 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Cancer is a major public health problem and is the second leading cause of death in the United States and worldwide; nearly one in six deaths are attributable to cancer. Approximately 20% of all cancers diagnosed in the United States are attributable to unhealthy diet, excessive alcohol consumption, physical inactivity, and body fatness. Individual cancers are distinct disease states that are multifactorial in their causation, making them exceedingly cumbersome to study from a nutrition standpoint. Genetic influences are a major piece of the puzzle and personalized nutrition is likely to be most effective in disrupting cancer during all stages. Increasing evidence shows that after a cancer diagnosis, continuing standard dietary recommendations may not be appropriate. This is because powerful dietary interventions such as short-term fasting and carbohydrate restriction can disrupt tumor metabolism, synergizing with standard therapies such as radiation and drug therapy to improve efficacy and ultimately, cancer survival. The importance of identifying dietary interventions cannot be overstated, and the American College of Nutrition’s commitment to advancing knowledge and research is evidenced by dedication of the 2017 ACN Annual Meeting to “Disrupting Cancer: The Role of Personalized Nutrition” and this resulting proceedings manuscript, which summarizes the meeting’s findings.
Introduction
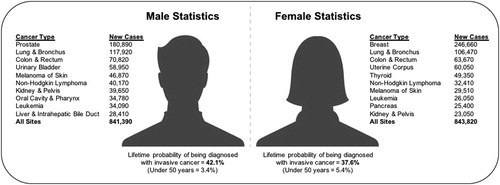
Cancer is a major public health problem and is the second leading cause of death in the United States and worldwide. Cancer is responsible for over 8.8 and 1.6 million deaths globally (Citation1) and domestically (Citation2), and nearly one in six deaths are attributable to cancer. In the United States, prostate, lung, and colorectal cancers account for 44% of all cases in men, with prostate cancer accounting for one in five new diagnoses. Breast, lung, and colorectal cancers account for one-half of all cases in women, with breast cancer accounting for 29% of new diagnoses (Citation2) (). Aside from its societal burden and loss of human potential, cancer has a large global economic impact currently estimated at over $1.16 trillion annually, which is expected to increase by 70% within 2 decades, posing a major challenge to health systems across the world (Citation3). The World Health Organization (WHO) estimates that around one-third of deaths from cancer are due to the five-leading behavioral and dietary risks: high body mass index (BMI), low fruit and vegetable consumption, lack of physical activity, tobacco use, and excessive alcohol intake (Citation3).
Figure 1. Leading estimated new cancer cases by sex in the United States, 2016.
*Adapted with permission from Siegel et al. (Citation2).
**Estimates are rounded to the nearest 10 and cases exclude basal cell and squamous cell skin cancers and in situ carcinoma except urinary bladder.
Approximately 20% of all cancers diagnosed in the United States are attributable to unhealthy diet, excessive alcohol consumption, physical inactivity, and body fatness (Citation1). Cancer is a heterogeneous and complex systems disease. Research on how nutrition influences cancer was relatively absent until the late 1970s, when large international differences in cancer rates suggested that certain components of the diet might play a major role in the cause and prevention of cancer. Today, evidence that overweight, obesity, and physical inactivity are causally related to cancer is sufficiently strong to support policy actions to reduce these hazards. Nutrition is an important part of both cancer prevention and treatment. Efforts to promote an overall healthy diet plentiful in fruits and vegetables are well justified, but the sustained effects on cancer risk are likely to be modest over time. As scientists, we have come to appreciate that most cancers are in fact distinct disease states that are multifactorial in their causation, making them exceedingly cumbersome to study from a nutrition standpoint. There are distinct differences (e.g., lack of a true placebo) between evidence that can be obtained for testing of drugs using randomized controlled trials (RCTs) and those needed for development of nutrient requirements and/or dietary guidelines to aide in the prevention or treatment of disease (Citation4). Control of body fat must be a high priority for cancer prevention, since it has become an established cause of most common cancers. Cancer treatment requires treating the tumor as well as patient as a whole. Unfortunately, recent evidence also shows that adherence to national dietary guidelines remains low (Citation5) and the majority of adults (60–87%) worldwide report consuming fewer than five servings of fruits and vegetables per day (Citation6).
Nutrition is not one size fits all. Genetic influences are a major piece of the puzzle and personalized nutrition is likely to be most effective in disrupting cancer during all stages. Increasing evidence shows that after a cancer diagnosis, continuing standard dietary recommendations may not be appropriate. This is because powerful dietary interventions such as short-term fasting and carbohydrate restriction can disrupt tumor metabolism, synergizing with standard therapies such as radiation and drug therapy to improve efficacy and ultimately, cancer survival. Public policies, campaigns, and prevention programs must be developed and integrated with activities for the prevention of other disease states (e.g., cardiovascular diseases, type 2 diabetes, etc). There must be an increased focus on increasing adherence to the U.S. Dietary Guidelines for Americans (Citation7). Achieving and maintaining a healthy body weight requires appropriate balance between energy intake and expenditure.
The four general recommendations of the American Cancer Society (ACS) guideline for nutrition and physical activity are summarized in (Citation8). The American College of Nutrition (ACN) supports the recommendations of the ACS but seeks to further expand guidance and messaging in several specific key areas that have recently emerged as favorable in preventing and/or disrupting cancer. Recommendation of specific foods with significant scientific research is warranted given the current cancer epidemic, accompanied with the low risk of harm and high potential for benefit. The intent of this article is to clarify and reinforce the notion that the ACN remains unified and committed to advancing research in the field of nutrition for the purpose of reducing the burden of cancer as well as other chronic disease states. The importance of identifying dietary interventions cannot be overstated, and our commitment is evidenced by dedication of the 2017 ACN Annual Meeting to “Disrupting Cancer: The Role of Personalized Nutrition” and this resulting proceedings manuscript. Sadly, science only has the power to inform and educate. Even though healthy choices are made by individuals, they must be better facilitated through social, economic, and policy environments. Community efforts are vital in creating environments that easily enable healthy food choices and opportunities for increased physical activity.
