Abstract
Stripe (yellow) rust, caused by the biotrophic fungal pathogen Puccinia striiformis f. sp. tritici (Pst), is emerging as a serious threat to wheat (Triticum aestivum L.) production in many regions of North America. Genetic resistance to stripe rust is typically conditioned by the products of resistance genes that detect pathogen-associated molecular patterns and initiate a cascade of signalling events, culminating in the generation of reactive oxygen species (ROS) and a hypersensitive response (HR) that eventually suppress the pathogen. Complete resistance with no symptoms (immunity response) has also been observed, but less frequently, in certain Pst-wheat interactions. However, such HR-independent resistance responses to Pst have not been well studied. Here we report that the Pst-resistant near isogenic line Avocet-Yr15 (carrying the Yr15 resistance gene) exhibited an immunity response to an isolate of Pst collected in Elora, Ontario, while the Avocet-S line (lacking Yr15) was highly susceptible to the same isolate. In particular, histochemical assays of defence-associated ROS generation and HR were negative during the early stages of the interaction between Pst and the resistant Avocet-Yr15. Microscopic analysis indicated that the pathogen was not able to penetrate the stomata of Avocet-Yr15. Moreover, RNA-sequencing of the immunity response of Avocet-Yr15 to Pst highlighted the activation of a global anti-oxidative-stress response consisting of genes involved in the maintenance of cell viability, redox homoeostasis and photosynthesis. These results provide insights into the molecular mechanisms controlling the immunity response to stripe rust of wheat.
Résumé
La rouille jaune, causée par l’agent pathogène fongique biotrophe Puccinia striiformis f. sp. tritici (Pst), semble être une sérieuse menace pour la production de blé (Triticum aestivum L.) dans de nombreuses régions d’Amérique du Nord. La résistance génétique à la rouille jaune est généralement conditionnée par les produits des gènes de résistance qui détectent les schémas moléculaires associés aux agents pathogènes et amorcent une série d’événements en cascade, aboutissant à la production d’espèces réactives de l’oxygène (ERO) et à une réaction d’hypersensibilité (RH) qui éventuellement supprime l’agent pathogène. La résistance totale sans symptômes (réponse immunitaire) a également été observée, mais plus rarement, dans le cadre d’interactions Pst-blé. Toutefois, de telles réactions de résistance indépendantes de la réaction d’hypersensibilité à Pst n’ont pas été étudiées en profondeur. Dans cette étude, nous soulignons que la lignée quasi-isogénique Avocet-Yr15 résistante à Pst (portant le gène de résistance Yr15) a affiché une réponse immunitaire à un isolat de Pst collecté à Elora, en Ontario, tandis que la lignée Avocet-S (sans Yr15) était extrêmement sensible au même isolat. Entre autres, des tests histochimiques de défense associés à la production d’ERO et à une RH étaient négatifs durant les premiers stades de l’interaction entre Pst et la lignée résistante. L’analyse microscopique a montré que l’agent pathogène était incapable de pénétrer les stomates de la lignée Avocet-Yr15. Qui plus est, le séquençage de l’ARN résultant de la réponse immunitaire de la lignée Avocet-Yr15 à Pst a mis en lumière l’activation d’une réaction générale au stress antioxydant induite par des gènes impliqués dans la maintenance de la viabilité de la cellule, de l’homéostasie rédox et de la photosynthèse. Ces résultats jettent une nouvelle lumière sur les mécanismes responsables de la réponse immunitaire à la rouille jaune du blé.
Introduction
Stripe (yellow) rust disease of wheat is caused by the biotrophic fungal pathogen Puccinia striiformis f.sp. tritici (Pst). It is becoming a major economic threat to wheat production in Canada and worldwide, causing considerable reductions in yield (Solh et al. Citation2012; Beddow et al. Citation2015; Xi et al. Citation2015; Brar et al. Citation2019). The use of resistant wheat varieties is one of the most effective approaches to manage the disease (Chen Citation2013). However, genetic resistance can be broken down by new virulent races of Pst (Chen Citation2014). Two distinct types of resistance responses have been reported, namely ‘adult plant resistance’ and ‘race-specific or all-stage resistance’ (Chen et al. Citation2013). Adult plant resistance usually starts expressing when wheat plants are at the full flag-leaf emergence stage (i.e., post seedling stage). Such resistance is often durable and broad-spectrum (i.e., non-race-specific), leading to partial or incomplete resistance to Pst (Chen et al. Citation2013). Race-specific or all-stage resistance, on the other hand, results in complete resistance, following the classical ‘gene-for-gene’ model of plant immune response to pathogens (Jones and Dangl Citation2006). This resistance manifests itself in three distinct yet interconnected levels, which include (i) the recognition of signature molecules derived from the invading pathogen, such as pathogen-associated molecular patterns (PAMPs) and effector proteins, by the plant surveillance system; (ii) the initiation of defence-associated signalling events, such as the generation of reactive oxygen species (ROS, like H2O2) and activation of kinase protein cascades; and eventually (iii) the induction of localized cell death and hypersensitive response (HR), culminating in the suppression of the infectious pathogen (Hammond-Kosack and Jones Citation1996; Jones and Dangl Citation2006). The HR is characterized as a type of rapid programmed cell death (PCD) at the site of pathogen entry, protecting the remaining plant tissue while depriving the pathogen access to vital nutrients from its host. It also leads to the activation of several downstream defence mechanisms such as cell wall fortification, biosynthesis of anti-microbial compounds (i.e., phytoalexins) and pathogenesis-related proteins (PRs) that collectively cause the cessation of pathogen growth and infection (Govrin and Levine Citation2000; Jones and Dangl Citation2006). To date, several resistance (R) genes that induce an HR-mediated resistance response to stripe rust have been identified and reported such as Yr1, Yr5, Yr10, Yr15 and Yr24 and more (Zeng et al. Citation2014). In particular, the R gene Yr15, originally identified from wild emmer wheat, confers resistance to nearly all Pst races originating from different locations worldwide, with only a few reports of compatible (i.e., virulent) cases (Peng et al. Citation2000; Qie et al. Citation2019). It encodes a signalling-associated kinase/pseudo-kinase tandem protein, named wheat tandem kinase 1 (WTK1). (Klymiuk et al. Citation2018). Macroscopic and microscopic observations revealed that the Yr15-mediated resistance mechanism is controlled by the induction of a strong PCD after the completion of fungal penetration through the stomata and the formation of feeding structures in the host leaf tissue (Klymiuk et al. Citation2018).
