Abstract
Objective: To evaluate the ability of a proprietary arabinogalactan extract from the larch tree (ResistAid, Lonza Ltd., Basel, Switzerland) to change the immune response in healthy adults to a standardized antigenic challenge (tetanus and influenza vaccines) in a dose-dependent manner compared to placebo.
Methods: This randomized, double-blind, placebo-controlled trial included 75 healthy adults (18–61 years old). Subjects were randomized to receive either 1.5 or 4.5 g/day of ResistAid or placebo for 60 days. At day 30, subjects were administered both tetanus and influenza vaccines. Serum antigenic response (tetanus immunoglobulin G [IgG], influenza A and B IgG and immunoglobulin M [IgM]) was measured at days 45 (15 days after vaccination) and 60 (30 days after vaccination) of the study and compared to baseline antibody levels. Frequency and intensity of adverse events were monitored throughout the study.
Results: As expected, all 3 groups demonstrated an expected rise in tetanus IgG levels 15 and 30 days following the vaccine. There was a strongly significant difference in the rise in IgG levels at day 60 in the 1.5 g/day group compared to placebo ( p = 0.008). In the 4.5 g/day group, there was significant rise in tetanus IgG at days 45 and 60 compared to baseline ( p < 0.01) but these values were not significant compared to placebo. Neither group demonstrated any significant elevations in IgM or IgG antibodies compared to placebo following the influenza vaccine. There were no clinically or statistically significant or serious adverse events.
Conclusions: ResistAid at a dose of 1.5 g/day significantly increased the IgG antibody response to tetanus vaccine compared to placebo. In conjunction with earlier studies, this validates the effect of ResistAid on the augmentation of the response to bacterial antigens (in the form of vaccine).
INTRODUCTION
The adaptive immune system (also called the acquired immune system) is composed of specialized cells and actions that are involved in the elimination or prevention of pathogenic challenges. The adaptive immune response provides the immune system with the ability to recognize and remember specific pathogens and to mount a stronger response each time a pathogen is encountered. Adaptive immunity is triggered in humans when a pathogen invades the innate immune system and generates a threshold level of antigen. The adaptive immune response has been exploited by modern medicine through the use of vaccines Citation[1]. By using live (attenuated) or inactivated pathogens or part of pathogens, vaccines trigger an immune response and development of vaccine-specific antibodies. The measurement of this response is frequently used as a way to measure the immunomodulatory effect of certain drug and dietary interventions Citation[2]. It is a validated model to assess the in vivo functional capacity of the human immune system Citation[3]. Vaccines used in clinical trials to measure antibody response have included tetanus and influenza vaccines.
Tetanus is an acute, often fatal, disease that causes painful tightening of the muscles, produced by an exotoxin (protein) secreted by the bacterium Clostridium tetani. C. tetani produces 2 exotoxins: tetanolysin and tetanospasmin. The latter is a neurotoxin and produces the clinical manifestations of the disease. Tetanus toxoid consists of formaldehyde-treated toxin (protein), which is a single antigen. Tetanus toxoid is a highly effective antigen, and a completed primary series generally induces protective levels of serum antitoxin that persists for 10 or more years Citation[4]. In a trial of 26 adults given a booster dose of tetanus toxoid, 81% of the subjects demonstrated a 2-fold or greater rise in serum antitoxin antibody levels Citation[5]. The antigenic response to tetanus toxin is approximately 80% immunoglobulin G (IgG) Citation[6]. A 4-fold increase in IgG levels is expected when comparing postvaccination to prevaccination results for previously unvaccinated individuals. For previously vaccinated individuals receiving a booster inoculation, the rise in IgG levels may be less than 4-fold.
Influenza is a respiratory tract infection caused by 3 types of RNA viruses: types A, B, and C. Each consists of 8 negative single-strand RNA segments encoding 11 proteins. The major surface glycoproteins of the virus are hemagglutinin (HA) and, to a lesser extent, neuraminidase. The antigenic drift of the HA protein results in the development of novel viral strains and a requirement for annual vaccination to keep up with the changes. The influenza vaccine contains 3 inactivated influenza viruses: one A (H3N2) virus, one regular seasonal A (H1N1) virus (in 2010 when this study took place this was replaced by the 2009 pandemic H1N1 virus), and one B virus. The vaccine produces antibody responses to both HA and neuraminidase. There is a rapid and robust influenza-specific response by antibody-secreting plasma cells that begins as early as 2 to 6 days after vaccination, peaks after 2 weeks, and then wanes over the next 6 months Citation[7]. Influenza-specific antibodies are predominately IgG and immunoglobulin M (IgM) in serum and IgA in oral fluid Citation[8].
