Abstract
BACKGROUND: Few natural products have demonstrated the range of protective and therapeutic promise as have turmeric and its principal bioactive components: curcumin, demethoxycurcumin, and bisdemethoxycurcumin. Success in translating this potential into tangible benefits has been limited by inherently poor intestinal absorption, rapid metabolism, and limited systemic bioavailability. Seeking to overcome these limitations, food ingredient formulators have begun to employ a variety of approaches to enhance absorption and bioactivity. Many of these strategies improve upon the age-old practice of consuming turmeric in fat-based sauces, such as in a fat-rich yellow curry. However, there exists uncertainty as to how the various commercially available offerings compare to each other in terms of either uptake or efficacy, and this uncertainty leaves physicians and nutritionists with a dearth of data for making recommendations to interested patients and consumers. Further complicating the issue are recent data suggesting that formulation strategies may not equally enhance the absorption of individual curcuminoids, a significant issue in that these curcuminoids exhibit somewhat different physiologic properties.
OBJECTIVE: This review introduces needed order to the curcumin marketplace by examining bioavailability studies on a number of commercial curcumin ingredients and evaluating them on a level playing field.
METHODS: The comparative analysis includes standard pharmacokinetic parameters and a new metric, relative mass efficiency (E). Relative mass efficiency allows for the comparison of different formulations even in cases in which the weight percentage of curcuminoids is vastly different.
RESULTS: A hydrophilic carrier dispersed curcuminoid formula exhibits 45.9 times the bioavailability of the standard purified 95 percent curcuminoid preparation and, based on relative mass efficiency, 1.5 times the bioavailability of the next best commercial ingredient, a cyclodextrin complex.
CONCLUSIONS: Delivery strategies can significantly improve the bioavailability of curcuminoids. Total formula mass is important for making practical formulation decisions about dosing, cost and space.
INTRODUCTION
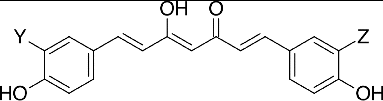
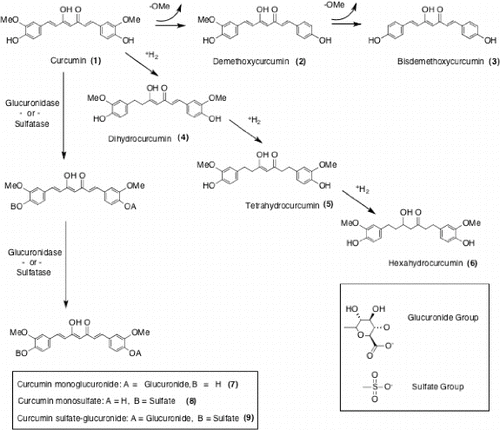
Curcumin (diferuloylmethane; 1,7-bis[4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione; CAS #: 458-37-7) is the principal curcuminoid found in turmeric (Curcuma longa L.), a widely used botanical in South Asian culinary and natural medicinal practice. Curcumin is not the only curcuminoid found in turmeric; it typically is accompanied by two minor curcumin analogues, demethoxycurcumin (CAS: 22608-11-3) and bisdemethoxycurcumin (CAS: 24939-16-0), which are found in commercially available turmeric extracts at near 1/4th and 1/20th the molar ratio of curcumin, respectively Citation[1]. These curcuminoids and their metabolites have become the subjects of controversy, as explained in more detail in the following paragraphs, due to widely reported poor bioavailability, rapid metabolism and excretion, and disparate claims about the pharmacokinetic benefits of different delivery systems. Presently, there is no consensus regarding how to evaluate the comparative absorption and utilization of commercially available curcumin products and there is no established metric for determining the cost–benefit ratio of various approaches to oral delivery. In addressing these and related issues, we introduce the concept of relative mass efficiency (E) as a useful measure of intestinal absorption for practitioners seeking to compare different preparations of the same active ingredient.
In an aqueous environment, such as the human gastrointestinal tract, curcuminoids are only sparingly soluble (see ). Poor water solubility and limited gastrointestinal absorption are two interrelated issues that plagued early attempts to determine curcuminoid bioavailability Citation[2]. Interestingly, turmeric-consuming cultures appear to have been long aware of at least one solution to this problem. In both culinary and Ayurvedic practice in South Asia, powdered turmeric is often combined with a source of fat, such as ghee, milk, or coconut milk. For example hot turmeric milk, or haldi ka doodh, is commonly recommended as a salubrious elixir. Implicit in this cultural wisdom is that fat facilitates the absorption of curcuminoids from the gut Citation[3].
Rapid degradation and metabolism of curcuminoids, both intestinal and hepatic, is another limitation with oral consumption Citation[4,5]. Nonenzymatic degradation occurs via auto-oxidation and pH-dependent lability. Enzymatic conversion to water-soluble metabolites via beta-glucuronidase and sulfatase occurs readily; in vitro work using isolated rat hepatocytes and liver microsomes indicates 90% curcuminoid metabolism via these two mechanisms after just 30 minutes Citation[6]. With curcumin (1), demethoxycurcumin (2), and bisdemethoxycurcumin (3) all containing two phenolic hydroxyl groups, there is more than one locus amenable to conjugation with glucuronide and sulfate groups. Only monoglucuronides, monosulfates, and sulfate-glucuronides (one of each) are typically observed as a result of human metabolism Citation[7]. For curcumin this leads to curcumin monoglucuronide (7), curcumin monosulfate (8), and curcumin sulfate-glucuronide (9). However, the phenolic groups are not the only moieties in curcuminoids susceptible to biotransformation. The extended alkene system, or alternatively the two α,β unsaturated ketones, are susceptible to both conjugation and reduction. Reduction results in colorless compounds, such as dihydrocurcumin (4) (CAS #76474-56-1), tetrahydrocurcumin (5) (CAS #: 36062-04-1), and hexahydrocurcumin (6) (CAS #: 36062-05-2), by destroying the extended conjugated system responsible for the typical yellow-orange color of curcuminoids Citation[8]. These reduced curcumin metabolites are also subject to conjugation with glucuronides or sulfate groups. In the liver, alcohol dehydrogenase and glutathione S-transferase, but not cytochrome p450 enzymes, have been credited with transforming curcuminoids into the reduced curcuminoid metabolites; lipoxygenases also appear to play a role Citation[9]. In the intestines, microbial metabolism also plays a role via NADPH-dependent reduction Citation[10].
