Abstract
ʻCandidatus Phytoplasma asterisʼ (CPa) has been identified along with ʻCandidatus Liberibacter asiaticusʼ (CLas) from citrus trees showing Huanglongbing (HLB) symptoms in Mexico. The low titre of CPa and CLas in infected plants is the major constraint to developing an accurate and sensitive method for their detection that would support an effective HLB diagnosis and management programme. This investigation reports the development and validation of a new TaqMan quantitative PCR to detect low titres of CPa in citrus trees with HLB symptoms based on the phytoplasma 16S rRNA gene region. The assay was used to monitor the titre of CPa at early and late stages of disease development in symptomatic and asymptomatic Mexican lime trees in Colima, Mexico. The concentration of CLas was determined by nested-qPCR. Only eight out of 39 sampled branches showed HLB symptoms at the early infection stage. All 39 branches were symptomatic at the late infection stage. The qPCR yielded an increase in the branch incidence and concentration of CLas (49 to 100% and 5 × 103 to 12 × 103 cells/100 ng of total DNA), while CPa showed a decreased incidence and concentration (72to 41% and 199 to 19 cells/100 ng of total DNA) throughout the disease development. Results confirm the occurrence of mixed infection of CLas and CPa in HLB-affected citrus trees, although their epidemiological role in co-infecting remains unknown.
Résumé
‘Candidatus Phytoplasma asteris’ (CPa) a été identifié au Mexique, parallèlement à ‘Candidatus Liberibacter asiaticus’ (CLas), sur des citrus affichant les symptômes du huanglongbing (HLB). Le faible titre du CPa et du CLas chez les plants infectés est la principale entrave au développement d’une méthode précise et sensible permettant leur détection, méthode qui appuierait un programme efficace de diagnostic et de gestion du HLB. Cette enquête traite du développement et de la validation d’une nouvelle PCRq TaqMan pour détecter les faibles titres du CPa chez les citrus affichant des symptômes de HLB, basée sur la région du gène 16S de l’ARNr du phytoplasme. Le biotest a été utilisé pour suivre le titre du CPa aux stades initiaux et terminaux du développement de la maladie chez les limettiers symptomatiques et asymptomatiques de Colima, au Mexique. La concentration du CLas était déterminée par PCRq emboîtée. Seulement 8 des 39 branches échantillonnées ont affiché les symptômes du HLB au stade précoce de l’infection. Les 39 branches étaient symptomatiques au stade tardif. La PCRq a généré une augmentation de l’incidence et de la concentration du CLas durant le développement de la maladie (de 49 à 100% et de 5 × 103 à 12 × 103 cellules/100 ng d’ADN total), tandis que le CPa a affiché une réduction de l’incidence et de la concentration (de 72 à 41% et de 199 à 19 cellules/100 ng d’ADN total). Les résultats confirment l’occurrence d’infection mixte causée par CLas et CPa chez les citrus touchés par le HLB, bien que leur rôle épidémiologique quant à la co-infection demeure inconnu.
Introduction
Huanglongbing (HLB) is the most destructive citrus pathosystem worldwide. Primarily known from Asia and Africa, HLB was introduced into the western hemisphere in 2004 (Gottwald Citation2010). HLB was detected in Mexico in 2009 and has rapidly spread across 22 of the 28 citrus-producing states, decreasing national Mexican lime (Citrus aurantifolia Christm., Swingle) production by 333 370 tons (25.4%) from 2008 to 2014. In 2008, Colima state contributed 48% of the national production, and almost 100% of the Mexican lime groves (18 632 ha) are affected by HLB, with reductions in productivity (by 58%) and yield (by 60%) (SIAP-SAGARPA Citation2014).
Although Koch’s postulates have not been fulfilled yet, it seems that two plant pathogens may be involved in the aetiology of HLB. Initially, HLB was only associated with Candidatus Liberibacter spp., a group of Gram-negative, phloem-limited α-proteobacteria given provisional Candidatus status (Jagoueix et al. Citation1996). Indeed, three Liberibacters have been reported, which are namely, Ca. Liberibacter asiaticus (CLas), Ca. Liberibacter africanus (CLaf) and Ca. Liberibacter americanus (CLam) (Bové Citation2006).
Candidatus Phytoplasma spp. have been associated with HLB-like symptoms in Brazil, China and Mexico. The Pigeon Pea Witches’-Broom phytoplasma (16SrIX group) was recorded in Brazil (Teixeira et al. Citation2008), while Ca. P. asterisʼ (CPa) was identified from HLB-affected citrus in southern China (Chen et al. Citation2009). Furthermore, a phytoplasma of the subgroup 16SrII-A was reported in an HLB-affected grapefruit (Citrus paradisi Macfad) orchard in Guangxi Province, China (Lou et al. Citation2014) and recently, two phytoplasma strains belonging to the subgroups 16SrI-B and 16SrI-S were identified from citrus groves with HLB-like symptoms in Mexico (Arratia-Castro et al. Citation2014).
Phytoplasmas are plant pathogenic bacteria of the Mollicutes class (Bertaccini Citation2007). They are cell wall-free organisms that reside in the phloem elements of infected plants and are naturally transmitted by sap-sucking insect vectors. Diseases associated with phytoplasmas occur worldwide in many plant species, including vegetable and fruit crops and ornamental plants. More than 300 distinct plant diseases have been attributed to phytoplasmas (Hoshi et al. Citation2007) and the list of diseases caused by phytoplasmas continues to grow. The titre of phytoplasma cells in the phloem of infected plants varies with the season and plant species, and it is often very low in woody hosts, which renders their effective diagnosis difficult (Marzachì Citation2004), similar to the diagnosis of Ca. L. species.