Arabinogalactans are high-molecular-weight, highly branched, water-soluble polysaccharides that contain units of d-galactose and l-arabinose Citation[9]. Dietary intake of arabinoglactans comes from plant food sources such as carrots, radishes, tomatoes, pears, and wheat. Gum arabic, a commonly used food additive, is composed of highly branched arabinogalactan Citation[10]. The mean estimated intake of arabinogalactan from the diet is approximately 10.474 g Citation[11]. The most common commercial source of arabinogalactans is from the wood of the larch tree ( Larix spp.). Larch arabinogalactan consists of galactose and arabinose in a 6:1 ratio. It is a long, densely branched nonstarch polysaccharide with a galactan backbone with side chains of galactose and arabinose.
An ex vivo study with human peripheral blood mononuclear cells found that larch arabinogalactan stimulated natural killer cell activity through a possible increase in interferon-gamma Citation[12]. A study with dogs demonstrated increased numbers of circulating white blood cell counts (primarily neutrophils and eosinophils) following oral administration of larch arabinoglactan Citation[13].
A randomized, double-blind, placebo-controlled study evaluated the immunomodulating effects of 4 different preparations of echinacea, a proprietary larch arabinogalactan (1.5 g/day), and a combination of larch arabinogalactan and one of the echinacea preparations Citation[14]. The study included 48 adult women (22–51 years old) who were divided into 6 groups of 8 women. After 4 weeks of treatment, there was a statistically significant increase in complement properdin in 2 of the echinacea groups and the group taking the larch arabinoglactan and echinacea combination. There was no significant increase in the group taking the larch arabinogalacton alone.
The proprietary arabinogalactan extract ResistAid (Lonza Ltd., Basel, Switzerland) was previously tested in a randomized, double-blind, placebo-controlled, parallel-group pilot study to determine immunomodulatory activity following vaccination against Streptococcus pneumonia Citation[15]. This 72-day study included 45 healthy adult subjects who had not previously received the vaccine. The primary end points were 7 different pneumococcal IgG antibodies (4, 6B, 9V, 14, 18C, 19F, and 23F). The secondary objective was to determine whether the ResistAid product (4.5 g/day) would stimulate other arms of the immune system to which there was no direct antigenic stimulus. Secondary endpoints included salivary immunoglobulin A (IgA), white blood cell counts, complement (C3 and C4), and inflammatory cytokine levels. Subjects were randomized using a block design. In response to the vaccine, pneumococcal IgG plasma levels increased. The arabinogalactan group demonstrated a greater IgG antibody (Ab) response than the placebo group in two Ab subtypes (18C and 23F) at both day 51 ( p = 0.006 and p = 0.002, respectively) and day 72 ( p = 0.008 and p = 0.041). Ab subtypes 18C and 23F also demonstrated change scores from baseline in favor of the arabinogalactan group at day 51 ( p = 0.033 and 0.001) and day 72 ( p = 0.012 and p = 0.003). Change scores from baseline and mean values were greater in the arabinogalactan group than placebo for most time points in Ab subtypes 4, 6B, 9V, and 19F, but this was not significant. There was no effect from the vaccine or arabinogalactan on salivary IgA, white blood cell count, inflammatory cytokines, or complement. The proprietary larch arabinogalactan used in this study may have a selective immunomodulatory effect on acquired or adaptive immunity shown as an increase in antibodies without clinically significant changes to total white blood cells, cytokines, or complement. It is possible that rather than acting as a general immunostimulant, arabinogalactan can act in a specific manner.