Table 1. American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention.
The context of this review is limited to the areas of expertise of the presenters/authors and is not encompassing of all nutritional domains related to cancer. Each section is reflective of the individual presenter/author’s presentation at the meeting and is not a reflection of a position by the American College of Nutrition. For additional information on ACN programs and meetings, visit www.americancollegeofnutrition.org.
Nutritional genomics and systems biology
The Systems Approach for Improved Clinical Outcomes in Cancer
A major challenge to effective cancer treatment is tumor heterogeneity—diverse genomic aberrations, perturbed molecular networks, and distinct subclonal cell types all contribute to intertumoral and intratumoral heterogeneity. At least 10 hallmarks of cancer have been delineated for therapeutic targeting (Citation9). It is imperative to stratify diseases of this complexity for impedance match with emerging therapeutic regimens for better clinical outcomes. Systems approaches employing advanced technologies in genomics (for oncogenic mutations), epigenetics and transcriptomics (for aberrant gene regulatory networks), proteomics (for cancer biomarkers), and single cell analysis (for tumor cell subpopulations) have been applied to dissect tumor tissue heterogeneity in leukemia, brain tumors, and colorectal cancer (Citation10–15). Moreover, cancer is a systemic disease that requires a comprehensive assessment of the whole body for better understanding of the disease pathophysiology and systematic impact from therapeutic interventions such as chemotherapy, radiation, targeted agents, and immune therapy.
Good nutrition is of particular importance for cancer patients because both the illness and related therapies can affect one’s appetite. Cancer and cancer treatments can also affect the body's ability to tolerate certain foods and use nutrients. Cancer treatment requires treating the tumor as well as the patient as a whole, of which nutrition is an integral component of the regimen (Citation16). A pioneering wellness study by the Institute for Systems Biology (ISB) examined 108 healthy individuals over the course of 9 months to obtain a multidimensional holistic view of health (Citation17). Participants were evaluated through whole genome sequencing. Quarterly measurements of blood chemistry, the metabolome, and the gut microbiome, as well as frequent physical activities. Clinically actionable possibilities were identified for all, with guided counseling by behavioral coaches to enhance one’s health. Similar principles and strategies are being adopted in disease cohorts including cancer, with the goal of alleviating the suffering and improving the wellness of cancer patients undergoing chemotherapy.
The future of healthcare is going to be individual focused, proactive wellness- and disease- care through personalized data clouds. This form of 21st century medicine, which we term predictive, preventive, personalized, and participatory (P4) medicine (Citation18, Citation19), entails coordinated efforts from practitioners, including physicians, nutritionists, scientists, and technologists. The P4 Healthcare Alliance was established to promote P4 medicine as a new medical specialty through training, certification, and advocacy. Thought leaders and stakeholders in P4 medicine from academic, nonprofit, and commercial institutions are expected to join forces in charting this exciting new territory in medicine and healthcare.
Nutritional Genetics and Cancer: Personalized Nutrition in Clinical Practice
The rapidly advancing field of nutritional genetics offers great promise for personalized nutrition in clinical practice. Nutritional genetics is the umbrella term for the field that covers two distinct phenomena: nutrigenetics and nutrigenomics. Nutrigenetics evaluates the impact that our genes have on our body’s response to nutrition. Nutrigenomics assesses the impact that nutrition has on the expression of our genes. As such, nutrigenetics and nutrigenomics are often considered the “two sides of the coin” of nutritional genetics.
There is a growing evidence base demonstrating genetic impact on overall dietary intake as well as response to specific macronutrients and micronutrients. Numerous systematic reviews and meta-analyses support the role of the rs9939609 variant on alpha-ketoglutarate-dependent dioxygenase (FTO) in both dietary intake (Citation20) and diet-related weight loss (Citation21). This variant also appears to influence the response to macronutrient intake, although the evidence is mixed (Citation22–24). More studies are needed and the effect sizes of the FTO associations are generally modest.
There appears to be a stronger genetic influence on some micronutrient concentrations. Variants in methylenetetrahydrofolate (MTHFR) are arguably the most notable genetic influence on micronutrient response, as they are common (particularly the C677T variant) and have a major impact on the methylation of folate and our need for folate intake (Citation25). Other micronutrients with well-established genetic influences include vitamin B12 (FUT2) (Citation26), vitamin D (VDR, CYP2R1, CYP27B1) (Citation27–29), and the carotenoids (BCM01, SCARB1) (Citation30, Citation31).
Nutrigenomics spans several distinct epigenetic processes, with nutritional influences on DNA methylation and histone deacetylation (HDAC) inhibition being the best-studied to date. Many common nutrients function as methyl donors (B vitamins, S-adenosyl-L-methionine (SAM-e), choline, epigallocatechin (EGCG), etc. (Citation32)) and HDAC inhibitors (sulforaphane, curcumin, quercetin, EGCG, etc. (Citation33)).
Many of the aforementioned nutrients with genetic influences have been shown to reduce the risk of a variety of cancers through a number of different mechanisms. While DNA methylation and HDAC inhibition are among these mechanisms and reducing the expression of oncogenes and increasing the expression of tumor suppressor genes plays a role in cancer prevention and improved prognosis, we do not yet have sufficiently extensive nor precise evidence to utilize nutritional genetics to prevent or treat cancer. As further evidence continues to accumulate, there is suggestive evidence that a generally nutrient-dense diet that is mindful of gene-diet interaction is the optimal dietary approach to cancer (Citation34).
Modulating the microbial landscape
The human gut microbiome is a collection complex ecosystem of organisms, containing two orders of magnitude more genes than the human genome (Citation35). Our microbiota plays a critical role in regulating diverse host processes including metabolism, inflammation, and tumorigenesis (Citation36, Citation37). Our microbiota are suggested to have a larger influence on interpersonal heterogeneity than do human genetics (Citation38). Each individual’s microbiome is unique: Even identical twins have different microbiota, and intra-individual variation is less than inter-individual variability (Citation38, Citation39). The composition of our microbiomes is influenced by a set of deterministic factors, including genetics and country of origin (Citation36, Citation40). However, lifestyle choices also play a strong role in regulating the microbiome. An individual’s medication history, diet, tendency to exercise, work history, and sleep all influence microbial communities (Citation40, Citation41).