Currently, there are a large number of quantitative polymerase chain reaction (qPCR) assays that have been developed to improve the diagnosis and quantification of phytoplasmas affecting different crops. Universal or group-specific qPCR assays using 16S rDNA Taqman probes have been developed for the generic detection and quantification of a wide range of phytoplasma strains (Liao et al. Citation2002; Christensen et al. Citation2004) or specific phytoplasmas such as those associated with apple proliferation (Baric & Dalla-Via Citation2004; Nikolić et al. Citation2010; Baric et al. Citation2011), grapevine yellows (Angelini et al. Citation2007; Hren et al. Citation2007), chrysanthemum yellows (Marzachì & Bosco Citation2005), Columbia basin potato purple top (Crosslin et al. Citation2006) and coconut lethal yellowing (Córdova et al. Citation2014). Primers and Taqman probes have been also designed based on the tuf (Wei et al. Citation2004), SecY (Hren et al. Citation2007; Herath et al. Citation2010), Map (Pelletier et al. Citation2009), and 23S rRNA (Hodgetts et al. Citation2009; Jawhari et al. Citation2015) genes. Other chemistries have also been used for detecting phytoplasma strains, such as SYBR Green (Galetto et al. Citation2005; Torres et al. Citation2005; Yvon et al. Citation2009), and EvaGreen (Frost et al. Citation2011; Monti et al. Citation2013). However, the detection of phytoplasmas in HLB-affected citrus has been limited to DNA amplification by PCR, followed by restriction fragment length polymorphism (RFLP) analysis. No reports are available on efficient methods to detect and quantify phytoplasmas from citrus hosts, which has become a major constraint for the implementation of an effective disease management approach.
This investigation reports on the development and validation of a new qPCR method to detect low titres of ‘Ca. P. asterisʼ (CPa) in a large number of citrus trees affected by HLB to improve the routine screening. The qPCR assay was used to monitor the titre of CPa over the course of 2 years (2012 and 2013) at both early and late infection stages in asymptomatic and symptomatic Mexican lime trees in Colima, Mexico. Nested PCR was also used for detection of CPa. The titre of CLas was monitored by nested-qPCR.
Materials and methods
Plant materials
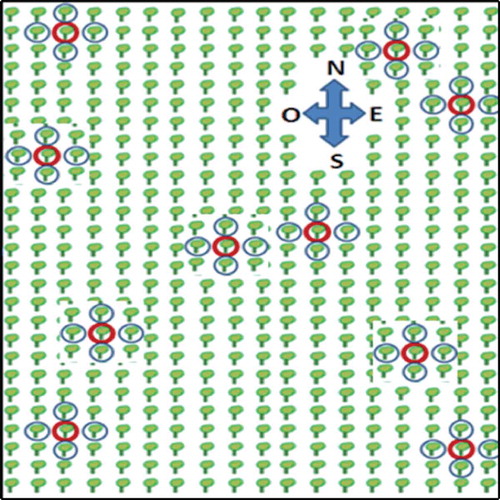
Citrus samples were collected from a commercial citrus grove in Tecomán, Colima, located on the Pacific coast of Mexico. In February 2012, a 10-year-old citrus grove showed an early infection stage with an HLB incidence of 5%. Ten Mexican lime trees (Citrus aurantifolia) showing light to moderate HLB symptoms (blotchy mottle and vein yellowing) were labelled and sampled. Branches from four asymptomatic trees, adjacent to each symptomatic central tree, were collected (one for each cardinal point), 50 trees in total (10 central and 40 surrounding trees) (). In February 2013, five out of 10 central trees previously labelled were re-sampled along with their four adjacent trees. At the time of this collection, all the sampled trees (central and adjacent trees) showed severe HLB symptoms such as asymmetric blotchy mottle covering 90–100% of the canopy, considering it as a late infection stage. For both sampling times, four branches of each central tree (one branch of each cardinal sector of the tree canopy), and one branch of each adjacent tree (the branch adjacent to the central trees) were collected. Citrus samples collected were transferred to the laboratory of Molecular Biology of Plant Pathogens of the Instituto Politécnico Nacional (CIIDIR-IPN-Sinaloa). Upon arrival, samples were stored at 4°C and processed within 48 h.
DNA extraction
Total nucleic acids were extracted from 20 mg of lyophilized and ground (TissueLyser II, QIAGEN, Hilden, Germany) leaf midribs and petioles, using a previously described CTAB protocol (Zhang et al. Citation1998; Arratia-Castro et al. Citation2014). Total DNA optical density and purity was measured at 260/230 nm and 260/280 nm using the NanoDrop ND-1000 UV-Vis Spectrophotometer (Thermo Scientific, Wilmington, DE). The quality of the DNA extracted was confirmed by electrophoresis in 1% agarose gels stained with ethidium bromide (0.2 μg mL−1) and visualized under a UV transilluminator. DNA concentration was adjusted to 20 ng μL−1 and stored at −20°C for further use.
Primers and probe design: specificity and sensitivity
Primers and a probe were designed for the detection of phytoplasmas based on the alignment of representative phytoplasma strains belonging to 16SrI, 16SrII, 16SrIII, 16SrIV, 16SrVI, 16SrXIII and 16SrXVII (Gundersen et al. Citation1994; Jomantiene et al. Citation1998; Harrison et al. Citation2002; Oshima et al. Citation2004; Arocha et al. Citation2005; Yue et al. Citation2008; Davis et al. Citation2013). Sequences were aligned using the Clustal W method (Thompson et al. Citation1994) and primers and probe were chosen from DNA regions suitable for high specificity due to the fact that the probe and the reverse primer were located in a hypervariable 16S rRNA region among different phytoplasma groups ().
Fig. 2 Multiple sequence alignment of the 16S rDNA regions showing the specificity of the PPTP1-AS2F (forward primer), PPTP1-AS2R (reverse primer) and PPTP1-AS2M (TaqMan probe) in relation to other phytoplasma isolates belonging to different ribosomal groups.

