperature and relative humidity at 30-min intervals were mounted at 1.2 m and launched in August 2010. Radiation sensors (LI-190 SA Quantum Sensor, LI-COR, Lincoln, NE) that were connected to data loggers (LI-1400 data logger, LI-COR) were placed in pairs at each site, with one sensor on the roadside transect and one sensor placed in the forest. Volumetric soil moisture sensors (SM200 soil moisture sensor, GP1 Data Logger, Delta-T Devices Ltd., England) were also placed in pairs at each site. Soil moisture sensors were installed at the sites using surface installation, which measured the top 5 cm of the soil. Soil temperature at 10-cm depth (digital MULTI-thermometer, Infrared Thermometers.net) was also measured in August 2010. Data were downloaded from the loggers in October. We used the environmental data to indicate the sites or transects where alders would have been the most vulnerable to water stress. This 3-month measurement period captured several weeks of sunny, high temperature weather during late summer and early autumn.
Ramet selection
Alders were inoculated between 30 June and 5 July 2008. At each site, we established a forested transect and a parallel roadside transect that bordered the Bonanza Creek Experimental Forest Road. The wooded transect was at least 25 metres from the roadside and began adjacent to the long-term study sites (Mulder et al., Citation2008). Along each transect, we selected 19 ramets that were within a diameter range of 15–28 mm. We chose ramets at 3–5 metre intervals to ensure that we were selecting genetically distinct individuals. Ramets were randomly assigned to a treatment (control, Isolate 1, Isolate 2). On each transect, eight ramets were inoculated with Isolate 1, eight ramets were inoculated with Isolate 2, and three ramets served as controls. For the three separate sites at Bonanza Creek, we inoculated 16 ramets per transect and 32 ramets per site, for a total of 96 inoculated ramets plus 18 control ramets.
Fungal isolates
Two V. melanodiscus isolates were used to produce inoculum: Jim's Landing 2 (06–08) and Helmaur 1 (06–12) (Adams, Citation2008), hereafter referred to as Isolate 1 and Isolate 2. Both were obtained from cankers on A. tenuifolia in Alaska and were collected and identified by Gerard Adams (Adams, Citation2008). These isolates were chosen for this study because they are of proven pathogenicity on A. tenuifolia and were associated with a canker disease outbreak (Stanosz et al., Citation2011). The same isolates have also been used in greenhouse inoculation studies with A. fruticosa (Rohrs-Richey et al., Citation2011) and in greenhouse and field inoculation studies with A. tenuifolia (Stanosz et al., Citation2011). Cultures were maintained on potato dextrose agar (PDA) (Fisher Scientific, Houston, TX) at 17 °C.
Inoculation procedure
Plugs of inoculum (10 mm × 5 mm) were cut from the actively growing margins of V. melanodiscus cultures on PDA. The inoculation site (on the stem 50 cm above the soil surface) was wiped with a cloth wetted with tap water and then with a cloth wetted with 95% EtOH. A scalpel was used to make a wound (10 mm × 5 mm) exposing the sapwood (Rohrs-Richey et al., Citation2011; Stanosz et al., Citation2011). An inoculum plug was positioned on the wound with mycelium facing the sapwood and secured with Parafilm (American National Can, Greenwich, CT). Control stems were similarly treated, except a sterile PDA plug was used. The Parafilm was removed 4 weeks after inoculation.
Assessing external and internal symptoms
All stems were evaluated in the field approximately 2 months after inoculation (5 September 2008). On this date, we measured the length of externally visible bark necrosis associated with wounding and inoculation. Stems were harvested 1 year later (August 2009) and evaluated for external and internal symptoms. The dimensions of externally visible necrotic bark were measured. The dimensions (length and width) of callus were also measured on each side of the inoculation point, as well as the dimensions of dead, exposed sapwood. Percentage girdle was based on external stem diameter measurements. We compared the stem diameter within the necrotic region (surrounding to inoculation point) to the stem diameter 50 mm from the inoculation point (beyond the length of necrosis). Other visible external disease symptoms were recorded, such as bark cracking. The internal canker dimensions were measured on the sapwood surface, which was exposed by shaving away the outer bark and underlying layers of periderm with a razor blade. On a subset of inoculated stems (n = 22), we peeled back the bark and cut deeply into the sapwood to determine the length of discoloured tissue extending from the margins of the internal parts of the canker.