STRATEGIES TO INCREASE BIOAVAILABILITY
In the past decade, there has been a significant amount of effort devoted to the development of curcumin formulations that can overcome poor bioavailability, stability limitations, and rapid metabolism (see ). The rationale for attempting to overcome the bioavailability and metabolic hurdles goes beyond the issue of achieving therapeutic blood levels per se and includes 2 further areas of interest: (1) whether higher concentrations of unmetabolized—that is, “free”—curcuminoids in vivo will unlock the protective and therapeutic potential demonstrated for curcuminoids in vitro and (2) whether curcuminoid metabolites have the same, diminished, and/or different biological utilities compared to the native curcuminoids. Logically, achieving significant and consistently measureable serum levels of free curcuminoids and/or their conjugates and metabolites is a necessary first step for exploring these other issues.
A multitude of methods seeking to modulate the pharmacokinetic and delivery profile of curcuminoids have been devised Citation[2,11]. These strategies can be grouped into 4 broad classes: (1) glucuronidation/metabolism interference via adjuvants; (2) liposomes, micelles, and phospholipid complexes; (3) nanoparticles; and (4) emulsifying or dispersing agents Citation[2]. These 4 classes are neither exhaustive nor mutually exclusive, especially when tissue targeting comes into play, but they do exemplify a variety of the strategies that are being evaluated in multiple areas of curcumin product development Citation[12,13]. Inasmuch as each of these strategies usually is intended to overcome one or more particular hindrance to bioavailability, such as poor water solubility, particle size issues, and/or instability in certain digestive environments, it is expected that delivery strategies that combine more than one basic method may produce additive or even superadditive benefits with regard to assimilation. Indeed, the following comparison of commercial curcumin products provides evidence that an approach that addresses several delivery issues may be more successful than are univocal strategies.
As with many areas of product development, the challenge is not limited to solving the problem at hand but also includes demonstrating that this has been done. It is important to compare different delivery solutions to determine areas of equivalency or superiority. Bioavailability assessments of curcumin formulations often have been unsuccessful in achieving either of these 2 objectives. On the front end, the inability to detect small quantities of unmetabolized curcuminoids in blood even after test subjects had ingested large quantities of turmeric and concentrated curcuminoid powders has presented one set of problems Citation[14,15]. Ingested quantities of concentrated curcuminoid powder below 10 g routinely yield undetectable blood levels of unmetabolized curcuminoids Citation[16,17]. This finding has increased our body of knowledge regarding the sample preparation techniques, analytical protocols, and data reporting that are necessary to improve testing capabilities. On the back end, it seems that there has been little appetite to run comparative trials between formulated products.
Insofar as the understanding of what constitutes proper protocols for assessing curcumin bioavailability has changed and improved over time, care needs to be exercised in relying upon earlier studies. The extant literature on the subject must be read critically with respect to blood sample preparation techniques, analytical protocols, and data reporting. A critical reading will involve at least 3 steps. First, inspection of blood sample preparation protocols is necessary to determine how the samples have been purposefully, or inadvertently, altered after sample collection. For example, have the samples been treated with enzymes to convert curcuminoid metabolites back into unmetabolized curcuminoids or, conversely, have metabolic reactions been allowed to continue unchecked after sample collection? Second, the details of analytical methods should be evaluated to establish which curcuminoids have been quantified and the detection limits of the analytical instrumentation. One critical aspect of this involves determining whether all curcuminoid metabolites and conjugates have been accounted for. Third, comparative absorption metrics need to be parsed in terms of the maximum concentration achieved (Cmax, ng/mL) or the total concentration over a time period (area under curve [AUC], ng/mL × h). Comparing Cmax values to AUC values is akin to comparing pomegranates and bananas.
It is important to understand whether increased absorption data have been reported in terms of the total weight of the formulation or in terms of curcuminoid equivalents. The current convention is to report findings in terms of curcuminoid equivalents. This is done to focus on the active constituents and permit some degree of comparison among ingredients that have not been compared directly until recently. Although useful in some respects, simply relying on comparisons of curcuminoid equivalents may inflate the overall estimation of the improvement the solution offers because it ignores the mass of the other non-curcuminoid ingredients (i.e., carriers, excipients, emulsifying agents) needed to facilitate bioavailability. Absorption reported as curcuminoid equivalents often leads to a larger, more impressive “XX times absorption” number but can be misleading when evaluating solid dosage forms and biological value because it neglects the added mass of non-curcuminoid material. In the following analysis, the concept of relative mass efficiency (E) is introduced as an alternative method of comparing data to assist health practitioners and product formulators in assessing which ingredients offer better biological value in terms of cost and space.
BIOAVAILABILITY ASSESSMENT OF COMMERCIAL PRODUCTS
In animals, a number of strategies have been evaluated using ingredients suited for pharmaceutical development. A formulation of curcumin using the surfactant polysorbate has been shown to increase the bioavailability of curcumin in mice Citation[18,19]. Another emulsion generating formulation containing 2 surfactants, Cremophor RH40 and Transcutol P, increased bioavailability in rats. Many other delivery formulations remain mostly curiosities for the food and nutritional product market because the ingredients used are either not approved for food use, depending on the regulatory jurisdiction, or suffer backlash in outlets that limit themselves to “natural” alternatives (e.g., exclude polysorbate). This section focuses only on products unencumbered by such concerns.