Primers and TaqMan probe were designed using Primer Express software (version 2.0; ABI, Foster City, CA), and synthesized at Applied Biosystems, Inc. (ABI, Foster City, CA). The 5′ end of the TaqMan® probe was labelled with the fluorescent reporter dye, 6-carboxylfluorescein (FAM). The 3′ end of the probe was labelled with a non-fluorescent quencher, minor groove binder (MGB). Primers and probe sequences were tested for homology to phytoplasma and other prokaryotic sequences using BLASTN analysis on GenBank through the NCBI database. Primers ‘HLB-AS2F’ (5′-TCCGGAATTATTGGGCGTAAAGG-3′), ‘HLB-AS2R’ (5′-ACAATGTTG AGCATTGCACTTAGAC-3′) and TaqMan probe HLB-AS2M (5′-FAM/TAGGCGGTTAAATAAGTTTTATG/MGB-3′) were selected because they showed homology to several phytoplasma sequences and little homology with other prokaryotic 16S rRNA genes. A specific phytoplasma fragment of 75 bp was amplified in the 16S rRNA region. The Applied Biosystems® Eukaryotic 18S rRNA Endogenous Control (VIC® ⁄ TAMRA Probe, Primer Ltd) was used as an endogenous control to normalize the DNA quantities and as a positive internal control to assess the quality of the DNA extracts.
The phytoplasma specificity of the synthesized primers and probe was assessed by qPCR against the DNA extracts of potato and citrus samples known from previous studies to be infected with one of the following 16Sr group phytoplasma strains: I, II, III and XIII, other HLB-associated Ca. Liberibacter species: CLas and ‘Ca. Liberibacter solanacearum’, and bacterial pathogens, including Ralstonia solanacearum, Clavibacter michiganensis subsp. michiganensis, Pseudomonas syringae pv. tomato and Xanthomonas campestris pv. vesicatoria, as well as the citrus endophytes Enterobacter sp., Bacillus sp. and Pantoea agglomerans.
In order to evaluate the sensitivity of the qPCR assay, 10-fold serial dilutions of a plasmid DNA containing target sequences in a range of 107 to 101 copies were used as template DNA and compared with nested PCR assays using R16mF2/R16mR1 primers in the first round and R16F2n/R16R2 for the second PCR.
Quantitative PCR for phytoplasmas
All real-time PCR reactions were performed in MicroAmp® Fast Reaction tubes using a 7500 Fast real-time PCR system (ABI, Foster City, CA). The concentrations of the primers and TaqMan probe were optimized to 900 nM and 200 nM, respectively. Five μL of the DNA template (20 ng μL−1) were used in a 20 μL final volume of the PCR mixture prepared with TaqMan® Universal PCR Master Mix containing ROX as the passive internal reference dye (Applied Biosystems, Foster City, CA). The PCR thermal cycling protocol included 50°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Quantification cycle (Cq) values were analysed using the ABI 7500 software version 2.0.5 with an automated threshold and baseline setting. All reactions were performed in triplicate and each run contained deionized sterile water as a negative control and Mexican HLB phytoplasma 16SrI-S strain (HLBpc-Sin-IS, AB858474) as a positive control.
Nested PCR for phytoplasmas
The sensitivity of the newly developed qPCR assay was compared with the nested PCR. Phytoplasmas were detected using two ‘universal’ phytoplasma primer pairs (R16mF2/R16mR1 in the first round and R16F2n/R16R2 for the nested PCR reaction) that amplify an approximate 1250 bp fragment of the 16S rDNA (Gundersen & Lee Citation1996). PCR amplification was carried out in an automatic thermocycler (C1000 Thermal Cycler, BioRad, USA), in a final volume of 25 μL containing: 1 U of Taq DNA polymerase (Invitrogen Life Technologies, Brazil), 0.2 mM of each dNTP and 0.4 pmol of each primer. Total genomic DNA from the Mexican phytoplasma strain, Potato purple top (PPT) subgroup 16SrI-S (PPT-BC15-IS, GenBank accession number FJ914638) was used as positive control (Santos-Cervantes et al. Citation2010). DNA was replaced by deionized sterile water for a PCR negative control. PCR products were electrophoresed on a 1% agarose gel stained with ethidium bromide and visualized under a UV transilluminator. Two representative amplicons of symptomatic and asymptomatic samples were cloned and completely sequenced twice in order to confirm the identity of detected phytoplasmas (ABI PRISM 377, Applied Biosystems, Foster City, CA).
Nested-quantitative PCR for the Ca. L. asiaticus
The nested-qPCR assay for detecting CLas was carried out with primers and probe as described by Lin et al. (Citation2010). Briefly, the PCR reaction mixture included the following; 0.5 pmol of outer primer pair (Las-OF [5′-CGGTGAATGTATTAAGCTGAGGCGTTCC-3′] and Las-OR [5′-TACCCACAACAAAATGAGATACACCAACAACTTC-3′]), 20 pmol of inner primer pair (Las-IF [5′-CGATTGGTGTTCTTGTAGCG-3′] and Las-IR [5′-AACAATAGAAGGATCAAGCATCT-3′]), 10 pmol of target probe Las-p [5′-6-FAM/AATCACCGAAGGAGAAGCCAGCATTACA/MGB-3′] and 5 μL of DNA template (20 ng μL−1). The PCR mixture was prepared using TaqMan® Universal PCR Master Mix (ABI). The PCR cycling protocol was: 50°C for 2 min and 95°C for 10 min and followed by 20 cycles at 95°C for 30 s, 67°C for 45 s and 72°C for 45 s for the first round of PCR and 35 cycles at 95°C for 30 s, 57°C for 45 s and 72°C for 45 s for the second round of PCR.
PCR reactions were carried out in MicroAmp® Fast Reaction tubes using the 7500 Fast real-time PCR system (ABI) in a final volume of 25 μL. Cq values were analysed using the ABI 7500 software version 2.0.5 with an automated threshold and baseline setting. All reactions were performed using deionized sterile water as a negative control and total genomic DNA from CL as strain (HLBlas-Col, AB859772) as a positive control.