Culturing from the inoculated stem
Segments (50–60 cm in length, centred on the inoculation point) were harvested during the last week of August 2009, placed in plastic bags, and refrigerated. Within several days of harvest, culturing of V. melanodiscus was attempted for each inoculated and control stem. The bark and callused tissue was peeled away, exposing the underlying sapwood. Four chips of wood from the distal margin of the canker (or distal to the wounding point on the control stems) were surface disinfested (95% EtOH rinse for 20 s) and placed on PDA. Cultures were then incubated in the dark at ambient laboratory temperature. Plates were checked daily for colony characteristics, including conidiomata, that are indicative of V. melanodiscus. Transfers were made from a subset of one-third of the positive cultures to re-isolate V. melanodiscus in pure culture.
Data analysis
Variation in canker length and percentage girdle was first explained by stem treatment (control or inoculated). For wounded and inoculated stems only, ANOVA was used to explain variation of internal and external disease symptoms according to site, transect, isolate, and interactions of the main effects. Normality of all variables was checked before entry into the designated model. The data on canker length was log transformed before analysis of variance. All analyses were performed using SAS (SAS Inst. version 9, Cary, NC).
Results
External disease symptoms
When stems were evaluated 8 weeks after inoculation, only two stems had external disease symptoms. These stems had developed necrotic lesions 1.0 cm and 0.7 cm in length that extended longitudinally from the point of inoculation. Both of the diseased stems were located along the roadside transect at BNZ-West. All other inoculated stems had only 1 mm or less bands of bark necrosis surrounding the inoculation point, a typical necrotic response to wounding that was visible on the control stems.
When the stems were harvested and evaluated for disease (in August 2009), more severe disease symptoms had developed. Beyond the length of the original wound, 36% of the stems had developed necrotic lesions ≤ 5 mm long and 19% of the stems had developed lesions ≥ 10 mm long. Thirty-four per cent of inoculated stems only had a typical wound response to inoculation (bark necrosis ≤ 1 mm). Percentage girdle ranged from 4 to 15%, and none of the lesions girdled the stem entirely ().
Table 2. Responses of Alnus fruticos a to wounding and inoculation with isolates of Valsa melanodiscus at Bonanza Creek LTER. Means ± SD
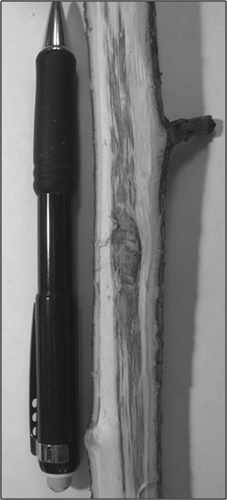
Severe symptoms included sunken, brown cankers in an elliptical area around the inoculation point (). Conidiomata were not seen on the surface of the cankers. Discolouration was most visible on stems with smoother, lighter green bark but was less distinct on stems with rougher bark. Longitudinal bark cracks were also common, with up to three longitudinal cracks (29.7 ± 3.5 mm) proximal to a canker.
Fig. 1. Response of Alnus fruticosa to inoculation with Valsa melanodiscus. Examples of canker development from the BNZ-South wooded transect (A) and roadside transect (B).

Regardless of the severity of disease symptoms, necrotic bark was surrounded, but not contained, by callus. The dimensions of callused tissue (16. 2 ± 2.9 mm long, 2.9 mm wide) were not large enough to close off the initial wound. At the inoculation site, the sapwood was dried out and exposed. The dimensions of exposed sapwood (13.4 ± 0.4 mm long, 6.1 ± 0.2 mm wide) were slightly greater than the dimensions of the initial stem wound (10 × 5 mm).
Lesions did not develop on the control stems, which had less than 1 mm wide band of necrotic tissue associated with the initial wound. Control stems developed callus (8.4 ± 1.7 mm long) in response to wounding, which was half the length of the callus produced on inoculated stems (15.3 ± 0.7 mm long) (F 1,110 = 26.86, P < 0.0001). The callused tissue on control stems extended in width (2.8 to 3.2 mm) on either side of the initial wound. Callusing entirely closed the wound in 53% of the control stems.