Diet plays an important role in regulating the microbiome over both long and short time scales. Long-term dietary patterns such as the consumption of vegetables, fiber, and omega-3 fatty acids are associated with increased microbial diversity and increased abundance of potential short-chain fatty acid (SCFA) fermenters (Citation42). In contrast, low-fiber diets are associated with decreased microbial diversity. Rapid dietary changes over a few days to a few weeks can affect the microbiome but do not lead to permanent changes (Citation42–44). Furthermore, the dietary influence on the microbiome is heterogeneous and is dependent on pre-intervention habits of each participant. A recent mouse study illustrates an interaction between diet and microbes in response to dietary fiber. Mice fed a low-fiber diet experienced a loss of bacterial diversity. However, some of these genera could be recovered when mice were returned to a high-fiber diet. In contrast, pups from several generations of mothers raised on a low-fiber diets lost these genera, even when they were returned to a high-fiber diet (Citation45).
Diets rich in dietary fiber have been associated with lower rates of colorectal cancer (Citation46). This effect has been attributed to two mechanisms: First, fiber is associated with increased transit time, which is hypothesized to decrease colonic exposure to carcinogens. Fiber is also thought to interact with the microbiome to produce tumor-suppressive metabolites. Recent work has implicated butyrate. Using a gnotobiotic mouse model (i.e., a mouse model in which only certain known strains of bacteria and other microorganisms are present), the relationship between SCFA fermenting bacteria, dietary fiber, and colorectal cancer was evaluated. Gnotobiotic mice allow direct perturbations of the microbiota while controlling for potentially confounding effects such as genetic background or food source. To evaluate the interaction between the microbiome and fiber in regulating colorectal cancer, gnotobiotic mice were inoculated with a consortium of four commensal bacteria with or without Butyrivibrio fibrisiolvens, a butyrate producer. Mice were fed high- or low-fiber chow. Colorectal cancer was then induced using the azoxymethane model. Tumor attenuation was seen only in mice that received both B. fibrisiolvens and dietary fiber, suggesting that fermentation to butyrate was required for the tumor-suppressive effect. The suppression could also be induced using a butyrate-fortified diet, supporting the role of microbial fermentation of dietary fiber in colorectal cancer prevention (Citation47).
Accumulating evidence suggests that the gut microbiome’s role in early cancer risk extends well beyond the activities of specific bacterial genera and is largely influenced by the wider microbial community of commensal bacteria. These key modulators of food digestion and SCFA metabolism stand at the interface of host metabolism and immunity to modulate inflammation, gut integrity, and tumorigenesis. Diets largely composed of fiber-rich plant foods are consistently recommended for cancer prevention at the primary (healthy persons without cancer), secondary (pre-cancer patients), and tertiary (cancer survivors) level (Citation48). Fiber-rich diets have been shown to expand commensal bacteria and limit pathogenic bacteria’s access to the gut epithelium with reciprocal changes in mucosal biomarkers of cancer risk (Citation49, Citation50).
A cluster of January 2018 Science publications revealed that cancer patients initiating immunotherapy can be stratified into responders and non-responders on the basis of the composition of their intestinal microbiomes (Citation51–54). Although targeted therapies and checkpoint inhibitor immunotherapies are changing the face of cancer treatment, particularly in patients with late-stage disease, cancer patient outcomes remain heterogeneous and many patients still ultimately succumb to this disease. The scientific and medical research community’s spotlight on the microbiome and its potential to improve personalized cancer medicine, coupled with the patient’s persistent interest in diet, brings to light new opportunities and priorities for diet-microbiome research in cancer patients. An improved understanding of modifiable patient behaviors (e.g., diet) and other host factors associated with clinical benefit from these treatments may improve their personalized use and provide new insight into resistance, relapse, and recurrence. Such modifiable factors, including diet, obesity, and the microbiome, which are known to impact inflammation and immune pathways, are increasingly recognized in cancer and cardiovascular disease prevention and deserve a closer look for cancer patients and survivors (Citation55–58).
Regarding the current state of science, we know something about what shapes the microbiome (e.g., diet, medications, alcohol use, disease, etc) and what differs or diverges in the microbiome of cancer patients, primarily sampled pre-treatment, as compared to healthy or cancer-free individuals. We still have a very limited understanding of what shapes the microbiome of cancer patients and/or how the microbiome changes in patients over the course of treatment. Despite growing recognition that factors beyond tumor genomics may influence therapeutic response in cancer patients, including the microbiome and obesity (Citation51–54, Citation58), we know remarkably little about diet in the context of cancer patients’ microbiomes or response to treatment.
Role of metabolism
Disrupting tumor metabolism in the oncology clinic
One approach to exploring the metabolic vulnerabilities of cancer during cancer therapy involves implementing the ketogenic diet and fasting in the oncology clinic. That this is both feasible and safe is shown by recent work. Observational and interventional trials demonstrate that fasting during cancer treatment is safe and may decrease adverse effects of anticancer drugs. A case series of 10 patients with various advanced cancers reported that fasts of 48–140 hours before chemotherapy infusions and 5–56 hours after chemotherapy infusions were not associated with any serious adverse effects. In fact, six patients reported less fatigue and weakness and fewer gastrointestinal side effects during chemotherapy cycles accompanied by fasting (Citation59). A dose escalation trial found that fasting for 24, 48, or 72 hours before administration of platinum-based chemotherapy was safe and feasible and decreased markers of hematologic toxicity, although not enough to reach statistical significance (Citation60). A study of six patients undergoing radiation therapy for cancer reported that while fat mass decreased, lean tissue mass was preserved with a ketogenic diet. Tumor regression occurred in five of six patients with early stage disease. One subject with metastatic small cell lung cancer experienced slight progression during the three cycles of combined chemotherapy plus the ketogenic diet, which progressed rapidly after ending the ketogenic diet (Citation61). A Veterans Affairs pilot study found that a low-carbohydrate diet was safe and feasible in humans with a variety of advanced cancers. Interesting to this study was that despite undergoing no other treatment, four of eleven evaluable patients achieved stable or improved disease status with the diet alone, with the best response obtained in those losing 10% or more of their body weight (Citation62). Spurred by encouraging results in animal studies, clinical trials are underway to evaluate the effectiveness of fasting and the ketogenic diet as adjuncts to cancer treatment. In the meantime, patients who wish to proceed with fasting or the ketogenic diet during cancer therapy can be assured that with supervision, these approaches are safe. Further studies enrolling a greater number of patients are recommended as data in this area is scarce.