It is hypothesized that the mechanism of this specific immunomodulation includes associated activation of the gut-associated lymphoid tissue as the long-chain-specific arabinogalactan passes through the gastrointestinal (GI) tract Citation[16]. Presentation of polysaccharides to immune effector cells may resemble the capsular antigens of some potentially pathogenic encapsulated bacteria and the chronic low level stimulation of the gut-associated lymphoid tissue may prepare the body for similar presence of comparable pathogens Citation[17]. Chronic low-level exposure to arabinogalactan in this manner may induce an immunomodulatory and immunostimlatory priming effect, allowing for faster response time of the immune system when a pathogenic antigen presents.
The current human clinical study was designed to test the hypothesis that the ingestion of ResistAid, a proprietary arabinogalactan extracted from larch ( Larix laricina), would selectively enhance the antibody response to the tetanus and influenza vaccines in a dose-related manner compared to placebo. The selected doses were 1.5 and 4.5 g, both of which had demonstrated effects in previous clinical studies.
METHODS
Study Sample
This study was conducted in accordance with Good Clinical Practice Guidelines and the ethical principles of the Declaration of Helsinki [Citation18]. The study protocol and material were approved by an institutional review board (Copernicus Group IRB, Cary, NC) and all subjects gave written informed consent prior to participation.
Table 1 Inclusion criteria, exclusion criteria, and study controls
This was a 60-day, 3-arm, randomized, double-blind, placebo-controlled, parallel-groups trial in healthy adults, conducted at one study center in Northridge, California (Staywell Research) and was designed and managed by the Medicus Research Contract Research Organization, also in Northridge, California. Subjects were recruited using existing databases and local advertising. Subjects were screened by phone prior to scheduling a screening visit. Inclusion criteria included assessment of being in good health, a body mass index between 18 and 30 kg/m2, and 18–60 years of age (). Subjects included in the study must not have had an influenza vaccine in the past year or a tetanus vaccine in the past 5 years. They were asked to maintain their normal diet and exercise routine during the study and females were required to use an approved birth control method during the study. Potential participants were excluded from the study if they had any major systemic, inflammatory, or chronic disease; any active infection or infection in the past month requiring antibiotics or antiviral medication; used immunosuppressive drugs in the prior 5 years; were known to have alcohol or drug abuse; were pregnant or lactating; or had any medical condition that in the opinion of the investigator might interfere with the subject's participation in the trial. They were excluded if they had an allergy to eggs. Subjects using dietary supplements designed to boost the immune system and/or multivitamins were required to discontinue these products for at least 2 weeks before entering the study.
Study Products
The intervention product tested was a proprietary arabinogalacton extract (ResistAid). The product is extracted from the wood of the larch tree ( Larix laricina) using a water extraction patented process [Citation19] (U.S. 5756098; EP 86608) in accordance with Hazard Analysis and Critical Control Points standards and in compliance with the monograph in the Food Chemicals Codex [Citation20]. ResistAid is a fine, dry, light brown powder with a neutral taste that dissolves quickly in water or juice. The larch arabinogalactan used in the ResistAid product has been designated Generally Recognized as Safe (GRAS) by the U.S. Food and Drug Administration [Citation21,22]. The placebo was maltodextrin (Maltrin M100, Grain Processing Corp., Muscatine, IA, USA).
The tetanus vaccine used in the study was the Massachusetts Biologic Labs Tetanus Diphth Tox AD NR SDV 0.5 mL 10/Pk.
The inactivated influenza vaccine used in the study was Fluzone (Sanofi Pasteur, Swiftwater, PA, USA) for the 2009–2010 influenza season (multidose vial, 5 mL). The vaccine formulation for the 2009–2010 season contains 3 strains of the influenza virus: the A/Brisbane/59/2007 (H1N1)-like virus, the A/Brisbane/10/2007 (H3N2)-like virus, and the B/Brisbane/60/2008-like virus. The 3 strains for the new influenza vaccine formulation were confirmed by the Food and Drug Administration's Vaccines and Related Biological Products Advisory Committee in February 2009 and correspond with recommendations made by the World Health Organization, also in February [Citation23]. Influenza vaccine is formulated each year to match the strains predicted to circulate during the upcoming season. This formulation for the 2009–2010 influenza season introduced a new B strain. The two A strains were unchanged from the 2008–2009 season formulation.