Internal disease symptoms
In association with the externally visible cankers, A. fruticosa developed internal lesions. The mean dimensions of internal lesions (24.4 ± 0.8 mm × 7.1 ± 0.2 mm) were much larger than the dimensions of the associated external cankers (6.5 ± 0.8 mm × 2.7 ± 0.2 mm). It was difficult to evaluate lesion extent at consistent depth within host tissue for all stem samples. Therefore, while internal lesions were consistently greater than external cankers, reported dimensions of internal lesions are only an estimate of lesion extent. Deep cuts into the xylem of a subset of inoculated stems revealed brown discolouration that extended longitudinally from the point of inoculation and also to the pith. For these stems, the length of discoloured sapwood (249. 8 ± 28.2 mm) extended far beyond the external margins of the length of externally visible canker (4.5 ± 0.9 mm) ().
V. melanodiscus was recovered from 91.5% of the inoculated stems. Development of a pink to red colouration of the agar was the quickest indicator of V. melandodiscus in culture. This occurred within 1 week and the agar became darker red as the colony expanded. Twenty-five per cent of the positive cultures developed pink-coloured agar in 3–4 days. Within several weeks, the colonies produced conidiomata. Pure cultures of V. melanodiscus were obtained from all of positive cultures from which subcultures were made. Cultures of fungi obtained from control stems did not have any indications of presence of V. melanodiscus.
Environmental conditions on roadside vs. forested transects
The roadsides at BNZ-South had the highest daily maximum temperatures and the greatest daily temperature fluctuations. During the 3-month measurement period, BNZ-South roadsides had the highest maximum air temperature (25.56 °C), while the maximum daily air temperature at other roadside sites did not exceed 22.09 °C (BNZ-East) and 22.47 °C (BNZ-West). The BNZ-South was also the only site where roadside air temperature reached higher maximum temperature than along the forested transect (22.86 °C). Air temperatures between the road and forested transects were similar at BNZ-East and West. During the 3-month measurement period, the BNZ-South roadside had a lower minimum temperature (−12.28 °C) than the roadsides at BNZ-East (−8.38 °C) and BNZ-West (−11.13 °C).
The BNZ-South roadsides also received higher daily maximum solar radiation (800–1000 μmol m−2 s−1) compared with the other roadside sites (400–500 μmol m−2 s−1). The forested transects consistently had lower maximum solar radiation, with the lowest levels at BNZ-West where daily highs typically did not exceed 200 μmol m−2 s−1. The high light, high temperature conditions along the BNZ-South roadside were also accompanied by low soil moisture. Volumetric soil moisture along the BNZ-South roadside remained between 11 to 13% for most of the measurement period, while soil moisture along the forested transect varied from 16 to 30%. Following a rain event, soil moisture increased to 38% along the forested transect but only 17% along the roadside.
Effect of site and roadside
Canker length was explained by site–transect interactions. At the BNZ-South and BNZ-West sites, alders on the roadside transects developed longer cankers than alders along forested transects (, ). Canker development was greatest along the BNZ-South roadside transects (12.1 ± 2.2 mm long). Canker length was also explained by isolate–site interactions. Longer cankers developed in response to inoculation with Isolate 1, but only at the BNZ-South and BNZ-West sites (, ).
Table 3. ANOVA results explaining variation in canker length and girdle following inoculation of Alnus fruticosa with Valsa melanodiscus
In addition to longer cankers at BNZ-South site, alders at the BNZ-South site also had the greatest incidence (47%) of longitudinal bark cracks, while the incidence of alders with cracked bark was lower (26%) at other sites.
Percentage girdle was only explained by roadside versus forested environment. Alders along the roadside transects had higher per cent girdle (8.6–14.8%) than the forested transects (4.2–8.9%) (, ). The length of necrotic sapwood and the length of callus tissue were not explained by site, transect or isolate type.
Discussion
Objective 1: response to inoculation
This is the first field study to confirm that V. melanodiscus is capable of inciting cankers on A. fruticosa. The sunken, brown elliptical cankers that developed on A. fruticosa were morphologically consistent with the response of A. tenuifolia to inoculation with V. melanodiscus (Stanosz et al., Citation2011). In association with cankers, longitudinal bark cracking was a common external disease symptom. Bark cracking is symptomatic of intercellular hyphal wedges growing through host periderm (Biggs et al., Citation1983) and colonizing adjacent regions of the stem (Schreiner, Citation1931).