Standard curves for quantification
The CPa-specific plasmid template used in the qPCR was developed by cloning a portion of the 16S rRNA region (HLB-CPa). A 1250 bp DNA fragment was amplified with two universal phytoplasma primers, R16mF2/R16mR1 and R16F2n/R16R2 (Gundersen & Lee Citation1996) by nested PCR and purified using the Wizard SV Gel and PCR Clean-Up System (Promega Corporation, Madison, WI). The plasmid used in the nested-qPCR for CLas harbouring the CLas-specific elongation factor Ts gene region (HLB-CLas) was constructed from a 470 bp DNA fragment amplified with the outer primers (Las-OF/Las-OR) (Lin et al. Citation2010) and purified as mentioned above. Both purified fragments were ligated into a pGEM-T Easy vector (Promega) and transformed into Escherichia coli JM109 competent cells. Plasmids from recombinant culture colonies were isolated and purified (Wizard Plus SV Minipreps DNA Purification System, Promega). The purified plasmids were quantified and fully sequenced in the ABI PRISM 377 sequencer, using the Dye cycle sequencing kit (ABI). The copy number for each plasmid was calculated by using the Avogadro’s number, 6.02 × 1023 molecules/mol, so each µg of the cloned plasmids was equivalent to 2.22 × 1011 and 2.61 × 1011 molecules/mol target DNA copies for HLB-CPa and HLB-CLas plasmids, respectively. Plasmids were serially diluted at the 10-fold interval and used to generate standard curves for absolute quantification for the qPCR assays. To estimate the number of CPa cells per 100 ng of total DNA, the estimated copy number was divided by 2 since each phytoplasma has two copies of the 16S rRNA gene (Schneider & Seemüller Citation1994; Marcone & Seemüller Citation2001). For CLas, the copy number was taken directly from the ABI 7500 software version 2.0, as CLas has one unique copy of the elongation factor Ts gene (Lin et al. Citation2010). The copy numbers obtained were normalized for the input amount of DNA using the qPCR assay for plant 18S rRNA. For the plant DNA, a linear standard curve was constructed using total DNA from a healthy Mexican lime plant. Total DNA of the sample was quantified by Nanodrop and was serially diluted 2-fold over a five point range (100 ng–6.4 pg). The titre of CLas and CPa was expressed as copy numbers of each pathogen per 100 nanograms (ng) of plant DNA.
Results
Validation of phytoplasma primers and probe specificity and sensitivity
To validate the qPCR primers and probe specificity, a series of in silico BLASTN searches against available NCBI prokaryotic sequences was performed. In silico analysis of the HLB-AS2F/HLB-AS2R primers and HLB-AS2M TaqMan probe demonstrated a strict sequence alignment with phytoplasma isolates. Additionally, qPCR assays were performed to test the specificity of the designed primers using different phytoplasma strains: 16SrI: 16SrI-B, 16SrI-S, 16SrI-T, 16SrI-U and 16SrI-V; and phytoplasma isolates from different ribosomal groups used as outgroups: 16SrII-C, 16SrII-M, 16SrIII-S, 16SrXIII-A and 16SrXIII-D. The specificity was further confirmed against ‘Ca. Liberibacter asiaticus’, ‘Ca. Liberibacter solanacearum’, R. solanacearum, C. michiganensis subsp. michiganensis, P. syringae pv. tomato, X. campestris pv. vesicatoria, Enterobacter sp., Bacillus sp. and P. agglomerans. No amplicons were produced using DNA extracts from citrus bacteria and endophytes (data not shown). Primer pairs only produced an amplification when using 16SrI phytoplasmas as a template, and not for 16SrII-C, 16SrII-M, 16SrIII-S phytoplasmas. Amplicons were also obtained for the 16SrXIII-A phytoplasma, which may be related to the close phylogenetic relationship between 16SrI and 16SrXIII phytoplasmas.
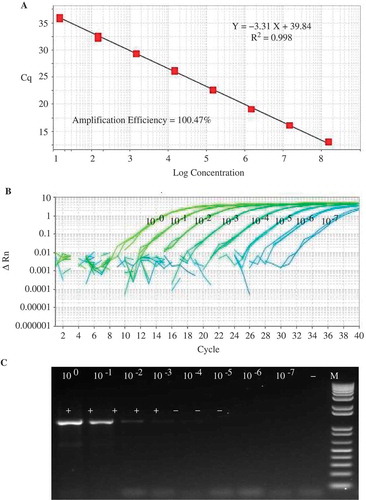
The sensitivity of the qPCR assay was compared with that of the nested PCR with R16mF2/R16mR1 and R16F2n/R16R2 primers. The obtained results showed a difference in sensitivity between the two detection systems. Amplifications from dilutions 100 to 10–4 corresponding to 16S rRNA gene copies of 148 500 000 to 14 850 were obtained by both methods. However, only the qPCR assay was able to detect 14.85 copies (). These results confirm that the qPCR assay offers a more accurate and sensitive method for quantification of phytoplasmas than the nested PCR.
Fig. 3 (Colour online) Comparison of the sensitivity between real time PCR (qPCR) and nested PCR analysis using a 10-fold serial dilution (100 to 10–7) of a plasmid DNA template developed by cloning a portion of the 16S rRNA region of CPa. (a) a molecular standard curve of qPCR using a plasmid DNA containing target sequences in a range of 108 to 10 copies with 100.47 amplification efficiency and 0.998 correlation coefficient; (b) quantitative PCR results using primers PPTP1-AS2F and PPTP1-AS2R and the TaqMan probe PPTP1-AS2M; and (c) nested PCR results using primers R16mF2/R16mR1 and R16F2n/R16R2.
Sequencing of the nested PCR products of Phytoplasmas
The 99.9% of the identity of the 16S rDNA sequences enabled us to confirm that the phytoplasma associated with symptomatic and asymptomatic samples collected in the HLB-diseased grove belongs to the aster yellows group (16SrI) ‘Candidatus Phytoplasma asteris’ subgroup S (16SrI-S).
Amplification efficiency of the quantitative PCR assays
The standard curves of CPa and CLas qPCR assays showed high efficiency in the dynamic range tested. The amplification efficiency of CPa TaqMan® PCR and nested CLas TaqMan® PCR was estimated based on the efficiency equation %AE = (10−1/slope − 1) × 100. The CPa qPCR assay resulted in standard curves with a very high correlation coefficient, R2 = 0.998 and a slope value of −3.31 (%AE = 100.47%). The standard curve generated by CLas nested-qPCR assay gave a linear regression of R2 = 0.994 with a slope value of −3.319 (%AE = 100.12). The efficiency for the plant 18S rRNA was 96%.