Typically, in studies on the wheat-stripe-rust interaction, a spectrum of different levels of resistance and susceptibility responses can be observed in the host. The resistance response ranges from immunity (i.e., complete resistance with no visible symptoms of HR) to HR-mediated resistance (i.e., HR-associated chlorotic spots with no traces of sporulation), while the susceptibility response ranges from moderate susceptibility (i.e., chlorotic or necrotic blotches with partial levels of sporulation) to severe susceptibility (i.e., heavily infected tissue with high levels of sporulation) (Singh et al. Citation2000; Chen Citation2013; Zeng et al. Citation2014; McIntosh et al. Citation2018; Klymiuk et al. Citation2018). HR-mediated defence mechanisms are typical resistance responses to the stripe rust pathogen in resistant wheat varieties (Yu et al. Citation2010; Dobon et al. Citation2016), including Yr15-harbouring wheat lines (Klymiuk et al. Citation2018); hence its characteristics have been well documented and discussed. However, mechanisms controlling the less-often-seen HR-independent immunity to stripe rust have not been well studied.
Here, we report that the near-isogenic wheat line Avocet harbouring Yr15 (Avocet-Yr15) is completely resistant to a local (Elora, ON) Pst isolate (UGW16001) through an HR-independent immunity response, whereas the control line lacking the Yr15 gene (Avocet-S) remains completely susceptible to that isolate. Microscopic and RNA-seq transcriptome analyses suggested a resistance model in which stomatal defence is coupled with a broad antioxidant reaction in the host to maintain redox homoeostasis, and photosynthesis underpins the observed immunity response. Our results shed light on molecular mechanisms controlling the immunity response to rust diseases in wheat.
Material and methods
Pathogen isolates and wheat lines
The stripe rust inoculum used in this study was a Pst isolate (UGW16001; Pst) collected from the wheat research fields at the Elora Research Station (University of Guelph) in Elora, ON, Canada in 2016 (Serajazari et al. Citation2018). Near-isogenic spring wheat lines of Avocet-Yr15, carrying the resistance gene Yr15, and its susceptible background parent Avocet-S (received from CYMMIT, Mexico) were used to study defence response to Pst. In order to generate an induced HR reaction to the Pst isolate and to compare the results with the immunity response, seedlings of the wheat cultivar ‘Thatcher’ were used and subjected to silicon (Si) treatment as an inducer of plant defence response to a wide range of phytopathogens (Ghanmi et al. Citation2004; Fauteux et al. Citation2005; Van Bockhaven et al. Citation2015). Si treatment was performed by supplementing 5 mM aqueous solution of sodium silicate (Na2SiO3) into the potting soil for two weeks before inoculation.