Cancer as a mitochondrial metabolic disease: implications for novel adjuvant therapeutics
The failure to manage cancer has been due in large part to the dogmatic belief that cancer is a constellation of genetic diseases (Citation63). Emerging evidence, however, suggests that cancer is primarily a mitochondrial metabolic disease involving disturbances in energy production through respiration and fermentation (Citation64). The disturbances in tumor cell energy metabolism are suggested to be linked to abnormalities in the structure and function of mitochondria that disrupt adenosine triphosphate (ATP) synthesis through oxidative phosphorylation (Citation65, Citation66). If this is indeed the case, all cancers could be considered a single disease with a common pathophysiological mechanism involving dysfunction of mitochondrial oxidative phosphorylation. Continuing this theory, gene mutations observed in various cancers and all other recognized cancer hallmarks may be considered downstream effects, and not causes, of the initial disturbance of cellular energy metabolism (Citation64, Citation67).
It has been proposed that cancer growth and progression might be managed following a whole-body transition from fermentable metabolites, primarily glucose and glutamine, to respiratory metabolites, primarily ketone bodies (Citation68). The potential mechanism for ketone diets as an adjuvant for cancer therapy has recently been narratively reviewed by experts (Citation69). Normal cells transition to ketone bodies for energy under low glucose conditions. Ketone body metabolism thus protects the brain against hypoglycemia. Tumor cells, on the other hand, cannot effectively use ketone bodies for energy due to their dysfunction in oxidative phosphorylation. Therapeutic fasting and calorie-restricted ketogenic diets have been suggested to lower cancer-provoking glucose and insulin-like growth factor (IGF-1) levels, while elevating ketone bodies (Citation70–73). The metabolic transition from glucose to ketone bodies reduces tumor angiogenesis and inflammation while enhancing tumor cell apoptosis. The Press-Pulse therapeutic paradigm used with the Glucose/Ketone Index () will facilitate the nontoxic management and prevention of cancer (Citation68, Citation74). As each individual is a unique metabolic entity, personalization of metabolic therapy as a broad-based cancer treatment and prevention strategy will require fine-tuning to match the therapy to an individual’s unique physiology. The efficacy of metabolic therapy for management of malignant cancer is seen in preclinical models and in humans with various cancers (Citation64,Citation68,Citation75–77) (). It is anticipated that metabolic therapies targeting glucose and glutamine while increasing therapeutic ketosis will significantly improve quality of life and overall survival for most cancer patients.
Figure 2. Relationship of plasma glucose and ketone body levels to brain cancer management. The glucose and ketone (β-OHB) values are within normal physiological ranges under fasting conditions in humans. This is referred to as the zone of metabolic management. As blood glucose falls and ketones rise, an individual is predicted to reach the zone of metabolic management. Tumor progression is predicted to be slower within the metabolic target zone than outside of the zone. This can be tracked using the Glucose/Ketone Index.*
*Adapted with permission from Meidenbauer et al. (2015) [X].
![Figure 2. Relationship of plasma glucose and ketone body levels to brain cancer management. The glucose and ketone (β-OHB) values are within normal physiological ranges under fasting conditions in humans. This is referred to as the zone of metabolic management. As blood glucose falls and ketones rise, an individual is predicted to reach the zone of metabolic management. Tumor progression is predicted to be slower within the metabolic target zone than outside of the zone. This can be tracked using the Glucose/Ketone Index.**Adapted with permission from Meidenbauer et al. (2015) [X].](/cms/asset/01cdb30d-77f2-4307-8ebe-9dd081528f41/uacn_a_1500499_f0002_c.jpg)
Table 2. The Press-Pulse Therapeutic Strategy and Glucose/Ketone Index.*
Breaking the obesity-cancer link: new targets and strategies
Over 125,000 cancer deaths per year in the US are attributed to overweight and obesity, and currently 38% of adults in the US are considered obese (BMI >30 kg/m2) with an additional 7.7% being classified as morbidly obese (BMI >40 kg/m2) (Citation78). By 2030, approximately 500,000 Americans per year will be diagnosed with obesity-related cancer unless corrective action is taken (Citation79). There is convincing evidence that obesity plays a causal factor for many types of cancer including colorectum, endometrium, kidney, esophagus, postmenopausal breast, gallbladder, pancreas, gastric cardia, liver, ovary, thyroid, meningioma, multiple myeloma, and advanced prostate cancers (Citation80). Emerging evidence supports the concept that metabolic reprogramming, inflammation, and genome instability (including epigenetic changes) underlie many of the hallmarks of cancer. In both obesity and metabolic syndrome, alterations occur in circulating levels of insulin and insulin-like growth factors, sex hormones, adipokines, inflammatory factors, several chemokines, lipid mediators and vascular associated factors (Citation81). In the case of cancer-associated metabolic reprogramming, cancer cells preferentially metabolize glucose through glycolysis rather than oxidative phosphorylation, even in the presence of oxygen (Citation9, Citation82, Citation83). Thus, the citric acid cycle intermediates are not used for adenosine triphosphate (ATP) production and are shuttled out of the mitochondria, providing precursors for nucleotide, amino acid, and lipid synthesis pathways for the dividing cell (Citation83). In this way, cancer cells readily take up and metabolize glucose to provide substrate for production of daughter cells. Levels of glucose uptake transporters (GLUT) and glycolytic enzymes are elevated in most cancers (Citation84).