A number of food-grade formulations designed to enhance the absorption of curcumin have been studied in human clinical trials Citation[20,21]. However, until recently, there have not been any published studies directly comparing different formulations with each other. Instead, researchers have chosen to compare a test formulation to basic curcuminoid powders when using an active control group. From the perspective of cost and the probability of a successful outcome, this is understandable inasmuch as these studies are typically commercially motivated. However, as a result, it can prove challenging to compare the data of one commercial formulation to the data of another because different metrics and tactics are used to determine and describe bioavailability. These limitations will become clearer in the following sections that examine individual commercial products, their bioavailability protocols, and the reported data.
Micronized Curcuminoids Plus Turmeric Oil
Standardized to contain 95% controlled particle-size curcuminoids as verified by independent analysis, the remainder of this proprietary formula is purported to be turmeric oil Citation[22]. A variety of terpenoid and similar compounds are typically found in turmeric oil, including turmerones, phelladrene, cineole, limonene, and zingiberene. These components may act as curcuminoid dispersal agents in aqueous media or potentially as adjuvants that interfere with the metabolic machinery that acts on curcuminoids (viz. piperine). This formulation has been studied in at least 2 small clinical trials Citation[23–25]. Both report bioavailability increases of 5-fold or more over controls ingesting 95% curcuminoid powder (C95) with uncontrolled particle size (see ).
Table 1. Commercial Curcuminoid Formulations Discussed in the Text
There are a few similar anomalies for all of the studies conducted with this formulation with respect to blood sample preparation, high-performance liquid chromatography (HPLC) analysis, and statistical evaluation. High blood levels of native curcumin (nonconjugated and unmetabolized) were reported despite the failure to treat blood samples with β-glucuronidase prior to analysis. Gram quantities of material were consumed in these trials. In the preponderance of other studies, native curcumin has been undetectable in blood samples even when quantities of curcuminoid several times larger were consumed alone or in conjunction with purported curcuminoid metabolism inhibitors, such as piperine Citation[7,16,26]. An insufficient description of the HPLC method used for the analysis adds to the problem. The lack of analytical details even makes it difficult to determine whether “curcumin” has been quantified individually or as a combination of the 3 curcuminoids.
Curcuminoid Phospholipid Complex
Curcuminoid phospholipid complex (CPC) is a patented formulation containing 18%–20% total curcuminoids, as verified by independent analysis, which also includes roughly 40% by weight of each phosphatidylcholine from soy lecithin and microcrystalline cellulose Citation[22,27]. The roughly 1:1 molar ratio of phosphatidylcholine to curcumin has been suggested to lead to the formation of P-O conjugates between phosphatidylcholine and curcuminoids at one of the phenolic hydroxy groups. These conjugates may subsequently allow the fatty acid hydrocarbons to protect the curcuminoid from intestinal metabolism and perhaps assist with cellular captation and pinocytosis Citation[28–31]. The same basic strategy has been successfully utilized to increase the absorption of other hydrophobic botanical constituents Citation[32].
One standalone human pharmacokinetic study evaluating CPC has been conducted and its results published. The clinical protocol, statistical analysis, and analytical methodology are all exemplary, making the results reported quite believable Citation[20]. Moreover, the results corroborate an independent pharmacokinetic study conducted with male Wistar rats, adding an additional level of credibility to the results Citation[33]. Nevertheless, 2 additional aspects are worth mentioning. First, the reporting of intestinal absorption gains of 20 times for curcumin and 30 times for total curcuminoids versus C95 is somewhat misleading because it is based on the curcuminoid equivalents, not the total mass, of the test materials. For CPC, which contains roughly 20% curcuminoids by weight, it may be more useful for formulators to compare the effect on plasma levels of equal quantities by mass. Second, this formulation appears to increase the bioavailability of the minor curcuminoids, demethoxycurcumin and bisdemethoxycurcumin, to a much greater extent than is true of curcumin itself. Alternatively, the formula may catalyze the conversion of curcumin to these analogues. The impact upon the ratio of curcuminoids appearing in plasma was so large that the researchers reported demethoxycurcumin species as the major curcuminoid present in plasma despite a natural presence in turmeric of only 25% that of curcumin. This finding clearly was a surprise to the researchers and the understanding about its importance may increase as knowledge of the differing biological activities of individual curcuminoids continues to grow.
The impact of carrier systems on the relative uptake of the individual curcuminoids has not been well studied even though it is of potential therapeutic importance. Indeed, this issue constitutes a major lacuna in the literature. The above standalone human pharmacokinetic study conducted using CPC does not examine the consequences of the changing ratios of curcuminoids appearing in serum with differing carrier formulations and therefore does not indicate how common or extensive these consequences are across commercially available products. Fortunately, a more recent study does provide comparative data. This is discussed below in the section entitled Hydrophilic Carrier Dispersed Curcuminoids.
Curcuminoid Cyclodextrin Complex
This preparation contains ca. 14% curcuminoids and is formulated with a roughly 2:1 γ-cyclodextrin : curcuminoid molar ratio directed at creating inclusion complexes at both phenolic ends of the curcuminoid molecules Citation[34–36]. This “capping” approach can best be classified as an adjuvant strategy that physically impedes glucuronidation and sulfation reactions. Dissolution data suggest that curcuminoid cyclodextrin complex (CCC) effectively uses a dispersion enhancement strategy. In addition, the properties of γ-cyclodextrin may allow it to act akin to micelles/liposomes in facilitating uptake via interaction with the intestinal epithelium.