Growth conditions, inoculation method, and disease evaluation
Seedlings of the Avocet-Yr15, Avocet-S and ‘Thatcher’ were inoculated with the Pst isolate at the two-leaf stage (2 weeks old). Urediniospores of Pst were reactivated with a heat shock treatment at 45°C for 5 min. The adaxial leaf surface of 10 plants was inoculated using a mixture of spores and talcum powder (1:20 ratio) and a small paintbrush. Control samples were treated only with talcum powder. To provide optimal conditions for spore germination and fungal penetration, inoculated wheat plants were kept in a dark dew chamber at 15°C for 48 h with 90% of relative humidity. Plants were then transferred to growth chambers at 21/17°C with 16/8 h light/dark regimes and 70% humidity and were monitored for two weeks for disease progression. Disease evaluation was done at 4, 7 and 14 days post-inoculation (dpi) following the McNeal infection-type scale (McNeal et al. Citation1971) with some modifications. Briefly, the 0–9 McNeal scale was modified to a 0–4 defence-response scale/scores with 0 (McNeal: 0) for ‘immunity’, i.e., complete resistance with no visible symptoms of HR; 1 (McNeal 1–2) for ‘HR-mediated resistance’; 2 (McNeal: 3–5) for ‘low susceptibility or moderate resistance’, i.e., chlorotic blotches with sporadic sporulation; 3 (McNeal: 6–7) for ‘moderate susceptibility’, i.e., moderate sporulation; and 4 (McNeal 8–9) for ‘severe susceptibility’, i.e., fully infected tissue covered with a high level of sporulation (Supplementary Fig. S2). A Disease Index (DI) was calculated for each plant based on the above-mentioned modified scores using the following formula: DI (%) = (score of 1st leaf + score of 2nd leaf)/(2 ✕ highest score) ✕ 100.
Histochemical analysis and visualization of host defence response
Inoculated leaf samples (first and second leaves) were cut and collected at 4, 7 and 14 dpi and were immediately fixed using 100% ethanol. The leaf samples harbouring fungal mycelia were stained with 0.1% trypan blue, and the accumulation pattern of hydrogen peroxide was determined by staining with 1 mg mL−1 diaminobenzidine (DAB) (visualized as a dark orange colour) following the procedures described previously (Seifi and Shelp Citation2019). All the leaf samples were mounted in 50% glycerol for microscopic analyses. The autofluorescence of deposited phenolic compounds in the cell wall was detected using an Olympus BX51 epi-fluorescence microscope (U-MWB2 GPF filter set, excitation filter 450–480 nm, dichroic beam splitter 500 nm, barrier filter BA515; Tokyo, Japan) according to Seifi et al. (Citation2013a). All the leaf samples were mounted in 50% glycerol for microscopic analyses.
RNA extraction, sequencing and data analysis
Control and Pst-inoculated leaf samples from Avocet-S and Avocet-Yr15 seedlings (i.e., four treatments in total) were collected at 4 dpi, flash-frozen, and stored at −80°C for later RNA extraction. For each treatment, leaf samples were excised from nine individual plants, pooled into three replicates (i.e., n = 3, and each replicate contained leaf samples from three individual plants). Total RNA was extracted using a NucleoSpin RNA Plant kit (Macherey-Nagel, Düren, Germany) following the manufacturer’s protocol. The quantity and quality of RNA samples were assessed at the Genomics Facility of the University of Guelph (Guelph, ON) using an Agilent 2100 BioAnalyser System (Agilent Technologies, Palo Alto, CA). High-quality RNA samples (RNA Integrity Number ≥ 6.5) were sequenced at the Genome Quebec Innovation Center of McGill University (Montreal, QC). RNA-seq data analysis was performed using the Galaxy platform (accessible at https://usegalaxy.org/) established on the High-Performance Cloud computing service provided by the Southern Ontario Smart Computing Innovation Platform (SOSCIP; University of Toronto, ON), following the pipeline previously described (Seifi and Shelp Citation2019). Briefly, quality control of data was performed using FastQC (accessible at http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Read filtering and Quality trimmings were done using the Trimmomatic tool (Bolger et al. Citation2014). Tophat2, Cufflinks, Cuffmerge, and Cuffdiff (Trapnell et al. Citation2012) were, respectively, used for read-alignment, mapping, transcriptome assembly, merging of assembly data, and differential gene expression analysis. The bread wheat whole genome sequence assembly TGACv1 (Triticum aestivum; http://plants.ensembl.org/Triticum_aestivum/Info/Index) was used as the reference genome for read alignment and mapping. The R package CummeRbund (Trapnell et al. Citation2012) was used for visualization of the data generated. Venn diagram generation was done using jvenn (Bardou et al. Citation2014) (accessible at http://jvenn.toulouse.inra.fr/app/example.html). The EnsemblPlants database (accessible at https://plants.ensembl.org/) was used for gene annotation analysis. ShinyGO (ShinyGO v0.61 (Ge et al. Citation2019); accessible at http://bioinformatics.sdstate.edu/go/)) was used for gene ontology enrichment (GOE) analysis, hierarchical clustering tree and network analysis.
Results
Avocet-Yr15 wheat exhibited immunity to the Pst isolate
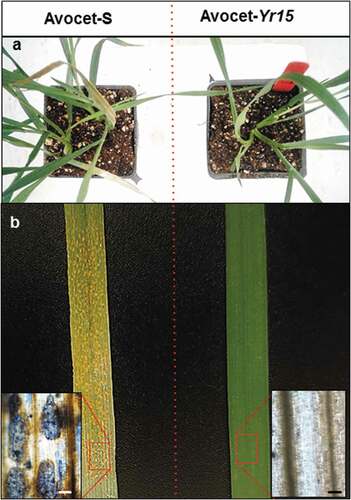
Inoculation of Avocet-Yr15 and Avocet-S plants with Pst-induced immunity and severe susceptibility, respectively (). No symptoms of typical defence-associated PCD and HR reactions (such as silicon-induced HR; Supplementary Fig. S1) were detected on the wheat leaf on Avocet-Yr15, while Avocet-S gradually developed severe susceptibility symptoms, including heavily infected leaf tissue entirely covered with pustules or uredinia (). At 14 dpi, no visible difference was detected between mock and Avocet-Yr15 inoculated plants. Microscopic analysis indicated a severe tissue collapse in Avocet-S leaves, accompanied by the release of urediniospores from uridinia, whereas Avocet-Yr15 plants remained symptomless with no sign of PCD or HR reaction (, bottom right), as is typically seen in leaf tissue undergoing HR (Supplementary Fig. S1).