White adipose tissue consists mainly of adipocytes, which serve to store neutralized triacylglycerides for use during periods of energy deficit, they are characterized by secretion of leptin, resisten, inflammatory cytokines and free fatty acids. White adipose tissue contains several types of stromal cells, including pre-adipocytes, vascular cells, fibroblasts, and a host of immune cells such as adipose tissue macrophages (Citation85). Brown adipose tissue on the other hand, which generates heat, is characterized by secretion of bone morphogenetic proteins, lactate, retinaldehyde, triiodothyronine (T3) and other factors associated with response to cold stress and increased energy expenditure (Citation86). Brown adipose tissues also produce adiponectin (but not leptin) and fibroblast growth factor-21, which can be anti-inflammatory and insulin sensitizing (Citation86). The increase in adipose tissue mass associated with obesity drives chronic inflammation in at least three ways:
Altered adipose secretome including levels of leptin, adiponectin, and sex hormones whose mechanisms have been previously reviewed (Citation87).
Adipose remodeling and lipid infiltration in other tissues – In a diseased state, white adipose tissue does not respond appropriately to changes in energy requirements, resulting in altered metabolic signaling characteristics by elevated adipokine and cytokine production (Citation88). Excess white adipose tissue promotes tumour cell proliferation through provision of circulating fatty acids (Citation89).
Crown-like structures – Obesity further drives subclinical inflammation in visceral and subcutaneous white adipose tissue, characterized by these rings of activated macrophages surrounding engorged or necrotic adipocytes. This adipocyte-macrophage interaction results in proinflammatory secretome from both cell types that activates cellular nuclear factor-κB (NF-κB), leading to increased levels of cytokines and other inflammatory factors and triggers of inflammation (Citation90).
As adipose tissue grows, so does the need for new blood vessels. This is mediated by vascular endothelial growth factor (VEGF) which is secreted by both adipocytes and tumour cells. The release of VEGF into circulation can interact with peripheral tissues and foster angiogenesis at tumour sites. New blood vessels provide nutrients and oxygen to cells within the primary tumour mass. Newly forming blood vessels provide a route into circulation for cells to metastasize to distal sites in the body.
Evidence for personalized nutrition across the cancer spectrum
Diet, epigenetics, and cancer prevention
The classic view of cancer etiology is that genetic alterations damage DNA structure and induce mutations resulting in nonfunctional proteins that lead to disease progression. More recently, the role of epigenetic alterations during cancer has gained increasing attention (Citation91–94). This has caused a paradigm shift in potential approaches for cancer prevention and therapy targets. Importantly, components of the diet are able to target epigenetic processes. The reversible acetylation of histones is an important mechanism of gene regulation. During cancer progression, specific modifications in acetylation patterns on histones are apparent (Citation95, Citation96). Targeting the epigenome, including the use of hydroxamic acid-based (HDAC) inhibitors, is a novel strategy for cancer chemoprevention. Recently, drugs classified as HDAC inhibitors have shown promise in cancer clinical trials (Citation97–102). Based on the similarity of sulforaphane (SFN) metabolites and other phytochemicals to known pharmacological HDAC inhibitors, it has been previously demonstrated that sulforaphane, a phytochemical derived from cruciferous vegetables, acts as an HDAC inhibitor in the prostate, causing enhanced histone acetylation, de-repression of P21 and Bax, and induction of cell cycle arrest/apoptosis, leading to cancer prevention (Citation103–106). Other epigenetic mechanisms, including DNA methylation, histone methylation, and noncoding RNA, also appear to be impacted with SFN. This work suggests that phytochemicals may have the ability to alter epigenetic events that lead to disease prevention. These mechanistic preclinical studies have provided a strong scientific foundation for ongoing human clinical trials. In human supplementation trials, we have directly compared the effects of the “whole food” (broccoli sprouts) to commercially available supplements. Significant decreases in bioavailability and impact on HDACs with supplements compared to the whole food have previously been illustrated (Citation107). Surprisingly, even when supplements are pretreated with myrosinase, the release of sulforaphane from its glucosinolate precursors in the supplement is limited. In a breast cancer human clinical trial, supplementation with broccoli sprout extracts resulted in decreases in HDAC activity and decreases in Ki-67 protein (otherwise known as MK167), a cell proliferation marker, compared to those given placebos (Citation108). These studies are significant because of the potential to qualify or change recommendations for high-risk cancer patients and thereby increase their survival through simple dietary choices incorporating easily accessible foods into their diets.
The role of nutrition in pediatric oncology
Nutrition is an important part of the health of all children, but it is especially important for children getting cancer treatment. There are several critical nutritional components to consider from a cancer control perspective in pediatric oncology. The American Cancer Society (ACS) has a comprehensive online guide specifically dedicated to nutrition for children with cancer (Citation109) Obesity at diagnosis is a negative prognostic indicator of several pediatric cancers including leukemia and bone tumours (Citation110). Case-controlled studies of childhood cancer indicate that maternal folate intake during conception and early pregnancy may be important in mitigating risk of some pediatric cancers. A recent meta-analysis reported multivitamins containing folic acid had protective effects against neuroblastoma, leukemia, and central nervous system (CNS) tumours (Citation111). However, multivitamins do not replace eating enough calories and protein, critical for the child’s continued growth and development. Eating well during cancer treatment may help a child 1) better tolerate treatment and its side effects; 2) stay closer to the treatment plan schedule; 3) heal and recover faster; 4) have less risk of injury or infection during treatment; 5) have better strength and energy; 6) do better at keeping up normal growth and development; 7) feel better and have better quality of life (e.g., less irritability, loss of sleep, etc) (Citation109).