In association with external canker development, internal lesions developed on the sapwood surface. We observed sapwood discolouration, which can be induced by hyphal colonization of the xylem (Biggs et al., Citation1983), and is a common response of hardwoods to colonization by Cytospora fungi (Adams et al., Citation2005). The extent of discolouration is typically correlated with the size of the external canker; however, as we previously observed in our greenhouse inoculations, discolouration continued beyond the length of the canker margin (Rohrs-Richey et al., Citation2011) and often extended to the pith. Whether V. melanodiscus was present throughout discoloured tissues of A. fruticosa is not known, and could be the subject of future studies.
In response to inoculation, A. fruticosa did not produce apparent disease symptoms until more than 2 months following inoculation. This suggests that A. fruticosa had greater resistance to extensive pathogen colonization at the time of inoculation. We inoculated alders during the first week of July, when alders typically approach peak rates of nitrogen fixation and plant growth (Mitchell & Ruess, Citation2009). In correspondence with peak growth rates, the plant might have been able to maintain resistance mechanisms that prevented or delayed canker advance, such as suberin and lignin production for mechanical barriers (Bloomberg, Citation1962; Bloomberg & Farris, Citation1963), non-specific wound healing (Maxwell et al., Citation1997), or synthesis of secondary metabolites (McPartland & Schoeneweiss, Citation1984; Boyer, Citation1995). Previous studies with Cytospora species on various hosts report that in places with long, cold winters, maximum canker expansion can be achieved by inoculation in the autumn (September–October) when tree defence mechanisms are compromised by seasonal dormancy (Adams et al., Citation2005).
Objective 2: The effect of roadsides on predisposition and susceptibility
We quantified environmental differences between the roadside and wooded sites, as previous studies have shown that A. fruticosa in open canopy environments (with high solar radiation and air temperatures) are more vulnerable to water stress under dry conditions (Rohrs-Richey, Citation2011). With a south-facing aspect, the BNZ-South roadside had the highest daily levels of solar radiation, the highest daily maximum air temperatures, and lower per cent volumetric soil moisture compared to other sites. This was also the only site where maximum air temperatures along the roadside transect were higher than maximum air temperatures along the wooded transect. Therefore, the environmental data indicate that A. fruticosa at the BNZ-South roadside transect would have been the most vulnerable to water stress during the course of the experiment.
The inoculation responses at BNZ-South support and refine our expectation that inoculated A. fruticosa on roadside transects would be more susceptible to the canker disease compared with similarly inoculated alders on forested transects. The extent of canker development at BNZ-South suggests that A. fruticosa has decreased disease resistance (enhanced susceptibility) in southern exposed, roadside environments. Depending on the isolate type, alders at BNZ-South developed cankers that were 50–400% longer than alders on the forested transect. Percentage girdle was also 61% greater on the roadside alders compared to the forest alders (). Therefore, this field experiment provides evidence that site-level conditions associated with increased vulnerability to water stress may also be related to increased susceptibility of A. fruticosa to Cytospora canker disease.
Isolate interactions
Currently, the extent of variation among isolates of the fungal pathogen is not known, and the variation in host response to infection and colonization is also not known. However, our results suggest that there is some variation in isolate aggressiveness, and that isolate aggressiveness could be influenced by site environment. For example, at BNZ-West, cankers associated with Isolate 1 were up to four times longer than cankers associated with Isolate 2 (). The environment at BNZ-West may have contributed to variation in canker length associated with the two isolates, as the seasonal climate was more humid, with lower air temperature and less solar radiation than the other sites (). However, our study was limited in scope, and future inoculation studies should include more pathogen isolates and a wider range of sites to determine variation in the aggressive of V. melanodiscus isolates on A. fruticosa.
Comparison of isolate response in A. tenuifolia and A. fruticosa
The isolates used in this experiment were cultured from cankers on A. tenuifolia, and we used these isolates because they are proven to be pathogenic and virulent on A. tenuifolia (Stanosz et al., Citation2011). Compared with A. fruticosa, experimental disease development appears to be more immediate and severe on A. tenuifolia, which developed lesions that ranged in length from 10 mm to 600 mm in response to inoculation with the same isolates used in this study (Stanosz et al., Citation2011). Although these isolates were more aggressive on A. tenuifolia, there is little reason to believe that the pathogen is locally adapted to A. tenuifolia. Isolates of V. melanodiscus across the state of Alaska show a high level of genetic variation (Adams, Citation2008), indicating that the disease epidemic on A. tenuifolia was not the result of a small group of highly aggressive genotypes that became dominant in the pathogen population. Instead, the vast extent of the canker disease outbreak on A. tenuifolia suggests that larger scale processes (such as climate warming) contributed to state-wide disease-related mortality (Ruess et al., Citation2009).