Pharmacokinetic data on this formulation appear to be limited to a confidential in vitro comparison (simulated intestinal absorption) to other marketed curcumin preparations and an unpublished animal study with scant details. There do not yet appear to be any published human bioavailability studies despite claims that one recently has been completed. Current marketing materials suggest that a trial found a 45-fold increase in bioavailability over C95 and at least a 4.5-fold increase over the next best commercial product Citation[37]. Details currently are limited so it is unclear which commercial products this formulation was compared against, although there is an indication that the comparator formulas were micronized curcuminoids plus turmeric oil (MCTO) and CPC. However, if the bioavailability reported from this CCC human trial is akin to that found from animal data using CCC, the same caveat should be mentioned as discussed above with CPC. Namely, the animal bioavailability data seem to have been reported in terms of curcuminoid equivalents instead of in terms of total mass, thus allowing for potential misinterpretation because it does not take into account the 86% non-curcuminoid mass of the formula. Moreover, the animal study is as of yet unpublished, so experimental details are unavailable for inspection. Marketing materials display data showing “10 to 20 times” greater concentration of curcuminoids in blood, yet without the details of the experiment, there are many aspects of the study and its findings that are unclear. For instance, the comparison material is not defined, nor is the method for curcuminoid analysis and absorption enhancement explained. If the numbers were instead reported on an equal weight basis (14% curcuminoids), the “10 to 20 times” greater concentration of curcuminoids in blood would change to “1.4 to 2.8 times.” Such equal weight comparisons may not make sense for a food product that is not sensitive to space or cost per kilogram restraints, but for nutritional products in a capsule or tablet dosage unit, the equal weight comparisons prove useful as a metric for health practitioners and formulators who often need to recommend one product over others that are superficially similar.
Lipid Curcumin Particles
This commercial offering appears to range from 20% to 30% total curcuminoid content, but no independent analysis was available for this review. In addition to curcuminoids, this formulation includes phospholipids (soy-derived), docosahexaenoic acid and/or vegetable stearic acid, ascorbyl (vitamin C) esters, and other inert ingredients. It is unclear why the curcuminoid content varies so greatly. In addition, it appears that particle size is not being controlled despite the allusion to nanoparticles in marketing materials. Lipid curcumin particles (LCP) may best be categorized as a micellular or “phytosome” formulation. The development of this formulation appears to have been driven by a goal of targeting brain tissue via passage across the blood–brain barrier. Tissue targeting is a difficult and contentious issue beyond the scope of this review.
One published human pharmacokinetic study has been conducted evaluating LCP Citation[38]. Methodological anomalies cast doubt on the reported data. A small number of subjects (6) were evaluated and the researchers reported “high interindividual variability in pharmacokinetics and nonlinear dose dependency was observed, suggesting potentially complex absorption kinetics” (p. 2095). In addition, the experimental and analytical protocols failed to allow detection of plasma curcuminoid levels after subjects ingested the control curcumin powder. Furthermore, only curcumin itself was evaluated, thus failing to account for either of the other 2 curcuminoids or any metabolites. Finally, blood was drawn from subjects at only one time point (8 hours post material ingestion), thus making impossible any assessment of curcuminoid bioavailability over time and instead, relying upon a single time point and Cmax instead of AUC. Without detecting curcumin levels in the control formulation and establishing a baseline, the researchers were unable to quantify the degree of increase in absorption with respect to LCP. However, it should be noted that marketing materials suggest that LCP increases absorption of free curcumin 65-fold without any clear indication as to how this number was reached Citation[39].
Dispersed Nanoparticle Curcumin
Mixing curcuminoids (10% curcumin, 2% other curcuminoids) with glycerin (46%), gum ghatti (4%), and water (38%) followed by wet milling and dispersion using high-pressure homogenization leads to dispersed nanoparticle curcumin (DNC). The particle size is controlled for a distribution from 100 to 1000 nm with a mean particle size of 190 nm. Such a controlled particle size nanoemulsion strategy has demonstrated promising results for increasing the oral bioavailability of other hydrophobic substances Citation[40–42].
Overall, the 2 human bioavailability studies with DNC utilized robust methodological, analytical, and data reporting protocols Citation[21]. The primary methodological shortcoming may be that plasma analysis was conducted solely for curcumin (and glucuronide conjugates), ignoring the minor curcuminoids, demethoxycurcumin and bisdemethoxycurcumin, and the metabolite tetrahydrocurcumin.
The 2 human bioavailability protocols are complementary in that the first study evaluated DNC predispersed in liquid at a dose of 30 mg, whereas the second study was administered in a solid dosage form (capsule) at 150 and 210 mg Citation[21,43]. These tests produced adequate support for the linear dose–response behavior touted for DNC up to 210 mg in humans and the suggestion that the absorption pathways do not become saturated at that level. Overall, the reported increase in curcumin bioavailability of ca. 27-fold on a curcumin equivalent basis (or 2.7-fold on an equal weight basis) seems to be well grounded in competently acquired experimental evidence and evolving theory Citation[44].
Hydrophilic Carrier Dispersed Curcuminoids
A curcuminoid formulation that was made water soluble by dispersing curcuminoids (20 wt%) and antioxidants (tocopherol and ascorbyl palmitate) onto water-soluble carriers such as polyvinyl pyrrolidone and cellulose derivatives and a small amount of fat in unknown proportions has been the subject of a recent patent application and comparative efficacy study in an animal model Citation[45,46].
A single dose comparator human bioavailability study was conducted that evaluated hydrophilic carrier dispersed curcuminoids (HCDC) versus C95, MCTO, and CPC. The researchers seem to have done their due diligence and were able to overcome many of the shortcomings encountered with previous curcuminoid trials with respect to blood sample preparation techniques, analytical protocols, and data reporting. This also appears to be the first published study that directly compares commercially available curcumin formulations beyond simply C95. In so doing, this seems to be the first study that demonstrates an effective solution for overcoming the poor bioavailability and metabolic lability of curcumin while also demonstrating that the test material (HCDC) is superior to other comparable, commercial formulations. shows these results, including a 45.9-fold greater bioavailability in serum than C95. As the only trial to date comparing multiple commercial curcuminoid formulations, it warrants additional discussion in the following section Citation[47,48].