Each child with cancer has their own unique nutritional needs. Cancer epidemiology and molecular epidemiology related to nutrition are emerging areas of research. It is essential to evaluate nutritional status of mothers and the children who develop cancer, so we can better understand its relationship. Nutritional status during chemotherapy may well affect pharmacokinetics and pharmacodynamics of several drugs that are utilized in treatment of pediatric malignancies (Citation112–115). At Both ends of the spectrum of malnutrition, that is, protein energy malnutrition and being underweight and stunted, or being overweight or obese, can influence how drugs are cleared. This has been well documented for methotrexate where undernutrition decreases drug clearance and is associated with increased toxicity. Obesity also affects clearance of ifosfamide, cyclophosphamide, and doxorubicin. The causes of cancer cachexia, characterized by systematic inflammation, negative protein and energy balance, and an involuntary loss of lean body mass, is multifactorial and may be related to disease, host phenotype, socioeconomic status, as well as the treatment used (Citation115–118).
Select micronutrients and phytonutrients
Copper depletion as a therapeutic strategy in breast cancer patients at high risk of recurrence
Dozens of human enzymes incorporate or utilize copper, taking advantage of the metal’s readiness to donate or accept electrons to catalyze key biochemical reactions. Tumours however, may be especially dependent on the metal. Copper has emerged as a critical component of the metastatic process, and multiple preclinical studies have shown copper depletion to be associated with inhibition of angiogenesis, the growth of blood vessels that can feed an expanding tumour (Citation119–123). Copper depletion may also reverse epithelial-mesenchymal transition (EMT) and downregulate expression of EMT-related genes, such as vimentin and fibronectin (Citation124). Furthermore, it is a vital component of several enzymes key to remodeling the tumor microenvironment and lung premetastatic niche, including lysyl oxidase (LOX), superoxide dismutase-1, and vascular adhesion protein-1. LOX is a copper-dependent amine oxidase, secreted by the primary tumour, that accumulates at premetastatic sites where it crosslinks collagen forming a scaffold for recruited bone marrow-derived CD11b+ myeloid cells. This scaffold for acts as a “premetastatic niche” and promotes tumour outgrowth of disseminated metastatic cells (Citation125–129). Copper may also bind and activate the enzyme Memo, which enables tumour cells to move independently and metastasize. It is also required for signaling by the mutant BRAF protein, which drives half of melanomas and many other cancers Therefore there has been interest to utilize copper depletion as a therapeutic strategy in patients with cancer.
Tetrathiomolybdate, a safe and well-tolerated oral copper chelator initially developed for the treatment of Wilson’s disease has been used as a therapeutic agent in a variety of solid tumors. What is notable about these phase 1 and 2 clinical trials is that the best response was stable disease in up to one-third of patients (Citation130–132). Building on the pre-clinical work by Pan and colleagues that suggested that copper depletion could prevent the development of overt metastases, a clinical trial was initiated in breast cancer patients with a high risk of recurrence (Stage 2 and stage 3 without any evidence of disease (NED) with 48% of the patients having a triple negative subtype) (Citation133). The concept was that a copper-depletion strategy targets would reduce the infrastructure critical for tumour progression (LOX and bone marrow-derived progenitor cells (i.e., VEGFR2* EPCs) in this high-risk population. It was found that copper depletion was safe, and well tolerated (Citation134, Citation135). At a median follow-up of 7.1-years the event free survival for all patients enrolled in the trial is 71% and for the TNBC patients, who are stage 4 NED it is 60%. This is encouraging because the median survival of stage 4 TNBC is 12 months (Citation136).
Vitamin D: its role in cancer prevention and treatment
Vitamin D is an unusual micronutrient in that its bioavailability derives from not only the diet but also biosynthesis in the skin in response to ultraviolet-B radiation exposure. Vitamin D is also unique in that it is a precursor to the potent steroid hormone calcitriol (otherwise known as 1,25-dihydroxyvitamin D3 (1,25(OH)2D3)), which has widespread actions throughout the body. The circulating form of vitamin D, serum 25-hydroxyvitamin D3 (25(OH)D3)), is converted to calcitriol in the liver, kidney and other organs that express the enzyme 1αhydroxylase (Citation137, Citation138). Calcitriol functions by binding and activating the nuclear vitamin D receptor (VDR), which is a member of the steroid-thyroid-retinoid receptor superfamily of ligand-activated transcriptions factors. VDRs are present in most cells in the body (Citation139). Therefore, calcitriol regulates as much as 3-5% of the human genome, exerting actions that may alter the body’s defenses and limit progression of multiple diseases, including cancer (Citation140). Although the kidney is the major source of circulating calcitriol, CYP27B1 (i.e., the gene providing the instructions for making 1αhydroxylase) is also expressed in multiple extrarenal sites, including cancer cells. This enables production of calcitriol to become localized where it can exert anticancer actions (Citation141). Calcitriol can function as both in an endocrine (systemic) manner or in an intracrine, autocrine or paracrine manner when it is locally synthesized. The presence of CYP27B1 in cancer cells suggests that dietary vitamin D may be used in cancer therapy since it is easily converted to 25(OH)D3 by the liver, and then locally to calcitriol within the cancer tissue, where it may exert anticancer actions (Citation142).
Epidemiological and early clinical trials on vitamin D intake and cancer are inconsistent, and randomized controlled trials in humans collectively do not yet support a beneficial role for vitamin D from the diet or supplements. However, accumulating science from preclinical and some clinical trials strongly suggest vitamin D deficiency is associated with an increased risk of developing cancer. Observational evidence collectively indicate that low serum 25(OH)D3 status is correlated with elevated rates of some cancers, particularly among populations living at higher altitudes (Citation143). Recent data from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort show a dose-response inverse relationship in serum 25(OH)D3 status and risk of colorectal cancer (Citation144). Data from breast cancer patients (n = 179) vs. control women (n = 179) recruited in a case-controlled study suggest that serum 25(OH)D3 levels <50 nmol and the bb BsmI VDR genotype were 6.82 times more likely to have developed breast cancer than subjects with serum 25(OH)D3 levels >50 nmol, as compared to either the BB or Bb Bsml VDR genotype (95% confidence interval (CI) 2.31–14.7, p < 0.001) (Citation145). A recent Canadian study showed women with serum 25(OH)D3 levels above 28.8 ng/mL at diagnosis had nearly half of the risk of dying with a hazard ratio of 0.58, (95% CI, 0.35–0.95) (Citation146). It should be noted that despite the observational, preclinical and laboratory research on vitamin D, several important gaps exist with respect to cancer survival, tumour subtypes, interactions between vitamin D and other factors including genetic variation, and vitamin D and cancer risk in black populations.