Randomization and Dosing
Subjects were randomly assigned to one of 3 treatment groups in blocks of 5 with 10 subjects randomized per block. The atmospheric noise method was used to generate the randomization schema [Citation24]. The treatment groups were as follows: (1) 1.5 g/day of ResistAid, (2) 4.5 g/day of ResistAid, or (3) placebo. In order to protect blinding, the study product was produced in identical opaque sachets that contained either 4.5 g of ResistAid, 1.5 g of ResistAid with 3.0 g placebo, or 4.5 g placebo. Subjects were instructed to mix sachets in an 8 oz. cold beverage to be taken once a day in the morning with breakfast.
Each box was labeled with perforated labels provided by the Medicus Research Contract Research Organization with subject-specific information including a unique randomization number. Subjects, the medical director, and research staff were blinded to the treatment assignment for the duration of the trial.
Study Procedure
Subjects were required to be present for 5 clinic visits during the 60-day study. At screening (visit 1), eligibility was determined based on the inclusion and exclusion criteria. For eligible subjects, blood was drawn to measure baseline influenza A and B IgM and IgG levels as well as tetanus IgG levels. Subjects were counseled not to change their diet or exercise level during the study and they received the first dose of the assigned study product during the visit. Product was dispensed and subjects received a study-dosing diary. On day 15, subjects were called to check on compliance and as a reminder of their next visit. On day 30 (visit 2), subjects were administered the tetanus and influenza vaccines via intramuscular injection.
All subjects returned the next day (visit 3) to observe the vaccination site. On day 45 (visit 4), subjects had blood drawn to measure influenza A and B IgM and IgG levels and tetanus IgG levels. The blood draw and antigen measures were repeated on day 60 (visit 5).
During study visits, subjects were questioned about changes in health status (including concomitant therapies) and vital signs were taken. Adverse event monitoring was completed at each visit beginning with visit 2. During visits 2, 4, and 5, dosing diaries were collected and study compliance assessed (interview, diaries, and product wrappers were returned). Study product was dispensed and new dosing diaries were provided. A urine pregnancy test was completed for all female subjects at visits 1, 2, and 4.
Outcome Measures
The primary end points were the changes in the markers of immune response to the tetanus and influenza vaccines. These end points were measured in plasma samples and included tetanus IgG (measured by enzyme immunoassay) and influenza A IgM, influenza A IgG, influenza B IgM, and influenza B IgG (all measured by antibody enzyme-linked immunosorbent assay). The antibodies were measured using plasma samples.
Safety assessment included vital signs (temperature, blood pressure, pulse, and respiratory rate) as well as detailed adverse event (AE) monitoring to assess the frequency and intensity of AEs. Safety monitoring also included assessment of the vaccination site during visit 3.
Statistical Analysis
Paired sample t tests were used for within-subject means comparisons and independent sample t tests for between group comparisons (placebo vs each of the active groups individually).
Excel 2003 was used for data entry, validation, restructuring, calculating changes in variables over time, reorganizing and reformatting results, and preparing graphs. Statistical analyses were performed using SPSS Base System version 18 (IBM, Chicago, IL).
RESULTS
Characteristics of the Study Population
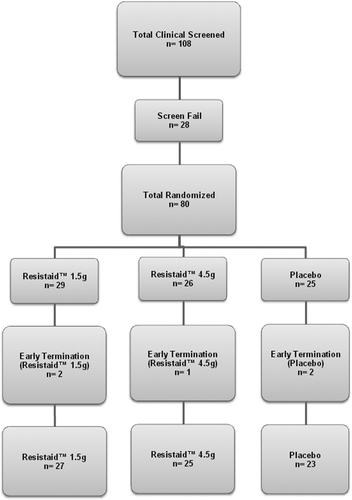
A total of 80 subjects were randomized for the study (see ). Seventy-five subjects completed the 60-day study: 1.5 g/day ( n = 27), 4.5 g/day ( n = 25), and placebo ( n = 23).
Five subjects (2 in the 1.5 g/day group, 1 in the 4.5 g/day group, and 2 in the placebo group) were lost to follow-up after visit 1 and never received the vaccines. They were not included in the analysis. The baseline characteristics of the subjects were not significantly different for gender, age, ethnicity, or marital status. The study began in May 2010 (first subject randomized) and ended in December 2010 (last subject out).