BIOAVAILABILITY ANALYSES USING MULTIPLE METRICS
From the previous section, it is apparent that multiple paths are being explored to surmount the 2-part challenge of inherently poor curcumin bioavailability and unlocking its physiological potential. It is also clear that data reported merely in terms of curcuminoid equivalents (as is customary) can be misleading. Such reporting categorically ignores the other ingredients needed to facilitate absorption and the mass of these matrix ingredients in the commercial formulations. Curcuminoid equivalent comparisons (see Relative Molar Absorption) are useful for understanding the impact that the matrix ingredients have on the overall bioavailability of the active ingredient(s). However, taken alone, curcuminoid equivalent comparisons are not particularly useful in analyzing the comparative costs or biological value of the overall formulas. Commercial ingredients are priced by weight, not by the amount of curcuminoid equivalents, and so must be evaluated in terms of overall mass and not merely the mass of the curcuminoid portion. Increasingly, finished product labels and even nutrition facts boxes muddle this distinction, with the potential effect of misleading both consumers and health practitioners. To generate accurate bioavailability data comparisons, the same experimental protocol must be used to evaluate the ingredients in question and preferably in a single study. Yet even when single-study comparator data are unavailable, thinking about the various formulations in terms of overall mass (see Relative Mass Efficiency) can be helpful as a qualitative ranking tool for making purchasing and prescribing decisions.
The recent study conducted by Jäger and colleagues was designed to measure the levels of all 3 major curcuminoids (curcumin, demethoxycurcumin, bisdemethoxycurcumin) and the major metabolite tetrahydrocurcumin Citation[47]. Plasma samples were incubated with both β-glucuronidase and sulfatase to liberate curcuminoid conjugates prior to analysis via HPLC-tandem mass spectrometry, allowing a superior limit of detection compared to HPLC-ultraviolet. An internal standard was employed to further improve the accuracy and reliability of the analytical data. Almost 5 times the quantity of curcuminoids was administered in the reference C95 material (curcuminoids = 1800 mg) compared to the test formulations (curcuminoids = 376 mg) to guard against undetectable plasma levels for C95. To account for this difference, the relative molar absorption (F) (Equation Equation(1)(1) ) was calculated by dividing the curcuminoid(s) plasma levels resulting from the test product (HCDC, MCTO, or CPC) by the plasma levels resulting from the reference product (C95) and then multiplying by the quotient of curcuminoid(s) in the reference product (1800 mg) and the curcuminoid(s) in the test product (376 mg). This correction is necessary because the amount of curcuminoids differed greatly between the reference product and the test products ingested by study subjects. Relative molar absorption can be approximated for 2 formulations not directly compared by evaluating them transitively using data comparing each to a similar standard ingredient. For example, based on unpublished data about the plasma concentration (PCCC) that resulted from a test quantity of CCC with known curcuminoid concentration (TCCCC) and relative to C95, a tentative value of FCCC = 45.0 can be established.
(1) where PT is the plasma concentration of curcuminoid(s) from the test formulation; PC95 is the plasma concentration of curcuminoid(s) from the reference formulation; TCC95 is the total mass of curcuminoid(s) in the reference formulation; and TCT is the total mass of curcuminoid(s) in the test formulation.
The relative molar absorption (F) (Equation Equation(1)(1) ) only represents the bioavailability as a function of curcuminoid equivalents, ignoring the non-curcuminoid ingredients and thus the total mass of the formula. This can be a useful metric for focusing on the active components of a finished product formulation. However, although F is the customary mode of reporting curcumin bioavailability data, it is not always the best or only metric that can be used. For health practitioners or formulators who are faced with dosage unit space limitations or who are attempting to compare the biological values of 2 curcumin formulations as a function of per kilogram costs, another metric is also of use (see ).
Table 2. Pharmacokinetic Parameters of Curcuminoid Concentrations Area under the Curve (AUC), Cmax, tmax, and Relative Molar Absorption for Each Treatment. Citation[47]
Table 3. Relative Efficiency of Curcumin Formulations to Increase Plasma Levels of Total Curcuminoids per Unit of Formulation Mass. CWPMCTO = 0.95, CWPCPC = 0.20, CWPHCDC = 0.20, CWPCCC = 0.14
The relative mass efficiency (E) (Equation Equation(2)(2) ), which relies on relative molar absorption (F), allows an apples-to-apples comparison of curcuminoid plasma levels achieved from an equivalent mass of different formulations, even when the weight percentage of curcuminoids is vastly different. For example, if EHCDC:MCTO = 7.4, that roughly means that 1 g of HCDC will lead to 7.4 times the total curcuminoids in plasma as 1 g of MCTO. Stated alternatively, the value of 1 kg of HCDC is roughly 7.4 times that of MCTO in terms of curcuminoid blood levels. Or from a space perspective, 1 kg of MCTO is equivalent to 135 g (i.e., 1 kg/7.4) of HCDC. A value greater than one indicates that test formulation 1 is of greater value, on an equal weight basis, than formulation 2 in terms of blood curcuminoid levels that will result. For the test formulas evaluated, it can be concluded that the HCDC formula was significantly better than all of the other products, even compared to CCC that is being marketed as possessing 4.5-fold greater bioavailability than the next best commercial product.
(2)
where FT1 is the relative molar absorption of test formulation 1; CWPT1 is the curcuminoid weight percentage of test formulation 1; FT2 is the relative molar absorption of test formulation 2; and CWPT2 is the curcuminoid weight percentage of test formulation 2.
In addition to the clarity provided by directly comparing commercial curcumin formulations, the work conducted by Jäger and colleagues confirms the nonuniform enhancement of the absorption of different curcuminoids in the CPC formulation Citation[47]. As in the CPC study, plasma levels of demethoxycurcumin were found to be higher than curcumin despite there being at least 4 times more curcumin present in the test formulation prior to ingestion Citation[20]. It is possible that the P-O conjugates that are theorized to exist between phosphatidylcholine and curcumin facilitate demethoxylation of curcumin and conversion to demethoxycurcumin instead of augmenting demethoxycurcumin, per se. This contrasts with the other 3 formulations in which plasma ratios of the 3 major curcuminoids correlated with the ratios found in the pre-ingested products, as one would expect. The implications of a major change in the ratio of the ingested curcuminoids as a result of changes in the delivery vehicle remain to be explored. It has been found that antioxidant activity decreases as methoxy groups are removed, but it remains unclear whether the biological utility of the 3 curcuminoids are comparable or merely different.