Evaluation of the CLas and CPA concentration in Mexican lime trees showing different stages of HLB disease development
End-point PCR was used to evaluate the 10 HLB-symptomatic trees collected during the early stage of symptom development in February 2012. By that time, CPa was detected in three out of 10 sampled trees, while nine of the trees were positive for CLas. The three CLas and CPa infected trees (mixed infected) and two of the CLas-positive trees were selected in order to quantify the level of CLas and CPa in early and late infection stages. Four trees surrounding each of the five CLas and/or CPa positive trees were sampled. The four branches of the five central HLB-infected symptomatic trees and a branch of its four adjacent trees were tested by qPCR. In total, 39 branches were included in each infection stage.
During the early stage of symptom development, six out of 19 branches of the central trees showed HLB-like symptoms, while the rest of the branches were asymptomatic (13 branches). Only two branches were symptomatic and 18 branches were asymptomatic in the adjacent trees. In total, of the 39 branches collected in 2012, eight were symptomatic and 31 asymptomatic. Quantitative PCR revealed that all symptomatic branches and 11 out of 31 asymptomatic branches were positive for CLas (), with a Cq mean value of 19.74 (sd ± 3.85) (4790 cells/100 ng of total DNA) and 24.37 (sd ± 5.07) (235 cells/100 ng of DNA), respectively (). For CPa, a total of four symptomatic branches and 24 asymptomatic branches were positive by the qPCR assay (). The Cq mean was 31.45 (sd ± 2.60) (199 cells/100 ng of total DNA) and 32.10 (sd ± 2.12) (106 cells/100 ng of DNA, respectively ().
Table 1. Real time PCR detection of ‘Ca. L. asiaticus’ and ‘Ca. P. asteris’ at early (2012) and late (2013) stages of HLB disease development in both symptomatic and asymptomatic Mexican lime trees in Colima, Mexico.
Fig. 4 Comparative detection of ‘Ca. L. asiaticus’ and ‘Ca. P. asteris’ by quantitative PCR of symptomatic and asymptomatic Mexican lime branches. Samples were collected from a commercial citrus grove in Tecomán, Colima, located in the Pacific coast of Mexico in February 2012 and February 2013. Concentration of CPa and CLas expressed as cells/100 ng of DNA. Different letters indicate statistically significant differences (P < 0.05), vertical lines are standard errors (SE). Differences were determined by Statgraphics Centurion XVI and significantly different means (P < 0.05) were separated by the LS means method.
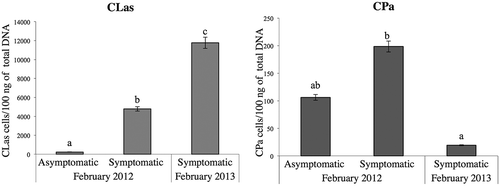
During the second year of the study, at late infection stage, the typical HLB symptoms of asymmetric blotchy mottle became more intense in all analysed branches (39), covering 90–100% of the canopy of central and adjacent trees, so that CLas was detected in all of them. The branches previously detected as positive for CLas () showed a faster disease progression and CLas was detected with an earlier Cq. This indicated an increase in the concentration of CLas. There was a decrease in the number of branches detected as positive for CPa during the second sampling period compared with the first sampling period. In some branches where CPa was detected during the early infection stage, CPa was not detected in the late infection stage of HLB disease development, even though the branches showed more intense HLB symptoms. Among the 39 symptomatic branches, all of them were positive for CLas and 16 trees were positive for CPa with a Cq mean of 18.08 (sd ± 1.40) (11 761 cells/100 ng of DNA) and 34.23 (sd ± 0.38) (19 cells/100 ng of DNA), respectively ().
Discussion
Currently, there is evidence that phytoplasmas can be associated with HLB symptoms (Teixeira et al. Citation2008; Chen et al. Citation2009; Arratia-Castro et al. Citation2014; Lou et al. Citation2014). Phytoplasmas are sometimes present in low concentrations in the plant and insect hosts (Wang & Hiruki Citation2001; Bertaccini & Duduk Citation2009). Therefore, detection methods for both ‘Ca. Liberibacter sp.’ and phytoplasmas with higher levels of specificity and sensitivity are a research priority. Routine phytoplasma detection is based on DNA amplification with nested PCR, followed by RFLP analysis. In this study, a qPCR method to simultaneously detect and quantify 16SrI phytoplasma strains in HLB-affected Mexican lime trees was developed. Furthermore, the assay detected other phytoplasma groups (16SrXIII), which may add to the suitability of the test for a broader detection spectrum. The qPCR assay was compared with the nested PCR, which has been widely used to detect phytoplasmas from citrus trees as well as from other important economic crops. The lowest detection limit of the nested PCR assay was 14 850 16S rRNA gene copies, while the qPCR showed a detection limit of 14.85 copies. This is 1000-fold more sensitive than the nested PCR, which is consistent with previous reports where the sensitivity of the qPCR was higher than that of the nested PCR (Torres et al. Citation2005; Crosslin et al. Citation2006; Nikolić et al. Citation2010). The qPCR assay developed in this study will definitively become a powerful tool for the early detection of CPa in HLB-affected citrus in support of an effective management of HLB.
CPa was more frequently detected in asymptomatic infected mature leaves of the Mexican lime branches at early and late infection stages, while CLas was detected equally in all symptomatic branches at both infection stages (). The distribution of the phytoplasma associated with Australian papaya dieback was investigated by conventional PCR at the different stages of the disease progression. Phytoplasmas can be detected in expanding leaves at an early infection stage, but not in mature leaves at all stages of the disease progression (Siddique et al. Citation1998). Similarly, CLas has been observed in a higher titre in young leaves than in mature leaves (Folimonova & Achor Citation2010). Using conventional and nested PCR, the distribution of the phytoplasma associated with Lethal yellowing (LY) was investigated at the different stages of the disease development (either symptomatic or asymptomatic). The young leaves showed a very high level of LY phytoplasma, low levels were found in the intermediate leaves, whereas no LY phytoplasma DNA was detected in the mature leaves. The detection percentage of LY phytoplasma DNA was lowest in asymptomatic infected palms (Oropeza et al. Citation2011). In this study, the detection percentage of CPa was higher in the asymptomatic mature leaves of the Mexican lime branches at an early infection stage. However, the CPa titre was higher in symptomatic than in the asymptomatic mature leaves at the early infection stage (). The difference between our study and the previous reports might be explained either by the sensitivity of the methods used or by differences of the phytoplasma strains or the host plant used.