Carotenoids and breast cancer
Carotenoids, essential for plant photosynthesis, are fat-soluble yellow-red pigments in fruit and vegetables. Of more than 600 identified, six carotenoids represent 90% of carotenoids found in the circulation (Citation147, Citation148), and are found in common components of the US diet: α-carotene (carrots), β-carotene (sweet potatoes and leafy greens), lutein and zeaxanthin (leafy greens), lycopene (tomatoes), and β-cryptoxanthin (citrus fruits). Anticarcinogenic activity of carotenoids is hypothesized to include metabolism to retinoids that contribute to cellular differentiation, antioxidation, immuno-enhancement, or the inhibition of tumorigenesis and malignant transformation (Citation149–153).
Breast cancer is the most common female malignancy worldwide (Citation154). Most identified risk factors are not easily modified, including age, menopausal status, family history, parity, age at menarche, and age at first birth. However, inverse associations with fruit and vegetable intake and, more specifically, carotenoid intake have been reported. In pooled analyses of 18 cohort studies, inverse associations were observed between intakes α-carotene, β-carotene, and lutein and zeaxanthin and the more aggressive estrogen receptor (ER)-negative tumors, but not ER-positive tumors (Citation155). The measurement of circulating carotenoids provides an integrated measure that captures cooking influences (Citation146, Citation156), geographic and seasonal variation of foods (Citation156), and individual variation in absorption while avoiding measurement error from recalled diet (Citation145) and inaccuracies of nutrient databases (Citation157).
Pooled data from eight cohort studies indicate circulating carotenoids may reduce subsequent risk of breast cancer. This study included 3055 women with breast cancer diagnosed after blood collection and 3956 matched controls (Citation158), adjusted for the following established breast cancer risk factors: menopausal status, age at menarche, parity, age at first birth, exogenous hormone use, BMI, current smoking status, race, personal history of benign breast disease, and family history of breast cancer. Comparing the highest versus lowest quintile of circulating carotenoid concentration, significant inverse associations were observed for α-carotene (RR =0.87, 95% CI =0.71–1.05), β-carotene (RR =0.83, 95% CI =0.70–0.98), lycopene (RR =0.78, 95% CI =0.62–0.99), and total carotenoids (RR =0.81, 95% CI =0.68–0.96). Several studies published subsequently also support these findings (Citation159–163).
Similar to the dietary intake results, the inverse associations between circulating carotenoid levels and breast cancer observed in the pooled analysis were stronger for ER-negative versus ER-positive breast cancers (e.g., highest vs. lowest quintile β-carotene ER-negative: RR =0.52, 95% CI =0.36–0.77, p-trend =0.001; ER-positive: RR =0.83, 95% CI =0.66–1.04, p-trend =0.06; p-heterogeneity =0.01) (Citation158). In an extended analysis in the Nurses’ Health Study with 2188 breast cancer cases and their matched controls, pre-diagnostic plasma carotenoid levels were associated with significant reduced risk of lethal or recurrent breast cancer (e.g., β-carotene highest vs. lowest quintile: RR =0.32, 95% CI =0.2–0.51, p-trend =0.001). In addition, other studies have reported lower risk of recurrence and death among women with higher carotenoid levels at the time of diagnosis and after diagnosis (Citation164, Citation165), suggesting a role for carotenoids in prognostic improvement as well.
Overall, current evidence consistently shows that circulating carotenoids are associated with lower risk of incident breast cancer, including aggressive and lethal tumors. While supplemental beta-carotene is not recommended, given harmful effects documented among smokers (Citation166), breast cancer risk reduction may be possible through dietary changes. Future studies should focus on the distinct role of carotenoids in ER-negative versus ER-positive breast cancers, as well as the prognostic role of carotenoids after breast cancer diagnosis.
Conclusions
Most research has focused on high-income countries and while many identified risk factors will have the same physiologic effects in low-income countries, the determinants may be different, in addition to other genetic differences across populations. A variety of factors influence dietary and lifestyle habits. Surveillance efforts through prospective studies is an important area of research. Early exposure to an energy-rich and nutrient-deplete diet alongside other suboptimal lifestyle behaviors may alter infant’s and children’s growth patterns and result in impaired metabolism, obesity and risk of developing cancer in adulthood. Lifelong and multigenerational prospective studies are greatly needed. Nutrient dense dietary patterns characterized by higher intakes of plant foods with lower intakes of added sugars, refined grains and starch, as well as saturated and trans-fats are likely to contribute to cancer prevention. The microbiota composition, largely determined by the diet, is also likely to contribute to cancer risk. There is suggestive and emerging evidence that carbohydrate restriction (i.e., ketogenesis) may help to disrupt cancer, without risk of adverse effects. Future government research funding allocation in the field of nutrition is needed, alongside a strategic research roadmap, and action from policy makers.
Acknowledgments
The authors would like to thank Christina West for her assistance in copy-editing and formatting the manuscript.
Disclosure statement
The authors declare no funding support for this manuscript.
References
- World Health Organization: "Cancer Fact Sheet." [cited 2017 Dec 21]. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin . 2016;66(1):7–30.
- International Agency for Research on Cancer: "World Cancer Report 2014." Geneva, Switzerland: WHO Press, 2014.