Tetanus IgG
All 3 groups demonstrated an increase in IgG levels at day 45. The increase appeared to peak at day 45 for the placebo group, while the 1.5 and 4.5 g/day groups continued to show a small increase at day 60. There was a strongly significant difference between the 1.5 g/day group and the placebo group in IgG levels at day 60 ( p = 0.008). There were no other significant differences between groups at any time point (see ).
Within-group changes in IgG levels from baseline were significant for the placebo group at day 60 ( p ≤ 0.01) and for the 4.5 g/day group at both days 45 and 60 ( p ≤ 0.01). There were no significant within-group changes in the 1.5 g/day group.
Influenza IgM and IgG Antibodies
All 3 groups demonstrated an expected physiological increase and peak in influenza A IgM by day 45 with a slight reduction at day 60 (see ). Both the 1.5 and 4.5 g/day groups were not statistically different than placebo at baseline or day 60. The 1.5 and 4.5 g/day groups were not statistically different than each other at any time point. The within-group changes from baseline to day 45 and day 60 were not significant for any group at any time point with the exception of a significant increase from baseline to day 60 in the 1.5 g/day group ( p = 0.002).
Fig. 2 Tetanus IgG Antibody Level. All 3 groups demonstrated an increase in IgG levels at day 45. There was a strongly significant difference between the 1.5 g/day group and the placebo group in IgG levels at day 60 (p = 0.008).
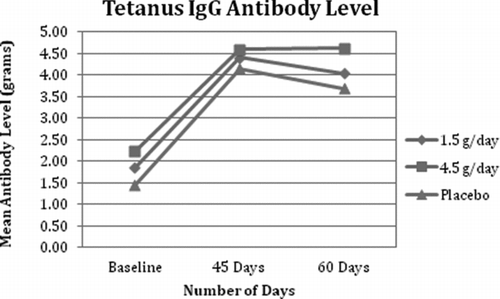
Fig. 3 Influenza (A) IgG Antibody Level. All 3 groups demonstrated an expected physiological increase and peak in influenza A IgM by day 45 with a slight reduction at day 60. All 3 groups demonstrated an expected rise in influenza A IgG following the vaccine, which peaked at day 45 for the 4.5 g/day and placebo groups and at day 60 for the 1.5 g/day group.
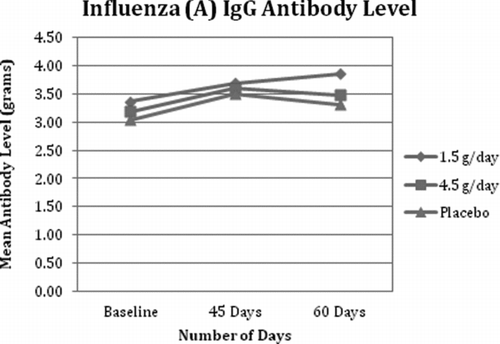
Fig. 4 Influenza (B) IgG Antibody Level. All 3 groups demonstrated an expected increase in influenza B IgM after vaccination.
All 3 groups demonstrated an expected increase in influenza B IgM after vaccination (see ). The 3 groups were not statistically different at any time point; however, there were statistically significant within-group changes from baseline.
All 3 groups demonstrated an expected rise in influenza A IgG following the vaccine, which peaked at day 45 for the 4.5 g/day and placebo groups and at day 60 for the 1.5 g/day group (see ). The placebo group was significantly lower than the 4.5 g/day at baseline ( p = 0.029); however, there were no significant differences between IgG levels in any of the 3 groups at day 45 or 60. The following within-group changes were statistically significant: (1) placebo group at day 45 ( p = 0.002) and day 60 ( p = 0.0001); (2) 1.5 g/day group at day 45 ( p = 0.006); and (3) 4.5 g/day group at day 45 ( p = 0.001) and day 60 ( p = 0.007).
All 3 groups demonstrated an expected rise in influenza B IgG after the vaccine with a peak at day 45 for the 4.5 g/day group and day 60 for the 1.5 g/day group and placebo group (see Table 5). There were no significant differences between the values in any of the 3 groups at any time point. The within-group changes were statistically significant for all 3 groups at day 45 and day 60.