Our results do not establish a clear association between the occurrence of CPa and CLas in Mexican lime trees with HLB disease at different stages of the disease development. This may be explained based on the percentage of detection of CPa (71.79%) during early infection stage, which is higher than that for CLas (48.71%). However, in the second sampling year, the progression of the HLB disease was faster, and the percentage of detection of CPa (41.02%) decreased considerably regardless of the progress of symptom expression, while the detection percentage for CLas increased to 100%. The lower detection of CPa in HLB-affected Mexican lime trees in late infection stage (February 2013) can be explained based on the lowest titre in addition to the irregular distribution of CPa in the phloem of woody trees (Seemüller et al. Citation1984a; Berges et al. Citation2000). There are reports on difficulties to detect CPa due to the variation of its titre depending on the season and the plant organ(s) affected (Seemüller et al. Citation1984b; Douglas Citation1986; Chen et al. Citation1992; Sinclair et al. Citation1992; Sahashi et al. Citation1995). Pear decline phytoplasmas are commonly detected at low or near zero concentration in the aerial parts of the trees during the winter due to low temperatures, and survive on roots to recolonize the stem and branches during the following spring (Errea et al. Citation2002). Based on the above, it may be more feasible to test the roots of HLB-affected trees for CPa during the winter months to increase the suitability of the tissue to effectively detect CPa.
In Brazil, CLam was the major Liberibacter species causing HLB in 2004, however, in 2008 the situation reversed and the symptomatic trees were found infected with CLas (Lopes et al. Citation2009). The authors hypothesized that the concentration of CLas in the HLB-affected trees were higher than that of CLam. It is possible that a similar situation is occurring for the HLB-affected Mexican lime trees, and that may explain why during the early infection stage, the incidence of CPa was higher than that of CLas. All HLB-symptomatic branches under study showed a high concentration of CLas during the late infection stage; while the percentage of detection of CPa decreased considerably. CPa concentration was low at both infection stages (199 cells/100 ng of DNA and 19 to cells/100 ng of DNA, respectively), whereas that for CLas was higher (4790–11 761 cells/100 ng of DNA) in symptomatic trees. The reason for the decreasing CPa multiplication from year to year is unknown. We could speculate that CLas is inhibiting CPa multiplication by competing for nutrients, or attachment sites, since both CLas and CPa are strictly restricted to the phloem sieve tubes. Another hypothesis could be that Mexican lime is not a good host plant for CPa multiplication or is vectored by an insect where its multiplication rate is higher. Similarly, in Brazil, Crotalaria juncea is the major source plant of the HLB phytoplasma and is transmitted by the leafhopper Scaphytopius marginelineatus to the sweet orange at a very low rate. The titre of the HLB phytoplasma in sweet orange was low (70 cells per ng DNA); in fact, the HLB phytoplasma titre was more than 1000 times higher in Crotalaria juncea than in sweet orange (Wulff et al. Citation2015).
This is the first report of a qPCR assay developed for the detection of phytoplasmas in HLB-affected Mexican lime trees. Since the level of mixed infection was high for CLas and CPa in the lime trees, this suggests that CPa may be a secondary aetiological agent of HLB, although its role in the mixed infection and disease development remains unknown. Further studies are in progress to decipher any putative antagonist effect related to the co-infection of CLas and CPa.
Acknowledgements
We thank Juan Francisco Félix Hinojosa for the editing of figures.
Additional information
Funding
References
- Angelini E, Luca Bianchi G, Filippin L, Morassutti C, Borgo M. 2007. A new TaqMan method for the identification of phytoplasmas associated with grapevine yellows by real-time PCR assay. J Microbiol Meth. 68:613–622.
- Arocha Y, Lopez M, Pinol B, Fernandez M, Picornell B, Almeida R, Palenzuela I, Wilson MR, Jones P. 2005. ‘Candidatus Phytoplasma graminis’ and ‘Candidatus Phytoplasma caricae’, two novel phytoplasmas associated with diseases of sugarcane, weeds and papaya in Cuba. Int J Syst Evol Microbiol. 55:2451–2463.
- Arratia-Castro AA, Santos-Cervantes ME, Fernández-Herrera E, Chávez-Medina JA, Flores-Zamora GL, Camacho-Beltrán E, Mendez-Lozano J, Leyva-López NE. 2014. Occurrence of ‘Candidatus Phytoplasma asteris’ in citrus showing Huanglongbing symptoms in Mexico. Crop Prot. 62:144–151.
- Baric S, Berger J, Cainelli C, Kerschbamer C, Letschka T, Dalla-Via J. 2011. Seasonal colonization of apple trees by ‘Candidatus Phytoplasma mali’ revealed by a new quantitative TaqMan real-time PCR approach. Eur J Plant Pathol. 129:455–467.
- Baric S, Dalla-Via J. 2004. A new approach to apple proliferation detection: a highly sensitive real-time PCR assay. J Microbiol Meth. 57:135–145.
- Berges R, Rott M, Seemüller E. 2000. Range of phytoplasma concentrations in various plant hosts as determined by competitive polymerase chain reaction. Phytopathology. 90:1145–1152.
- Bertaccini A. 2007. Phytoplasmas: diversity, taxonomy, and epidemiology. Front Biosci. 12:673–689.
- Bertaccini A, Duduk B. 2009. Phytoplasma and phytoplasma diseases: a review of recent research. Phytopathol Mediterr. 48:355–378.
- Bové JM. 2006. Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J Plant Pathol. 88:7–37.
- Chen J, Chang CJ, Jarre RL. 1992. DNA probes as molecular markers to monitor the seasonal occurrence of walnut witches’ broom mycoplasmalike organism. Plant Dis. 76:1116–1119.