Conclusions and further research
It remains unclear if a specific set of conditions facilitated the outbreak of canker disease on A. tenuifolia, or if these conditions could be relevant to disease development on A. fruticosa. The limited canker development in this study indicates that V. melanodiscus will not be associated with extensive disease-related mortality on A. fruticosa in the mixed stands that we examined. However, our study suggests that site conditions associated with water stress vulnerability could be related to increased disease development on A. fruticosa. This should be investigated in other environments where Cytospora canker mortality has also been detected on A. fruticosa, such as natural clearings and alpine environments (USDA, Citation2011). With the potential for stress-reduced resistance in this pathosystem coupled with the fast pace of high-latitude climate change in Alaska, the Cytospora canker disease on A. fruticosa should continue to be monitored.
Acknowledgements
This research was partially supported by a grant from the USDA Forest Service, Forest Health Protection. Funding was also provided by fellowships to J.K. Rohrs-Richey from Alaska's Experimental Program to Stimulate Competitive Research (EPSCoR). Helpful comments on the manuscript were provided by Christa P.H. Mulder, Roger W. Ruess and Barbara A. Roy.
References
- Adams, G.C., Catal, M., & Trummer, L. (2009). Distribution and severity of alder phytophthora in Alaska. Fourth Sudden Oak Death Science Symposium. June 15–18 Santa Cruz, CA http://www.suddenoakdeath.org/sodsymposium4/pdf/book_of_abstracts.pdf (http://www.suddenoakdeath.org/sodsymposium4/pdf/book_of_abstracts.pdf)
- Adams , G.C. , Wingfield , M.J. , Common , R. and Roux , J. 2005 . Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus . Stud. Mycol. , 52 : 1 – 147 .
- Adams , G.C. , Roux , J. and Wingfield , M.J. 2006 . Cytospora species (Ascomycota, Diaporthales, Valsaceae); introduced and native pathogens of trees in South Africa . Australas. Plant Patho. , 35 : 521 – 549 .
- Adams , G. 2008 . “ Final Report: Searching for invasive pathogens of Alnus incana in the Alaskan alder mortality. Grant #06DG11100100209 ” . In Internal document on file at USDA Forest Service , 1 – 22 . Anchorage : Forest Health Protection .
- Bier , J.E. 1953 . The relation of bark moisture to the development of canker diseases caused by native facultative parasites . Can. J. Bot. , 37 : 1140 – 1142 .
- Biggs , A.R. , Davis , D.D. and Merrill , W. 1983 . Histopathology of canker on Populus caused by Cytospora chrysosperma . Can. J. Bot. , 61 : 563 – 574 .
- Bloomberg , W.J. 1962 . Cytospora canker of poplars: the moisture relations and anatomy of the host . Can. J. Bot. , 40 : 1281 – 1293 .
- Bloomberg , W.J. and Farris , S.H. 1963 . Cytospora canker of poplars: bark wounding in relation to canker development . Can. J. Bot. , 41 : 303 – 310 .
- Boyer , J.S. 1995 . Biochemical and biophysical aspects of water deficits and predisposition to disease . Ann. Rev. Phytopathol. , 33 : 251 – 274 .
- Chapela , I.H. 1989 . Fungi in healthy stems and branches of American beech and aspen: a comparative study . New Phytol. , 113 : 65 – 75 .
- Christensen , C.M. 1940 . Studies on the biology of Valsa sordida and Cytospora chrysosperma . Phytopathology , 63 : 451 – 472 .
- Filip , G.M. , Parks , C.A. and Starr , G.L. 1992 . Incidence of wound-associated infection by Cytospora sp. in mountain alder, red-osier dogwood, and black hawthorn in Oregon . Northwest Sci. , 66 : 194 – 198 .
- Guyon , J.C. , Jacobi , W.R. and Mcintyre , G.A. 1996 . Effects of environmental stress on the development of Cytospora canker in aspen . Plant Dis. , 80 : 1320 – 1326 .