Fig. 1 Susceptibility and resistance responses to Puccinia striiformis f. sp. tritici isolate UGW16001 in the wheat lines Avocet-S and Avocet-Yr15 at 21 dpi. (a) Top views of infected Avocet-S and Avocet-Yr15 plants. (b) Macroscopic and microscopic appearance of disease symptoms (left) and immunity response (right). Heavy sporulation can be seen in the pustule/uredinia formed on the Avocet-S leaf (left). Fungal structures were stained with trypan blue. Scale bars = 50 µm
Pst failed to penetrate the Avocet-Yr15 stomata
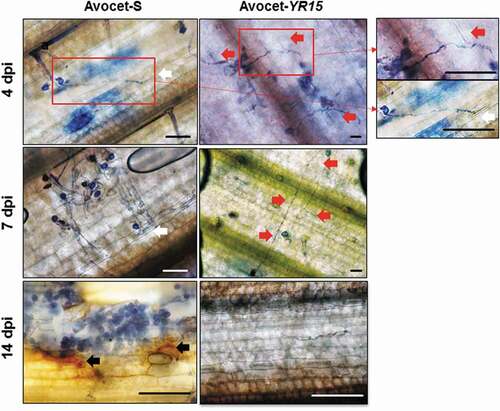
Microscopic analysis of the infected area on the adaxial surface of Avocet-Yr15 and Avocet-S leaves was performed at different time-points post-inoculation to monitor resistance and susceptibility responses to the Pst isolate, respectively (). Germinated spores of Pst with initial development of fungal mycelia were similarly observed on both resistant and susceptible genotypes at 4 dpi, with germ tubes elongating towards the stomata of their hosts. Notably, while the fungal pathogen was able to successfully locate and penetrate the stomata on the Asvocet-S leaf (, white arrows), its ingress into the stomata of Avocet-Yr15 seemed to fail around nearby stomata, forcing the Pst hyphae to elongate further in search for another entry point (, red arrows). At 14 dpi, the mycelial mass of the pathogen was considerably depleted, as only small traces of fungal hyphae were observed on Avocet-Yr15 (, red ellipse), while sporulation of the pathogen (i.e., the release of urediniospores from uredinia) were evident on Avocet-S (, bottom left). Visualization of H2O2 generation with DAB showed no particular area with a high level of defence-associated ROS accumulation on Avocet-Yr15. In contrast, due to the disintegration of the leaf tissue, areas with a high level of H2O2 accumulation were observed around the pustules in Avocet-S at 14 dpi ( black arrows).
Fig. 2 Microscopic analysis of the growth of Puccinia striiformis f. sp. tritici isolate UGW16001 germ-tube on the surface of leaves of the wheat lines Avocet-S and Avocet-Yr15 at 4, 7 and 14 dpi. White arrows indicate successful penetration into the Avocet-S leaf. Red arrows indicate prevention of penetration into the Avocet-Yr15 leaf. Black arrows show areas with brownish/orange colouration indicating diaminobenzidine (DAB)-stained accumulation of H2O2. Fungal structures were stained with trypan blue. Scale bars = 50 µm
RNA-seq analysis indicated transcriptional modifications associated with immunity against Pst in Avocet-Yr15
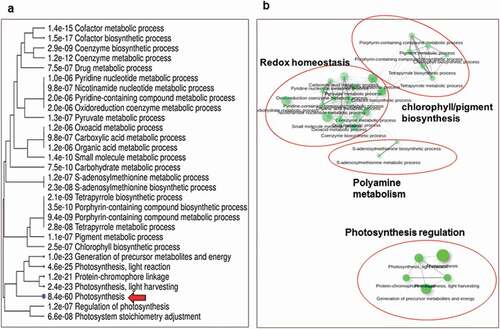
It has been previously shown that 3–5 dpi is the best window to capture the transcriptional difference between susceptible and resistance responses in the Pst-Avocet interaction (Dobon et al. Citation2016). Therefore, global patterns of gene expression in the ‘resistant’ interaction, i.e., Pst-inoculated and mock-inoculated Avocet-Yr15 plants (labelled as ‘RI’ and ‘RM’, respectively) and the susceptible interaction, i.e., Pst-inoculated and mock-inoculated Avocet-S plants (labelled as ‘SI’ and ‘SM’, respectively) were evaluated by high-throughput RNA-sequencing at 4 dpi. Differential gene expression analysis identified 517 genes (DEGs) that were uniquely upregulated (cut-offs: log2 fold change ≥ 2, fpkm ≥ 1, p < 0.05) in the resistant interaction (RI vs. RM, shown as RI/RM), compared with the susceptible interaction (SI vs. SM, shown as SI/SM (). GOE analysis revealed functional categories, defined by high-level GO terms, which are involved in the observed immunity response to Pst in Avocet-Yr15 (). Notably, genes involved in stress and immune responses, catabolic process, detoxification and regulation of biological quality were among the most represented function categories (). Moreover, key biological processes controlling asymptomatic immunity in Avocet-Yr15 were clustered into several interconnected groups, among which pathways involved in photosynthesis regulation, chlorophyll biosynthesis, redox homoeostasis (response to oxidative stress) and polyamine metabolism (biosynthesis) were highly represented, with the photosynthesis group being the most significant one (. summarizes further information regarding 12 important candidate genes determined using the Ensembl Plant Database (Bolser et al. Citation2016). Complete lists of the GOE analysis of the DEGs are provided in Supplementary Tables S1 and S2.