- Chen J, Pu X, Deng X, Liu S, Li H, Civerolo E. 2009. A phytoplasma related to ‘Candidatus Phytoplasma asteris’ detected in citrus showing huanglongbing (yellow shoot disease) symptoms in Guangdong, P. R. China. Phytopathology. 99:236–242.
- Christensen NM, Nicolaisen M, Hansen M, Schulz A. 2004. Distribution of phytoplasmas in infected plants as revealed by real-time PCR and bioimaging. Mol Plant-Microbe Interact. 17:1175–1184.
- Córdova I, Oropeza C, Puch-Hau C, Harrison N, Collí-Rodríguez A, Narvaez M, Nic-Matos G, Reyes C, Sáenz L. 2014. A real-time PCR assay for detection of coconut lethal yellowing phytoplasmas of group 16SrIV subgroups A, D and E found in the Americas. J Plant Pathol. 96:343–352.
- Crosslin JM, Vandemark GJ, Munyaneza JE. 2006. Development of a real-time, quantitative PCR for detection of the Columbia Basin potato purple top phytoplasma in plants and beet leafhoppers. Plant Dis. 90:663–667.
- Davis RE, Zhao Y, Dally EL, Lee I-M, Jomantiene R, Douglas SM. 2013. ‘Candidatus Phytoplasma pruni’, a Novel Taxon Associated with X-Disease of Stone Fruits, Prunus spp.: multilocus Characterization Based on 16S rRNA, secY, and Ribosomal Protein Genes. Int J Syst Evol Microbiol. 63:766–776.
- Douglas SM. 1986. Detection of mycoplasma like organisms in peach and chokecherry with X-disease by fluorescence microscopy. Phytopathology. 76:784–787.
- Errea P, Aguelo V, Hormaza JI. 2002. Seasonal variations in detection and transmission of pear decline phytoplasma. J Phytopathol. 150:439–443.
- Folimonova SY, Achor DS. 2010. Early events of citrus greening (Huanglongbing) disease development at the ultrastructural level. Phytopathology. 100:949–958.
- Frost KE, Willis DK, Groves RL. 2011. Detection and variability of aster yellows phytoplasma titer in its insect vector, Macrosteles quadrilineatus (Hemiptera: Cicadellidae). J Econ Entomol. 104:1800–1815.
- Galetto L, Bosco D, Marzachì C. 2005. Universal and group-specific realtime PCR diagnosis of Flavescence dorée (16SrV), Bois noir (16SrXII) and apple proliferation (16SrX) phytoplasmas from field-collected plant hosts and insect vectors. Ann Appl Biol. 147:191–201.
- Gottwald TR. 2010. Current epidemiological understanding of citrus Huanglongbing. Annu Rev Phytopathol. 48:119–139.
- Gundersen DE, Lee IM. 1996. Ultrasensitive detection of phytoplasmas by nested- PCR assays using two universal primer pair. Phytopathol Mediterr. 35:144–151.
- Gundersen DE, Lee I-M, Rehner SA, Davis RE, Kingsbury DT. 1994. Phylogeny of mycoplasmalike organisms (Phytoplasmas): a basis for their classification. J Bacteriol. 176:5244–5254.
- Harrison NA, Womack M, Carpio ML. 2002. Detection and characterization of a lethal yellowing (16SrIV) Group phytoplasma in Canary Island date palms affected by lethal decline in Texas. Plant Dis. 86:676–681.
- Herath P, Hoover GA, Angelini E, Moorman GW. 2010. Detection of elm yellows phytoplasma in elms and insects using real-time PCR. Plant Dis. 94:1355–136060.
- Hodgetts J, Boonham N, Mumford R, Dickinson M. 2009. Panel of 23S rRNA gene-based real-time PCR assays for improved universal and group-specific detection of phytoplasmas. Appl Environ Microbiol. 75:2945–2950.
- Hoshi A, Ishii Y, Kakizawa S, Oshima K, Namba S. 2007. Host-parasite interaction of phytoplasmas from a molecular biological perspective. Bull Insectology. 60:105.
- Hren M, Boben J, Rotter A, Kralj P, Gruden K, Ravnikar M. 2007. Real-time PCR detection system for Flavescence dorée and Bois noir phytoplasmas in grapevine: comparison with conventional PCR detection and application in diagnostics. Plant Pathol. 56:785–796.
- Jagoueix S, Bové JM, Garnier M. 1996. PCR detection of the two ‘Candidatus’ liberobacter species associated with greening disease of citrus. Mol Cell Probes. 10:43–50.
- Jawhari M, Abrahamian P, Sater AA, Sobh H, Tawidian P, Abou-Jawdah Y. 2015. Specific PCR and real-time PCR assays for detection and quantitation of ‘Candidatus Phytoplasma phoenicium’. Mol Cell Probes. 29:63–70.
- Jomantiene R, Davis RE, Maas J, Dally EL. 1998. Classification of new phytoplasmas associated with diseases of strawberry in Florida, based on analysis of 16S rRNA and ribosomal protein gene operon sequences. Int J Syst Evol Microbiol. 48:269–277.
- Liao XL, Zhu SF, Chen HY, Huang WS, Luo K, Zhao WJ, Ma RQ. 2002. Establishment of real-time fluorescent PCR method with TaqMan probe for phytoplasma detection and identification. Acta Phytopathol Sin. 32:361–367.
- Lin H, Chen C, Doddapaneni H, Duan Y, Civerolo EL, Bai X, Zhao X. 2010. A new diagnostic system for ultrasensitive and specific detection and quantitation of ‘Candidatus Liberibacter asiaticus’, the bacterium associated with citrus Huanglongbing. J Microbiol Meth. 81:17–25.
- Lopes SA, Frare GF, Bertolini E, Cambra M, Fernandes NG, Ayres AJ, Marin DR, Bové JM. 2009. Liberibacters associated with citrus huanglongbing in Brazil: ‘Candidatus Liberibacter asiaticus’ is heat tolerant, ‘Ca. L. americanus’ is heat sensitive. Plant Dis. 93:257–262.