Adverse Events
There were no clinically significant or serious adverse events during the study. A total of 13 adverse events were reported during the study. In the placebo group, there were 5 AEs reported: upper respiratory tract infection (URI; 2 reports), sinus headache, hypertension, and lower abdominal pain. In the 1.5 g/day group there were 7 reported AEs: URI (3 reports), food poisoning, gastroenteritis, nausea, and headache. In the 4.5 g/day group there was one report of dizziness and no URIs reported. None of the adverse events in any group were attributed to the study product.
DISCUSSION
The present study employed a model antigenic stimulation using a vaccine-specific serum antibody production to evaluate the immunomodulatory effects of proprietary larch arabinogalactan product (ResistAid) in a healthy adult population. The IgM antibodies are the acute antibodies that provide short-term response to the antigen (in this case the vaccine). It is expected that they will rise and fall in a relatively short period of time (1 to 4 weeks). It is the IgG antibodies that provide the long-term protection and are a more significant immune marker. These tend to rise more slowly than the IgM antibodies but continue to rise for a longer period of time.
The study employed 2 different doses of arabinogalactan, 1.5 and 4.5 g/day, with the hypothesis that there would be a dose–response effect. We had previously observed an increase in IgG in response to the pneumococcal vaccine with the dose of 4.5 g/day Citation[15]. A previous clinical study that measured levels of complement properdin reported that a dose of 1.5 g possibly augmented an effect due to echinacea species Citation[14]. In the present study, the 1.5 g/day dose was found to significantly increase tetanus IgG antibody response at day 60 compared to placebo ( p = 0.008). This is a confirmation that the ResistAid product confers a benefit in increasing the antibody response to a standard antigenic challenge. The ResistAid 4.5 g/day group showed statistically significant increases from baseline for this same vaccine and continued to show elevations in IgG levels at day 60 even when both the placebo and 1.5 g/day groups had already peaked, but this group did not show a statistically significant difference compared to the placebo group.
There were no significant differences between either ResistAid dose and placebo in the influenza antibodies. Both IgM and IgG were tested for influenza A and influenza B. Based on these results and on the prior results of the pneumococcal vaccine study Citation[15], it appears that the ResistAid product confers a benefit in preparing the body to deal with bacterial antigens but perhaps not with viral antigens. As one considers other purported mechanisms of action in the GI tract for the product, the above may become clearer. The product may stimulate the Peyer's patches in the gut as it traverses the length of the intestines. The polysaccharide may have a structure similar to that of these potentially pathogenic bacteria and therefore provide a low level of stimulation, which keeps an array of antibodies ready in case the actual antigen appears. If the structure of the polysaccharide is similar to that of bacteria, then it may not be similar to the structure of viruses and therefore may not confer the same benefit in that case. Another plausible explanation may be the noted prebiotic activity for larch arabinogalactan Citation[25].
Prebiotics are noted to have immunomodulating activity, in part by increasing lactic acid bacteria and increasing production of short-chain fatty acids in the GI tract Citation[26]. A combination of short-chain galactooligosaccharides and long-chain fructooligosaccharides was shown to influence immune response to an influenza vaccine in mice Citation[27]. Supplementation with the prebiotic mix increased vaccine-specific delayed-type hypersensitivity (DTH) response when given prior to the primary vaccination. Supplementation after day 8 did not affect the DTH response. The study found a positive correlation between percentages of cecal lactobacilli at day 9 and DTH responses. A placebo-controlled study also found an effect on regulatory T-cells following influenza vaccine in mice supplemented with a prebiotic combination consisting of short-chain galactooligosaccharides, long-chain fructooligosaccharides, and pectin hydrolysate-derived acidic oligosaccharides Citation[28]. The study found that the prebiotic mixture depleted CD25+ regulatory T-cells, which resulted in enhanced Th1 vaccine responsiveness.
However, the results in animal studies have not been duplicated thus far in human studies examining the immunomodulating effect of prebiotics following vaccination. In a randomized, placebo-controlled trial, healthy elderly adults (≥70 years old) were randomized to receive 6 g/day of a prebiotic fructooligosaccharide mixture 70% raftilose and 30% raftiline or placebo for 28 days Citation[29]. At week 2 of the study, all subjects were vaccinated with influenza and pneumococcal vaccine. Though a slight increase in influenza A antibodies (saliva secretory IgA) was observed, there was no effect on serum influenza A and B and pneumococcal IgG or IgM levels in the prebiotic group compared to placebo.