- Kamiri , L.K. and Laemmlen , F.F. 1981 . Effects of drought stress and wounding on Cytospora canker development on Colorado blue spruce . J. Arboric. , 7 : 113 – 116 .
- Karim , M.N. and Mallik , A.U. 2008 . Roadside revegetation by native plants. I. Roadside microhabitats, floristic zonation and species traits . Ecol. Engin. , 32 : 222 – 237 .
- Kepley , J.B. and Jacobi , W.R. 2000 . Pathogenicity of Cytospora fungi on six hardwood species . J. Arboric. , 26 : 326 – 333 .
- Maxwell , D.L. , Kruger , E.L. and Stanosz , G.R. 1997 . Effects of water stress on colonization of poplar stems and excised leaf disks by Septoria musiva . Phytopathology , 87 : 381 – 388 .
- Mcpartland , J.M. and Schoeneweiss , D.F. 1984 . Hyphal morphology of Botryosphaeria dothidea in vessels of unstressed and drought-stressed stems of Betula alba . Phytopathology , 74 : 358 – 362 .
- Mitchell , J.S. and Ruess , R.W. 2009 . Seasonal patterns of climate controls over nitrogen fixation by Alnus viridis subsp. fruticosa in a secondary successional chronosequence in interior Alaska . Ecoscience , 16 : 341 – 351 .
- Mulder , C.P.H. , Roy , B.A. and Güsewell , S. 2008 . Herbivores and pathogens on Alnus viridis subsp. fruticosa in interior Alaska: effects of leaf, tree, and neighbor characteristics on damage levels . Botany , 86 : 408 – 421 .
- Rohrs-Richey , J.K. , Mulder , C.P.H. , Winton , L.M. and Stanosz , G.R. 2011 . Physiological performance of an Alaskan shrub (Alnus fruticosa) in response to disease (Valsa melanodiscus) and water stress . New Phytol. , 189 : 295 – 307 .
- Rohrs-Richey , J.K. 2011 . Biotic pest damage of green alder (Alnus fruticosa): susceptibility to a stem disease (Valsa melanodiscus) and functional changes following insect herbivory , Fairbanks , Alaska, USA : Ph.D. Thesis. University of Alaska .
- Ruess , R.W. , McFarland , J.M. , Trummer , L.M. and Rohrs-Richey , J.K. 2009 . Disease-mediated declines in N-fixation inputs by Alnus tenuifolia to early successional floodplains in Interior and South-Central Alaska . Ecosystems , 12 : 227 – 248 .
- Schreiner , E.J. 1931 . Two species of Valsa causing disease in Populus . Amer. J. Bot. , 18 : 1 – 29 .
- Sinclair , W.A. , Lyon , H.H. and Johnson , W.T. 1987 . Diseases of trees and shrubs , 194 – 202 . Ithaca , NY : Cornell University Press .
- Stanosz , G.R. , Trummer , L.M. , Rohrs-Richey , J.K. , Smith , D. , Adams , G.C. and Worrall , J.J. 2011 . Response of Alnus tenuifolia to inoculation with Valsa melanodiscus . Can. J. Plant Pathol. , 33 : 202 – 209 .
- Uliassi , D.D. and Ruess , R.W. 2002 . Limitations to symbiotic nitrogen fixation in primary succession on the Tanana River floodplain, Alaska . Ecology , 83 : 88 – 103 .
- USDA . 2009 . Forest health conditions in Alaska – 2008 , Anchorage , AK : USDA Forest Service, Alaska Region, Forest Health Protection . Report R10-PR-21Compiled by M. Lamb and T. Wurtz
- USDA . 2010 . Forest health conditions in Alaska – 2009 , Anchorage , AK : USDA Forest Service, Alaska Region, Forest Health Protection . Report R10-PR-21Compiled by M. Lamb and L. Winton
- USDA . 2011 . Forest health conditions in Alaska – 2010 , Anchorage , AK : USDA Forest Service, Alaska Region, Forest Health Protection . Report R10-PR-23Compiled by M. Lamb and L. Winton
- Worrall , J. J. 2009 . Dieback and mortality of Alnus in the southern Rocky Mountains, USA . Plant Dis. , 93 : 293 – 298 .
- Worrall , J.J. , Adams , G.C. and Tharp , S.C. 2010 . Summer heat and an epidemic of cytospora canker of Alnus . Can. J. Plant Pathol. , 32 : 376 – 387 .