- Lou B, Bai X, Bai Y, Deng C, RoyChowdhury M, Chen C, Song Y. 2014. Detection and Molecular Characterization of a 16SrII‐A Phytoplasma in Grapefruit (Citrus paradisi) with Huanglongbing‐like Symptoms in China. J Phytopathol. 162:387–395.
- Marcone C, Seemüller E. 2001. A chromosome map of the European stone fruit yellows phytoplasma. Microbiology. 147:1213–1221.
- Marzachì C. 2004. Molecular diagnosis phytoplasmas. Phytopathol Mediterr. 43:228–231.
- Marzachì C, Bosco D. 2005. Relative quantification of chrysanthemum yellows (16SrI) phytoplasma in its plant and insect host using real-time polymerase chain reaction. Mol Biotechnol. 30:117–127.
- Monti M, Martini M, Tedeschi R. 2013. EvaGreen Real-time PCR protocol for specific Candidatus Phytoplasma mali detection and quantification in insects. Mol Cell Probes. 27:129–136.
- Nikolić P, Mehle N, Gruden K, Ravnikar M, Dermastia M. 2010. A panel of real-time PCR assays for specific detection of three phytoplasmas from the apple proliferation group. Mol Cell Probes. 24:303–309.
- Oropeza C, Cordova I, Chumba A, Narváez M, Sáenz L, Ashburner R, Harrison N. 2011. Phytoplasma distribution in coconut palms affected by lethal yellowing disease. Ann Appl Biol. 159:109–117.
- Oshima K, Kakizawa S, Nishigawa H, Jung HY, Wei W, Suzuki S, Arashida R, Nakata D, Miyata S, Ugaki M, Namba S. 2004. Reductive evolution suggested from the complete genome sequence of a plant-pathogenic phytoplasma. Nat Genet. 36:27–29.
- Pelletier C, Sala P, Gillet J, Cloquemin G, Very P, Foissac X, Malembic-Maher S. 2009. Triplex real-time PCR assay for sensitive and simultaneous detection of grapevine phytoplasmas of the 16SrV and 16SrXII-A groups with an endogenous analytical control. Vitis. 48:87–95.
- Sahashi N, Nakamura H, Yoshikawa N, Kubono T, Shoji T, Takahashi T. 1995. Distribution and seasonal variation in detection of phytoplasma in bark phloem tissues of single Paulownia trees infected with witches’ broom. Ann Phytopathol Soc Japan. 61:481–484.
- Santos-Cervantes ME, Chávez-Medina JA, Acosta-Pardini J, Flores-Zamora GL, Méndez-Lozano J, Leyva-López NE. 2010. Genetic diversity and geographical distribution of phytoplasmas associated with potato purple top disease in Mexico. Plant Dis. 94:388–395.
- Schneider B, Seemüller E. 1994. Presence of two sets of ribosomal genes in phytopathogenic mollicutes. Appl Environ Microbiol. 60:3409–3412.
- Seemüller E, Kunze L, Schaper U. 1984a. Colonization behavior of MLO, and symptom expression of proliferation diseased apple trees and decline diseased pear trees over a period of several years. Z Pflanzenkr Pflanzenschutz. 91:525–532.
- Seemüller E, Schaper U, Zimbelmann F. 1984b. Seasonal variation in the colonization patterns of mycoplasmalike organisms associated with apple proliferation and pear decline. Z Pflanzenkr Pflanzenschutz. 91:371–382.
- SIAP-SAGARPA, Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. 2014. Servicio de información Agroalimentaria y Pesquera. Available from: http://www.siap.gob.mx/cierre-de-la-produccion-agricola-por-estado/
- Siddique ABM, Gurthrie JN, Walsh KB, White DT, Scott PT. 1998. Histopathology and within-plant distribution of the phytoplasma associated with Australian papaya dieback. Plant Dis. 82:1112–1120.
- Sinclair WA, Griffiths HM, Davis RE, Lee IM. 1992. Detection of ash yellows mycoplasma like organisms in different tree organs and in chemically preserved specimens by DNA probe vs. DAPI. Plant Dis. 76:154–158.
- Teixeira DC, Wulff NA, Martins EC, Kitajima EW, Bassanezi R, Ayres AJ, Eveillard S, Saillard C, Bové JM. 2008. A phytoplasma closely related to the pigeon pea witches’-broom phytoplasma (16SrIX) is associated with citrus huanglongbing symptoms in the estate of São Paulo, Brazil. Phytopathology. 98:977–984.
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680.
- Torres E, Bertolini E, Cambra M, Montón C, Martín MP. 2005. Real time PCR for simultaneous and quantitative detection of quarantine phytoplasmas from apple proliferation (16SrX) group. Mol Cell Probes. 19:334–340.
- Wang K, Hiruki C. 2001. Molecular characterization and classification of phytoplasmas associated with canola yellowsand a new phytoplasma strain associated with dandelions. Plant Dis. 85:76–79.
- Wei W, Kakizawa S, Suzuki S, Jung HY, Nishigawa H, Miyata S, Oshima K, Ugaki M, Hibi T, Namba S. 2004. In planta dynamic analysis of onion yellows phytoplasma using localized inoculation by insect transmission. Phytopathology. 94:244–250.
- Wulff NA, Teixeira DC, Martins EC, Toloy RS, Bianco LF, Colletti DAB, Kitajima EW, Bové JM. 2015. Sunn hemp, a major source-plant of the phytoplasma associated with huanglongbing symptoms of sweet orange in São Paulo State, Brazil. J Cit Pathol. Available from: iocv_journalcitruspathology_26956
- Yue HN, Wu YF, Shi YZ, Wu KK, Li YR. 2008. First report of Paulownia witches’-broom phytoplasma in China. Plant Dis. 92:1134.
- Yvon M, Thébaud G, Alary R, Labonne G. 2009. Specific detection and quantification of the phytopathogenic agent ‘Candidatus Phytoplasma prunorum’. Mol Cell Probe. 23:227–234.
- Zhang YP, Uyemoto JK, Kirkpatrick BC. 1998. A small-scale procedure for extracting nucleic acids from woody plants infected with various phytopathogens for PCR assay. J Virol Meth. 71:45–50.