Table 1. Selected candidate differentially expressed genes (DEGs) associated with the immunity response of the wheat line Avocet-Yr15 to Puccinia striiformis f. sp. tritici isolate UGW16001
Fig. 3 Transcriptome analysis of the defence responses of the wheat lines Avocet-Yr15 and Avocet-S to challenge by Puccinia striiformis f. sp. tritici isolate UGW16001. (a) Venn diagram showing the unique and overlapping sets of genes significantly (P < 0.05) and differentially (log2 fold change ≥ 2) upregulated in the various interactions (i.e., SI: Avocet-S and Pst-inoculated; SM: Avocet-S and mock-inoculated; RI: Avocet-Yr15 and Pst-inoculated; RM: Avocet-Yr15 and mock-inoculated). The set of 517 differentially expressed genes (DEGs) exclusively belonging to RI/RM was selected for the downstream gene ontology enrichment analysis. (b) Enriched ‘Biological Process’ gene ontology terms of the selected genes
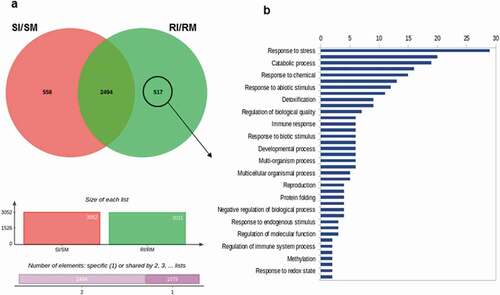
Fig. 4 (a) Hierarchical clustering tree of related gene ontology (GO) terms indicating the correlation among significant pathways associated with immunity of the wheat line Avocet-Yr15 to Puccinia striiformis f. sp. tritici isolate UGW16001. A larger size of the blue circles indicates more significant p-values. (b) Biological network analysis visualizing relationships among groups of enriched gene-sets detected by gene ontology enrichment (GOE) analysis. The thickness of the connecting lines reflects percent of overlapping genes. Size of the nodes corresponds to number of genes
Discussion
In this study, we present evidence of immunity in Avocet-Yr15 wheat to a local Pst isolate. Microscopic and histochemical analyses showed that the defence response to stripe rust, in this particular case, is not mediated by the induction of PCD and HR. Furthermore, it was revealed that the infectious mycelia of Pst were unable to effectively penetrate through the stomata on the surface of Avocet-Yr15 during the early stages of the interaction, whereas successful stomatal localization and penetration were seen in the susceptible Avocet-S plants. Likewise, RNA-seq-based differential gene expression and GOE analyses detected no traces of the induction of PCD and HR reactions in the resistant interaction compared to the susceptible one (i.e., RI/RM vs. SI/SM). Instead, the analysis of the Avocet-Yr15/Pst interaction highlighted genes involved in ‘stress/immune response’ () through biological processes attributed to maintaining tissue/cell viability and basic metabolic functions such as photosynthesis, redox homoeostasis and polyamine biosynthesis ().
While HR-mediated defence is widely known as the predominant defence response to stripe rust in resistant wheat varieties (Yu et al. Citation2010; Klymiuk et al. Citation2018), immune reactions have also been observed in much lower frequency in certain interactions between Pst isolates and wheat genotypes. Notably, in the case of wheat lines carrying Yr15, Klymiuk et al. (Citation2018) have shown that, in addition to HR being the main defence response in resistant hosts, in a few interactions with certain Pst isolates, such as isolate Pst#5006 on the line B70 and isolate AU85569 on Avocet-Yr15, immunity responses were also observed.
Generally, foliar pathogens establish infections by entering the host tissue through natural openings, such as the stomata and hydathodes, or through wounds (Melotto et al. Citation2017). Entry of the stripe rust pathogen into the host, which occurs by hyphal growth through stomata during the early stages of the infection (mainly during the first 24 h after inoculation), plays a vital role in its virulence and host colonization (Cantu et al. Citation2013). Some resistant plants have evolved defence mechanisms to close stomata upon timely sensing of PAMP signals derived from the pathogen growing on the surface of the leaf, prior to the actual penetrative attack (Melotto et al. Citation2017). A similar stomatal defence response seems to be activated in Avocet-Yr15 in response to Pst infection, preventing the pathogen from initiating the penetration phase ().