Variables that affect the immune response to vaccines include age, gender, race, body mass index, and genetic characteristics [Citation2,Citation30]. One of the goals of this study was to determine the effect of the intervention on a relatively broad population—healthy adults from age 18 to 60 years old. The between-subject variability in response to vaccination is normally quite high and using a larger study population in future studies may clarify the clinical indications we have observed so far. In addition, because gender and age differences may affect immunity, these potentially confounding variables could be examined in future studies.
CONCLUSION
Daily ResistAid supplementation at a dose of 1.5 g/day for 30 days before the administration of the tetanus vaccine significantly increased the tetanus IgG antibody response compared to placebo. The 4.5 g/day dose of ResistAid also increased the IgG antibody response to the tetanus vaccine and this increase continued to rise by day 60; however, these values did not reach statistical significance. Neither group demonstrated any significant elevations in IgM or IgG antibody response to the influenza vaccine. The results suggest that ResistAid induces an elevated response to bacterial antigens (in the form of vaccine), but not viral antigens.
Acknowledgments
Published with license by Taylor & Francis Group, LLC
REFERENCES
- Twigg , H L . 2005 . Humoral immune defense (antibodies): recent advances . Proc Am Thoracic Soc , 2 : 417 – 421 .
- Albers , R , Antoine , J M , Bourdet-Sicard , R , Calder , P C , Gleeson , M , Lesourd , B , Samartin , S , Sanderson , I R , Van Loo , J , Vas Dias , F W and Watzl , B . 2005 . Markers to measure immunomodulation in human nutrition intervention studies . Br J Nutr , 94 : 452 – 481 .
- Cummings , J H , Antoine , J M , Azpiroz , F , Bourdet-Sicard , R , Brandtzaeg , P , Calder , P C , Gibson , G R , Guarner , F , Isolauri , E , Pannemans , D , Shortt , C , Tuijtelaars , S and Watzl , B . 2004 . PASSCLAIM—gut health and immunity . Eur J Clin Nutr , 43 ( suppl 2 ) : 118 – 173 .
- 1991 . Diphtheria, tetanus, and pertussis: recommendations for vaccine use and other preventive measures. Recommendations of the Immunization Practices Advisory Committee . MMWR Morb Mortal Wkly Rep , 40 : 1 – 28 .
- Sanofi Pasteur . Tetanus toxoid for booster use only. Rep. U.S. Food and Drug Administration, 01 Dec 2005. Web 23 Sept 2013 . Accessed at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM166873.pdf
- Schauer , U , Stemberg , F , Rieger , C H , Büttner , W , Borte , M , Schubert , S , Möllers , H , Riedel , F , Herz , U , Renz , H and Herzog , W . 2003 . Levels of antibodies specific to tetanus toxoid, Haemophilus influenza type B, and pneumococcal capsular polysaccharide in healthy children . Clin Diagn Lab Immunol , 10 : 202 – 207 .
- Cox , R J , Haaheim , L R , Ericsson , J C , Madhun , A S and Brokstad , K A . 2006 . The humoral and cellular responses induced locally and systemically after parenteral influenza vaccination in man . Vaccine , 24 : 6557 – 6580 .
- Brokstad , K A , Cox , R J , Olofsson , J , Jonsson , R and Haaheim , L R . 1995 . Parenteral influenza immunization induces a rapid systemic and local immune response . J Infect Dis , 171 : 198 – 203 .
- Kelly , G S . 1999 . Larch arabinogalactan: clinical relevance of a novel immune-enhancing polysaccharide . Altern Med Rev , 4 : 96 – 103 .
- D’Adamo , P D . 1990 . Larch arabinoglactan . J Naturopath Med , 6 : 33 – 37 .
- US Food and Drug Administration . Department of Health and Human Services: “CFR—Code of Federal Regulations Title 21—Food and Drugs . Section 170.3. Accessed at: <http://cfr.vlex.com/source/code-federal-regulations-food-drus-1070/section/01.02.71>
- Hauer , J and Anderer , F A . 1993 . Mechanism if stimulation of human natural killer cell cytotoxicity by arabinogalactan from Larix occidentalis . Cancer Immunol Immunother , 36 : 237 – 244 .