DISCUSSION
There is more to “increased bioavailability” than a relative-fold absorption number. Such numbers often have been derived using experimental protocols that have shortcomings. The data derived from a bioavailability study are only as sound as the experimental protocol. From an analytical standpoint, protocols for future curcumin studies should include 3 key points: (1) Use an established and standardized HPLC method that, at a minimum, analyzes for the 3 curcuminoids while also considering quantifying levels of the metabolite tetrahydrocurcumin as well. (2) Blood samples should be taken at regular intervals over at least a 12-hour period to establish adequate AUC blood concentrations. (3) Use a large amount of standardized curcumin powder as a control to ensure that some blood levels are achieved to adequately set a baseline and then normalize using the equation for relative molar absorption (F) described above.
Although using the same experimental protocol is useful for achieving parity, the ideal remains direct bioavailability comparisons in a single comparator trial by a single research group. Moreover, comparing bioavailability in terms of the active ingredients and ignoring the total mass of the formula is not always the best means for evaluating commercial ingredients, which often consist of only a small percentage of actives. As may sometimes also be true in determining dosing, total formula mass is important for making practical formulation decisions about cost and space. The parameter relative mass efficiency (E) is designed to assist in making such evaluations. For example, ECPC : MCTOequal to 1.3 tells us that CPC will increase blood concentrations 30% greater than MCTO when consumed in equal amounts. This could be interpreted to mean that if the commercial cost of CPC is more than 130% that of MCTO, it may be overpriced in terms of biological value.
It should be understood that this perspective is not limited to commercial curcumin ingredients or even other multicomponent formulations, in general. Relative mass efficiency (E) also allows a comparison of individual molecules or salts in which the active moiety of interest only represents a portion of the overall mass. For example, interested readers may want to consider the E for magnesium oxide (60% Mg) versus magnesium citrate (11% Mg) when evaluating common claims that magnesium citrate is a more bioavailable form of magnesium. This may be true but becomes significantly less compelling when taking into account the weight percentage of the active constituent (i.e., Mg++) versus the total formula weight. Of course, this is not to say that relative molar absorption (F) is not a useful metric. It is, especially when the cost and space of the entire formulation are not important relative to the active constituents or when attention is focused solely upon the compound(s) of interest. Relative mass efficiency (E), however, can provide additional perspective relevant to understanding the practical implications of the compound(s) and formulations of interest.
With regard to the formulations tested, the values calculated for relative mass efficiency (E) metric indicate that the HCDC formula yields superior results on an equal weight basis as well as on a curcuminoid equivalent basis. On the former basis, HCDC exhibits 1.5 times the bioavailability of the next best commercial ingredient (i.e., CCC). On the latter basis, HCDC was 45.9 times more available than C95 (purified curcuminoids), whereas CCC has been claimed to be 45 times more available than C95. The seeming discrepancy is a result of the different concentrations of curcuminoids found in HCDC (20%) and CCC (14%). Although both of these numbers are significant to clinicians in determining dosing, the larger number generated for relative curcuminoid absorption may mislead unless approached with care.
There is a general dearth of understanding about the biological importance of the minor curcuminoids, demethoxycurcumin and bisdemethoxycurcumin, and of the curcuminoid metabolites. Despite the conviction of some researchers, it appears that curcumin metabolites retain at least some biological activity Citation[49–58]. The phenomenon in which turmeric extracts result in undetectable levels of free curcumin in plasma yet produce clinically significant effects would seem to be evidence for the biological relevance of curcuminoid metabolites Citation[7,59–62]. Indeed, the preponderance of the evidence indicates that only small amounts of free curcumininoids enter the circulation and such “free” components are rapidly conjugated or otherwise transformed. This may not exert a great impact on the use of curcumin to promote gut health, but in other areas the impact may not be trivial. For instance, it is doubtful that glucuronidated conjugates of curcuminoids are able to pass the blood–brain barrier Citation[5]. The picture is further complicated in that the curcumin metabolite, tetrahydrocurcumin, recently has become commercially available as a dietary ingredient Citation[63–66]. Efficacy aside, curcuminoid metabolites and conjugates represent an important piece of the pharmacokinetic landscape and should not be ignored in conducting bioavailability analyses. Recognizing that these other molecular species are important and should be measured individually, as some researchers have begun to do, is one way to gain a better understanding of their activity.
OUTLOOK
Fat has been added to turmeric preparations in the kitchen and at the apothecary's workbench since time immemorial. There is likely more than a smidgeon of cultural wisdom in such practices with respect to simple, qualitative bioavailability. But as a wag once observed, “[t]he times they are a-changin’.” This is certainly true of the formulation science for food and supplement ingredients, which has outpaced the ability to convey the benefits to consumers. There are gaps between the elements required on product labels and the information required to assess the claims appearing on those same labels. Curcumin ingredients are just one example. But inasmuch as curcumin ingredients are deployed in a wide variety of products and consumed in ever increasing amounts, they are an important bellwether for industry standards. Health practitioners and product formulators need to understand the differences among these ingredients in terms of space, cost, and biological effects themselves if they have any hope of conveying these differences to patients and consumers. For a dietary supplement industry whose products are now regularly categorized as “experience goods,” which, like wine, you need to try to see how they work, and in which consumers express significant doubt regarding label claims, more transparency could be beneficial Citation[67]. For innovative curcumin ingredients, clearer bioavailability claims and data substantiating them will lead to increased acceptance by the wider audiences expected to consume them.