Since several immune-response-associated genes (such as SAR-associated DIR1 and PR10) were upregulated in Pst-inoculated Avocet-Yr15 compared with mock-inoculated controls (i.e., RI/RM; and ). We speculate that Avocet-Yr15 was able to sense the presence of the rust pathogen growing on its leaves in a timely manner, and trigger a properly timed defence response, thus preventing the pathogen from colonizing the apoplast. Such stomatal-defence-mediated immunity has been observed in incompatible interactions between the stripe rust pathogen and non-host plants (An et al. Citation2016). For instance, inoculation of the non-host broad bean plant results in the impairment of Pst penetration through stomata, accompanied by strong activation of several layers of defence reactions in the host (Cheng et al. Citation2012). Likewise, successful penetration by Pst is significantly reduced in Pst-inoculated Arabidopsis plants, leading to a symptomless appearance in the host (Cheng et al. Citation2013).
The induction of polyamine biosynthesis and its accumulation in the plant tissue plays a protective role in response to a broad spectrum of biotic and abiotic stresses, particularly when the tissue undergoes oxidative stress (Alcázar et al. Citation2006; Seifi and Shelp Citation2019). In particular, the increase in arginine decarboxylase results in higher levels of polyamines, leading to higher tolerance to pollutants and heavy metal contamination by alleviating oxidative damage and preventing chlorophyll degradation (An and Wang Citation1997; Yu et al. Citation2015). Notably, the pronounced representation of polyamine biosynthesis pathways (such as arginine decarboxylase and s-adenosylmethionine biosynthetic genes), along with several antioxidant agents (such as superoxide dismutase, glutathione, and ascorbate peroxidases) in Avocet-Yr15 ( and ) during challenge by Pst, suggests that the plant is able to maintain intracellular and cellular redox equilibrium during the defence response to the fungal pathogen. Such a broad antioxidant response coupled with a significant overrepresentation of pathways regulating photosynthesis and chlorophyll biosynthesis () may explain the observed HR-independent resistance in Avocet-Yr15 and the symptomless and fully green phenotype even after 21 dpi (). Induction of such an anti-cell-death state in a plant host to maintain cell viability and basic metabolisms has been termed as the ‘endurance response’, which is typically triggered in resistant hosts in response to necrotrophic pathogens (Seifi et al. Citation2013b). However, here we observe that, in the absence of infectious penetration of the host tissue by a biotrophic pathogen, an endurance-like response has been activated in Avocet-Yr15. This observation suggests that the Yr15-mediated HR, entailing strong PCD in the host tissue (Klymiuk et al. Citation2018), is only triggered when the infectious mycelium of Pst breaks into the leaf tissue and invades the mesophyll cells. The absence of an invasive penetration, therefore, may explain why the induction of the WTK1 gene was not detected in our transcriptomic data. Moreover, conducting RNA-seq analysis in Avocet-Yr15 plants at only one time-point (4 dpi) may have also contributed to such results.
Collectively, our results favour a model in which the Pst isolate was prevented from effectively penetrating Avocet-Yr15 tissue, forcing the germ tube of the fungal pathogen to elongate further in search for an ingress point. Ultimately, elongation does not provide access, even at the later stages of the interaction (21 dpi), resulting in the death and total elimination of the pathogen. On the host side, Avocet-Yr15 appears to be alerted of the presence of the pathogen growing on the leaf surface, possibly by perceiving released PAMPs, leading to strong induction of genes related to the immune system and stress response. The prevention of penetration into the mesophyll, however, negates the need for the plant tissue to undergo an HR-mediated defence reaction which entails the cell death of the host tissue. Rather, the resistant host activates a broad antioxidant response in the attacked tissue to protect basal metabolism and photosynthesis, while maintaining redox homoeostasis.
Supplemental Table 2
Download MS Excel (7.3 KB)Supplemental Table 1
Download MS Excel (7.1 KB)Supplemental Figure 2
Download MS Power Point (492.8 KB)Supplemental Figure 1
Download MS Power Point (2.2 MB)Acknowledgements
The publication is dedicated to the memory of Professor Alireza Navabi who was the principal investigator of this study and led the Wheat Breeding Group from 2014-2019 at the Department of Plant Agriculture, University of Guelph.
Supplemental material
Supplemental data for this article can be accessed online here: https://doi.org/10.1080/07060661.2021.1907448.
Additional information
Funding
References
- Alcázar R, Marco F, Cuevas JC, Patron M, Ferrando A, Carrasco P, Tiburcio AF, Altabella T. 2006. Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett. 28:1867–197. doi:https://doi.org/10.1007/s10529-006-9179-3.