- Grieshop , C M , Flickinger , E A and Fahey , G C . 2002 . Oral administration of arabinogalactan affects immune status and fecal microbial populations in dogs . J Nutr , 132 : 478 – 482 .
- Kim , L S , Waters , R F and Burkholder , P M . 2002 . Immunological activity of larch arabinogalactan and echinacea: a preliminary, randomized, double-blind, placebo-controlled trial . Altern Med Rev , 7 : 138 – 149 .
- Udani , J , Singh , B B , Barrett , M L and Singh , V J . 2010 . Proprietary arabinogalactan extract increases antibody response to pneumonia vaccine: a randomized double-blind, placebo-controlled, pilot study in healthy volunteers . J Nutr , 9 : 32
- Spahn , T W and Kucharzik , T . 2004 . Modulating the intestinal immune system: the role of lymphotoxin and GALT organs . Gut , 53 : 456 – 465 .
- Kelly , G . 1999 . Larch arabinogalactan: clinical relevance of a novel immune-enhancing polysaccharide . Altern Med Rev , 4 : 96 – 103 .
- World Medical Association: . “ Ethical Principles for Medical Research Involving Human Subjects ” . World Medical Association Declaration of Helsinki . 1 Oct. 2008. Web. 23 Sept. 2013. Accessed at: <http://www.wma.net/en/30publications/10policies/b3/17c.pdf>
- Price Christopher , H , Hedtke , D , Richards , G N and Tempesta , M S . Methods for the Extraction of Phytochemicals from Fibrous Plants in the Absence of Solvent , Larex International, Inc . assignee. Patent 5756098. 26 May 1998. Print
- Hazard Analysis and Critical Control Points - FDA Code 2009 . Annex 4. N.p., n.d. Web. 23 Sept. 2013. Accessed at: http://www.fda.gov/Food/GuidanceRegulation/RetailFoodProtection/FoodCode/ucm188363.htm
- Agency Response Letter GRAS Notice No. GRN 000084. CFSAN/Office of Premarket Approval, 19 Feb. 2002. Web. 23 Sept. 2013. Accessed at: <http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm154598.htm>
- Agency Response Letter GRAS Notice No. GRN 000047. CFSAN/Office of Premarket Approval, 06 June 2000. Web. 23 Sept. 2013. Accessed at: <http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm153766.htm>
- “Advisory Committees.” 2009 Meeting Materials, Vaccines and Related Biological Products Advisory Committee. U.S. Food and Drug Administration, 15 Dec. 2009. Web. 23 Sept. 2013. Accessed at: <http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/ucm129568.htm>
- Haahr , M . Random.org: True random number service . Web resource, 2006. Accessed at: http://www.random.org
- Robinson , R R , Feirtag , J and Slavin , J L . 2001 . Effects of dietary arabinogalactan on gastrointestinal and blood parameters in healthy human subjects . J Am Coll Nutr , 20 : 279 – 285 .
- De Vrese , M and Schrezenmeir , J . 2008 . Probiotics, prebiotics, and synbiotics . Adv Biochem Engin/Biotechnol , 111 : 1 – 66 .
- Vos , A P , Knol , J , Stahl , B , M’rabet , L and Garssen , J . 2010 . Specific prebiotic oligosaccharides modulate the early phase of a murine vaccination response . Int Immunopharmacol , 10 : 619 – 625 .
- van't Land , B , Schijf , M , van Esch , B , van Bergenhenegouwen , J , Bastiaans , J , Schouten , B , Boon , L and Garssen , J . 2010 . Regulatory T-cells have a prominent role in immune modulated vaccine response by specific oligosaccharides . Vaccine , 28 : 5711 – 5717 .
- Bunout , D , Hirsch , S , Pía de la Maza , M , Muñoz , C , Haschke , F , Steenhout , P , Klassen , P , Barrera , G , Gattas , V and Petermann , M . 2002 . Effects of prebiotics on the immune response to vaccination in the elderly . Parenter Enteral Nutr , 26 : 372 – 376 .
- Thomas , C and Moridani , M . 2010 . Interindividual variations in the efficacy and toxicity of vaccines . Toxicology , 278 : 204 – 210 .