Author Contributions
The article was written through contributions of both authors. Both authors have given approval to the final version of the article.
FUNDING
Financial support was provided by OmniActive Health Technologies, Inc.
REFERENCES
- Ringman JM, Frautschy SA, Teng E, Begum AN, Bardens J, Beigi M, Gylys KH, Badmaev V, Heath DD, Apostolova LG, Porter V, Vanek Z, Marshall GA, Hellemann G, Sugar C, Masterman DL, Montine TJ, Cummings JL, Cole GM: Oral curcumin for Alzheimer's disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res Ther 4:1–8, 2012.
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB: Bioavailability of curcumin: problems and promises. Mol Pharm 4:807–818, 2007.
- Mattes RD: Fat taste in humans: is it a primary? In: Montmayeur JP, le Coutre J (eds): “Fat Detection: Taste, Texture, and Post Ingestive Effects.” Boca Raton, FL: CRC Press, 2010. Accessed at http://www.ncbi.nlm.nih.gov/books/NBK53546/
- Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ: Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev 11:105–111, 2002.
- Pan MH, Huang TM, Lin JK: Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos 27:486–494, 1999.
- Wahlström B, Blennow G: A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh) 43:86–92, 1978.
- Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, Brenner DE: Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev 17:1411–1417, 2008.
- Holder GM, Plummer JL, Ryan AJ: The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica 8:761–768, 1978.
- Hoehle SI, Pfeiffer E, Solyom AM, Metzler M: Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J Agric Food Chem 54:756–764, 2007.
- Hassaninasab A, Hashimoto Y, Tomita-Yokotani K, Kobayashi M: Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc Natl Acad Sci U S A 108:6615–6620, 2011.
- Kumar A, Ahuja A, Ali J, Baboota S: Conundrum and therapeutic potential of curcumin in drug delivery. Crit Rev Ther Drug Carrier Syst 27:279–312, 2010.
- Dhule SS, Penfornis P, Frazier T, Walker R, Feldman J, Tan G, He J, Alb A, John V, Pochampally R: Curcumin-loaded γ-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomedicine 8:440–451, 2012.
- Yallapu MM, Jaggi M, Chauhan SC: Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today 17:71–80, 2012.
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY: Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 21:2895–2900, 2001.
- Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ: Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev 14:120–125, 2005.
- Lao CD, Ruffin MT IV, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE: Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 17:6–10, 2006.
- Kanai M, Yoshimura K, Asada M, Imaizumi A, Suzuki C, Matsumoto S, Nishimura T, Mori Y, Masui T, Kawaguchi Y, Yanagihara K, Yazumi S, Chiba T, Guha S, Aggarwal BB: A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol 68:157–164, 2011.
- Yu H, Huang Q: Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions. J Agric Food Chem 60:5373–5379, 2012.
- Schiborr C, Kocher A, Behnam D, Jandasek J, Toelstede S, Frank J: The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol Nutr Food Res 68:157–164, 2014.
- Cuomo J, Appendino G, Dern AS, Schneider E, McKinnon TP, Brown MJ, Togni S, Dixon BM: Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod 74:664–669, 2011.
- Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, Wada H, Katanasaka Y, Kakeya H, Fujita M, Hasegawa K, Morimoto T: Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull 34:660–665, 2011.
- Türeli AE: “Characterization of Curcumin Formulations” [Confidential Report]. Homburg, Germany: MJR PharmJet GmbH. 2011.
- Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S: A pilot cross-over study to evaluate human oral bioavailability of BCM-95CG (Biocurcumax), a novel bioenhanced preparation of curcumin. Indian J Pharm Sci 70:445–449, 2008.
- Benny M, Antony B: Bioavailability of BIOCURCUMAXTM (BCM–095TM). Spice India 19:11–15, 2006.
- Mukkadan JK: Bioavailability of CURCU-GELTM Softsules→ Containing (BCM-95). 2007. Unpublished results.
- Volak LP, Hanley MJ, Masse G, Hazarika S, Harmatz JS, Badmaev V, Majeed M, Greenblatt DJ, Court MH: Effect of an herbal extract containing curcumin and piperine on midazolam, flurbiprofen, and acetaminophen pharmacokinetics in healthy volunteers. Br J Clin Pharmacol 17:1411–1417, 2012.
- Giori A, Franceschi F: “Phospholipid Complexes of Curcumin Having Improved Bioavailability.” Patent Application WO 2007/101551, September 13, 2007.
- Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK: Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal 15:1867–1876, 1997.
- Semalty A, Semalty M, Rawat MS, Franceschi F: Supramolecular phospholipids–polyphenolics interactions: the PHYTOSOME strategy to improve the bioavailability of phytochemicals. Fitoterapia 81:306–314, 2010.
- Aura AM: Microbial metabolism of dietary phenolic compounds in the colon. Phytochem Rev 7:407–429, 2007.
- Possemiers S, Bolca S, Verstraete W, Heyerick A: The intestinal microbiome: a separate organ inside the body with the metabolic potential to influence the bioactivity of botanicals. Fitoterapia 82:53–66, 2011.
- Kidd P, Head K: A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos). Altern Med Rev 10:193–203, 2005.
- Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ: Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol 60:171–177, 2007.
- Singh R, Tønnesen H, Vogensen S, Loftsson T, Másson M: Studies of curcumin and curcuminoids. The stoichiometry and complexation constants of cyclodextrin complexes as determined by the phase-solubility method and UV-Vis titration. J Incl Phenom Macrocycl Chem 66:335–348, 2010.
- Desai K: “Curcumin Cyclodextrin Combination for Preventing or Treating Various Diseases.” US Patent Application US20100179103, July 15, 2010.
- Yallapu MM, Jaggi M, Chauhan SC: beta-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf B Biointerfaces 79:113–125, 2010.
- CAVAMAX®. W8 curcumin-complex cyclodextrins. Accessed at: http://www.wacker.com/cms/en/products/product/product.jsp?product=13747
- Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, Agarwal MG: Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem 24:2095–2099, 2010.