- An LZ, Wang XL. 1997. Changes in polyamine contents and arginine decarboxylase activity in wheat leaves exposed to ozone and hydrogen fluoride. J Plant Physiol. 150:184–187. doi:https://doi.org/10.1016/S0176-1617(97)80200-3.
- An T, Cai Y, Zhao S, Zhou J, Song B, Bux H, Qi X. 2016. Brachypodium distachyon T-DNA insertion lines: a model pathosystem to study nonhost resistance to wheat stripe rust. Sci Rep. 6:1–9. doi:https://doi.org/10.1038/srep25510.
- Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C. 2014. jvenn: an interactive Venn diagram viewer. BMC Bioinformatics. 15:293. doi:https://doi.org/10.1186/1471-2105-15-293.
- Beddow JM, Pardey PG, Chai Y, Hurley TM, Kriticos DJ, Braun HJ, Park RF, Cuddy WS, Yonow T. 2015. Research investment implications of shifts in the global geography of wheat stripe rust. Nat Plants. 1:1–5. doi:https://doi.org/10.1038/nplants.2015.132.
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30:2114–2120. doi:https://doi.org/10.1093/bioinformatics/btu170
- Bolser D, Staines DM, Pritchard E, Kersey P. 2016. Ensembl Plants: Integrating tools for visualizing, mining, and analyzing plant genomics data. Methods Mol Biol. 1374:115–140.
- Brar GS, Fetch T, McCallum BD, Hucl PJ, Kutcher HR. 2019. Virulence dynamice and breeding for resistance to stripe, stem, and leaf rust in Canada since 2000. Plant Dis. 103:2981–2995. doi:https://doi.org/10.1094/PDIS-04-19-0866-FE.
- Cantu D, Segovia V, MacLean D, Bayles R, Chen X, Kamoun S, Dubcovsky J, Saunders DGO, Uauy C. 2013. Genome analyses of the wheat yellow (stripe) rust pathogen Puccinia striiformis f. sp. tritici reveal polymorphic and haustorial expressed secreted proteins as candidate effectors. BMC Genomics. 14:270. doi:https://doi.org/10.1186/1471-2164-14-270.
- Chen X. 2013. Review Article: high-temperature adult-plant resistance, key for sustainable control of stripe rust. Am J Plant Sci. 04:608–627. doi:https://doi.org/10.4236/ajps.2013.43080.
- Chen X, Coram T, Huang X, Wang M, Dolezal A. 2013. Understanding molecular mechanisms of durable and non-durable resistance to stripe rust in wheat using a transcriptomics approach. Curr Genom. 14:111–126. doi:https://doi.org/10.2174/1389202911314020004.
- Chen XM. 2014. Integration of cultivar resistance and fungicide application for control of wheat stripe rust. Can J Plant Pathol. 36:311–326. doi:https://doi.org/10.1080/07060661.2014.924560.
- Cheng Y, Zhang H, Yao J, Han Q, Wang X, Huang L, Kang Z. 2013. Cytological and molecular characterization of non-host resistance in Arabidopsis thaliana against wheat stripe rust. Plant Physiol Biochem. 62:11–18. doi:https://doi.org/10.1016/j.plaphy.2012.10.014.
- Cheng Y, Zhang H, Yao J, Wang X, Xu J, Han Q, Wei G, Huang L, Kang Z. 2012. Characterization of non-host resistance in broad bean to the wheat stripe rust pathogen. BMC Plant Biol. 12:96. doi:https://doi.org/10.1186/1471-2229-12-96.
- Dobon A, Bunting DCE, Cabrera-Quio LE, Uauy C, Saunders DGO. 2016. The host-pathogen interaction between wheat and yellow rust induces temporally coordinated waves of gene expression. BMC Genomics. 17:380. doi:https://doi.org/10.1186/s12864-016-2684-4.
- Fauteux F, Rémus-Borel W, Menzies JG, Bélanger RR. 2005. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol Lett. 249:1–6. doi:https://doi.org/10.1016/j.femsle.2005.06.034.
- Ge SX, Jung D, Yao R. 2019. ShinyGO: a graphical enrichment tool for animals and plants. Bioinformatics. 36:2628–2629. doi:https://doi.org/10.1093/bioinformatics/btz931.
- Ghanmi D, McNally DJ, Benhamou N, Menzies JG, Bélanger RR. 2004. Powdery mildew of Arabidopsis thaliana: a pathosystem for exploring the role of silicon in plant-microbe interactions. Physiol Mol Plant Path. 64:189–199. doi:https://doi.org/10.1016/j.pmpp.2004.07.005.
- Govrin EM, Levine A. 2000. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol. 10:751–757. doi:https://doi.org/10.1016/S0960-9822(00)00560-1.
- Hammond-Kosack KE, Jones JDG. 1996. Resistance gene-dependent plant defense responses. Plant Cell. 8:1773–1791. doi:https://doi.org/10.1105/tpc.8.10.1773.
- Jones JDG, Dangl L. 2006. The plant immune system. Nature. 444:323–329. doi:https://doi.org/10.1038/nature05286.