- The Longvida® breakthrough. Accessed at: http://longvida.com/the-breakthrough/
- Ankola DD, Viswanad B, Bhardwaj V, Ramarao P, Kumar MN: Development of potent oral nanoparticulate formulation of coenzyme Q10 for treatment of hypertension: can the simple nutritional supplements be used as first line therapeutic agents for prophylaxis/therapy? Eur J Pharm Biopharm 67:361–369, 2007.
- Mu L, Feng SS: A novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin E TPGS. J Control Release 86:33–48, 2003.
- Bala I, Bhardwaj V, Hariharan S, Kharade SV, Roy N, Ravi Kumar MN: Sustained release nanoparticulate formulation containing antioxidant-ellagic acid as potential prophylaxis system for oral administration. J Drug Target 14:27–34, 2006.
- Kanai M, Imaizumi A, Otsuka Y, Sasaki H, Hashiguchi M, Tsujiko K, Matsumoto S, Ishiguro H, Chiba T: Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother Pharmacol 69:65–70, 2012.
- Inkyoa M, Tahara T, Iwaki T, Iskandar F, Hogan CJ Jr, Okuyama K: Experimental investigation of nanoparticle dispersion by beads milling with centrifugal bead separation. J Colloid Interface Sci 304:535–540, 2006,
- Deshpande JV, Kulkarni SK: “Water Soluble Composition Comprising Curcumin Having Enhanced Bioavailability and Process Thereof.” US Patent Application 20130274343, 2013.
- Kulkarni SK, Akula KK, Deshpande J: Evaluation of antidepressant-like activity of novel water-soluble curcumin formulations and St. John's wort in behavioral paradigms of despair. Pharmacology 89:83–90, 2012.
- Jäger R, Lowery RP, Calvanese A, Joy JM, Purpura M, Wilson JM: Comparative absorption of curcumin formulations. Nutr J 13:1–8, 2014.
- Juturu V: Absorption of different curcumin formulations. International Standard Randomised Controlled Trial Number Register: ISRCTN28011391.
- Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, Steward WP, Gescher A: Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester–induced prostaglandin E2 production. Cancer Res 61:1058–1064, 2001.
- Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB: Curcumin, demethoxycurcumin, bisdemothoxycurcumin, tetrahydrocurcumin, and turmerones differentially regulate anti-inflammatory and antiproliferative responses through a ROS-independent mechanism. Carcinogenesis 28:1765–1773, 2007.
- Sugiyama Y, Kawakishi S, Osawa T: Involvement of the beta-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin. Biochem Pharmacol 52:519–525, 1996.
- Pfeiffer E, Hoehle SI, Walch SG, Riess A, Sólyom AM, Metzler M: Curcuminoids form reactive glucuronides in vitro. J Agric Food Chem 55:538–544, 2007.
- Kim JM, Araki S, Kim DJ, Park CB, Takasuka N, Baba-Toriyama H, Ota T, Nir Z, Khachik F, Shimidzu N, Tanaka Y, Osawa T, Uraji T, Murakoshi M, Nishino H, Tsuda H: Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis 19:81–85, 1998.
- Pari L, Amali DR: Protective role of tetrahydrocurcumin (THC) an active principle of turmeric on chloroquine induced hepatotoxicity in rats. J Pharm Pharm Sci 8:115–123, 2005.
- Murugan P, Pari L: Effect of tetrahydrocurcumin on plasma antioxidants in streptozotocin–nicotinamide experimental diabetes. J Basic Clin Physiol Pharmacol 17:231–244, 2006.
- Ryu MJ, Cho M, Song JY, Yun YS, Choi IW, Kim DE, Park BS, Oh S: Natural derivatives of curcumin attenuate the Wnt/β-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochem Biophys Res Commun 377:1304–1308, 2008.
- Fiala M, Liu PT, Espinosa-Jeffrey A, Rosenthal MJ, Bernard G, Ringman JM, Sayre J, Zhang L, Zaghi J, Dejbakhsh S, Chiang B, Hui J, Mahanian M, Baghaee A, Hong P, Cashman J: Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer's disease patients are improved by bisdemethoxycurcumin. Proc Natl Acad Sci USA 104:12849–12854, 2007.
- Mahattanadul S, Nakamura T, Panichayupakaranant P, Phdoongsombut N, Tungsinmunkong K, Bouking P: Comparative antiulcer effect of bisdemethoxycurcumin and curcumin in a gastric ulcer model system. Phytomedicine 16:342–351, 2009.
- Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP: Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res 7(7):1894–1900, 2001.
- Mohammadi A, Sahebkar A, Iranshahi M, Amini M, Khojasteh R, Ghayour-Mobarhan M, Ferns GA: Effects of supplementation with curcuminoids on dyslipidemia in obese patients: a randomized crossover trial. Phytother Res 27:374–379, 2013.
- Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, Steward WP: Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10:6847–6854, 2004.
- Metzler M, Pfeiffer E, Schulz SI, Dempe JS: Curcumin uptake and metabolism. Biofactors 39:14–20, 2013.
- Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, Hu S, Faull KF, Teter B, Cole GM, Frautschy SA: Curcumin structure–function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. J Pharmacol Exp Ther 326:196–208, 2008.
- Pari L, Murugan P: Influence of Tetrahydrocurcumin on tail tendon collagen contents and its properties in rats with streptozotocin–nicotinamide induced type 2 diabetes. Fundam Clin Pharmacol 21:665–671, 2007.
- Pari L, Murugan P: Antihyperlipidemic effect of curcumin and tetrahydrocurcumin in experimental type 2 diabetic rats. Ren Fail 29:881–889, 2007.
- Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, Steward WP, Gescher A: Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester–induced prostaglandin E2 production. Cancer Res 61:1058–1064, 2001.
- Thompson Reuters: “National Survey of Supplement Consumers for National Public Radio (The Shots).” New York: Thompson Reuters, 2010.