- Klymiuk V, Yaniv E, Huang L, Raats D, Fatiukha A, Chen S, Feng L, Frenkel Z, Krugman T, Lidzbarsky G, et al. 2018. Cloning of the wheat Yr15 resistance gene sheds light on the plant tandem kinase-pseudokinase family. Nat Commun. 9:1–12. doi:https://doi.org/10.1038/s41467-018-06138-9.
- McIntosh R, Mu J, Han D, Kang Z. 2018. Wheat stripe rust resistance gene Yr24/Yr26: a retrospective review. Crop J. 6:321–329. doi:https://doi.org/10.1016/j.cj.2018.02.001.
- McNeal FH, Konzak CF, Smith EP, Tate WS, Russell TS. 1971. A uniform system for recording and processing cereal research data. Washington: USDA-ARS Bull; p. 34–121.
- Melotto M, Zhang L, Oblessuc PR, He SY. 2017. Stomatal defense a decade later. Plant Physiol. 174:561–571. doi:https://doi.org/10.1104/pp.16.01853.
- Peng JH, Fahima T, Röder MS, Huang QY, Dahan A, Li YC, Grama NE. 2000. High-density molecular map of chromosome region harboring stripe-rust resistance genes YrH52 and Yr15 derived from wild emmer wheat, Triticum dicoccoides. Genetica. 109:199–210. doi:https://doi.org/10.1023/A:1017573726512.
- Qie Y, Liu Y, Wang M, Li X, See DR, An D, Chen X. 2019. Development, validation, and re-selection of wheat lines with pyramided genes Yr64 and Yr15 linked on the short arm of chromosome 1B for resistance to stripe rust. Plant Dis. 103:51–58. doi:https://doi.org/10.1094/PDIS-03-18-0470-RE.
- Seifi HS, Curvers K, De Vleesschauwer D, Delaere I, Aziz A, Höfte M. 2013a. Concurrent overactivation of the cytosolic glutamine synthetase and the GABA shunt in the ABA-deficient sitiens mutant of tomato leads to resistance against Botrytis cinerea. New Phytol. 199:490–504. doi:https://doi.org/10.1111/nph.12283.
- Seifi HS, Shelp BJ. 2019. Spermine differentially refines plant defense responses against biotic and abiotic stresses. Front Plant Sci. 10:117. doi:https://doi.org/10.3389/fpls.2019.00117.
- Seifi HS, Van Bockhaven J, Angenon G, Höfte M. 2013b. Glutamate metabolism in plant disease and defense: friend or foe? Mol Plant Microbe Interact. 26:475–485. doi:https://doi.org/10.1094/MPMI-07-12-0176-CR.
- Serajazari M, Hilker B, Sing Sidhu H, Follings J, Navabi A. 2018. Host-pathogen interaction in the winter wheat-stripe rust pathosystem in Ontario. In: 4th Canadian Wheat Symposium and 9th Canadian Workshop on FHB. Winnipeg, Manitoba, Canada.
- Singh RP, Nelson JC, Sorrells ME. 2000. Mapping Yr28 and other genes for resistance to stripe rust in wheat. Crop Sci. 40:1148–1155. doi:https://doi.org/10.2135/cropsci2000.4041148x.
- Solh M, Nazari K, Tadesse W, Wellings CR. 2012. The growing threat of stripe rust worldwide. In: Borlaug Global Rust Initiative (BGRI) conference, Beijing, China. p. 1–4
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 7:562–578. doi:https://doi.org/10.1038/nprot.2012.016.
- Van Bockhaven J, Spíchal L, Novák O, Strnad M, Asano T, Kikuchi S, Höfte M, De Vleesschauwer D. 2015. Silicon induces resistance to the brown spot fungus Cochliobolus miyabeanus by preventing the pathogen from hijacking the rice ethylene pathway. New Phytol. 206:761–773. doi:https://doi.org/10.1111/nph.13270.
- Xi K, Kumar K, Holtz MD, Turkington TK, Chapman B. 2015. Understanding the development and management of stripe rust in central Alberta. Can J Plant Path. 37:21–39. doi:https://doi.org/10.1080/07060661.2014.981215.
- Yu X, Wang X, Wang C, Chen X, Qu Z, Yu X, Han Q, Zhao J, Guo J, Huang L, et al. 2010. Wheat defense genes in fungal (Puccinia striiformis) infection. Funct Integr Genomics. 10:227–239. doi:https://doi.org/10.1007/s10142-010-0161-8.
- Yu Y, Jin C, Sun C, Wang J, Ye Y, Lu L, Lin X. 2015. Elevation of arginine decarboxylase-dependent putrescine production enhances aluminum tolerance by decreasing aluminum retention in root cell walls of wheat. J Hazard Mater. 299:280–288.
- Zeng QD, Han DJ, Wang QL, Yuan FP, Wu JH, Zhang L, Wang XJ, Huang LL, Chen XM, Kang ZS. 2014. Stripe rust resistance and genes in Chinese wheat cultivars and breeding lines. Euphytica. 196:271–284. doi:https://doi.org/10.1007/s10681-013-1030-z.