- Blumberg J, Heaney RP, Huncharek M, Scholl T, Stampfer M, Vieth R, Weaver CM, Zeisel SH. Evidence-based criteria in the nutritional context. Nutr Rev 2010;68(8):478–484.
- US Department of Health and Human Services, US Department of Agriculture: "Scientific Report of the 2015 Dietary Guidelines Advisory Committee." [cited 2017 Dec 28]. Available from: http://health.gov/dietaryguidelines/2015-scientific-report/.
- Murphy MM, Barraj LM, Herman D, Bi X, Cheatham R, Randolph RK. Phytonutrient intake by adults in the United States in relation to fruit and vegetable consumption. J Acad Nutr Di2012;112(2):222–229.
- US Department of Health and Human Services, US Department of Agriculture: "2015–2020 Dietary Guidelines for Americans, 8th edition." [cited 2017 Dec 22]. Available from: https://health.gov/dietaryguidelines/2015/guidelines/.
- Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2012;62(1):30–67.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674.
- Majeti R, Becker MW, Tian Q, Lee TL, Yan X, Liu R, Chiang JH, Hood L, Clarke MF, Weissman IL. Dysregulated gene expression networks in human acute myelogenous leukemia stem cells. Proc Natl Acad Sci U S A 2009;106(9):3396–3401.
- Yan X, Ma L, Yi D, Yoon JG, Diercks A, Foltz G, Price ND, Hood LE, Tian Q. A CD133-related gene expression signature identifies an aggressive glioblastoma subtype with excessive mutations. Proc Natl Acad Sci U S A 2011;108(4):1591–1596.
- Chiang JH, Cheng WS, Hood L, Tian Q. An epigenetic biomarker panel for glioblastoma multiforme personalized medicine through DNA methylation analysis of human embryonic stem cell-like signature. Omics 2014;18(5):310–323.
- Lin EH, Yan X, Xie F, Wu D, Scarborough JD, Tang J, Pritchard CC, Yeung RS, Hood LE, Tian Q. PI3K/APC pathway and cyclin-dependent kinase pathway to predict complete responders in CRC patients treated with ADAPT therapy. J Clin Oncol. 2015;33e14642–e14642.
- Tian Q, Sangar V, Price ND. Emerging proteomic technologies provide enormous and underutilized potential for brain cancer research. Mol Cell Proteomics 2016;15(2):362–367.
- Yang Z, Keel SB, Shimamura A, Liu L, Gerds AT, Li HY, Wood BL, Scott BL, Abkowitz JL. Delayed globin synthesis leads to excessive heme and the macrocytic anemia of Diamond Blackfan anemia and del(5q) myelodysplastic syndrome. Sci. Transl. Med 2016;8(338):338ra67.
- Bloch AS, Grant B, Hamilton KK, Thomson CA. American Cancer Society Complete Guide to Nutrition for Cancer Survivors: Eating Well, Staying Well During and After Cancer. Atlanta, GA: American Cancer Society, 2010.
- Price ND, Magis AT, Earls JC, Glusman G, Levy R, Lausted C, McDonald DT, Kusebauch U, Moss CL, Zhou Y, et al. A wellness study of 108 individuals using personal, dense, dynamic data clouds. Nat Biotechnol 2017;35(8):747–756.
- Tian Q, Price ND, Hood L. Systems cancer medicine: towards realization of predictive, preventive, personalized and participatory (P4) medicine. J Intern Med 2012;271(2):111–121.
- Hood L, Tian Q. Systems approaches to biology and disease enable translational systems medicine. Genomics Proteomics BioinformaticsBioinformatics 2012;10(4):181–185.
- Livingstone KM, Celis-Morales C, Lara J, Ashor AW, Lovegrove JA, Martinez JA, Saris WH, Gibney M, Manios Y, Traczyk I, et al. Associations between FTO genotype and total energy and macronutrient intake in adults: a systematic review and meta-analysis. Obes Rev 2015;16(8):666–678.
- Xiang L, Wu H, Pan A, Patel B, Xiang G, Qi L, Kaplan RC, Hu F, Wylie-Rosett J, Qi Q. FTO genotype and weight loss in diet and lifestyle interventions: a systematic review and meta-analysis. Am J Clin Nutr 2016;103(4):1162–1170.
- de Luis DA, Aller R, Izaola O, Primo D, Urdiales S, Romero E. Effects of a high-protein/low-carbohydrate diet versus a standard hypocaloric diet on weight and cardiovascular risk factors: role of a genetic variation in the rs9939609 FTO gene variant. J Nutrigenet Nutrigenomi2015;8(3):128–136.
- de Luis DA, Aller R, Izaola O, de la Fuente B, Conde R, Sagrado MG, Primo D. Evaluation of weight loss and adipocytokines levels after two hypocaloric diets with different macronutrient distribution in obese subjects with rs9939609 gene variant. Diabetes Metab Res Rev 2012;28(8):663–668.
- Huang T, Qi Q, Li Y, Hu FB, Bray GA, Sacks FM, Williamson DA, Qi L. FTO genotype, dietary protein, and change in appetite: the Preventing Overweight Using Novel Dietary Strategies trial. Am J Clin Nutr 2014;99(5):1126–1130.
- Molloy AM, Daly S, Mills JL, Kirke PN, Whitehead AS, Ramsbottom D, Conley MR, Weir DG, Scott JM. Thermolabile variant of 5,10-methylenetetrahydrofolate reductase associated with low red-cell folates: implications for folate intake recommendations. Lancet 1997;349(9065):1591–1593.
- Hazra A, Kraft P, Selhub J, Giovannucci EL, Thomas G, Hoover RN, Chanock SJ, Hunter DJ. Common variants of FUT2 are associated with plasma vitamin B12 levels. Nat Genet 2008;40(10):1160–1162.
- Yao P, Sun L, Lu L, Ding H, Chen X, Tang L, Xu X, Liu G, Hu Y, Ma Y, et al. Effects of genetic and nongenetic factors on total and bioavailable 25(OH)D responses to vitamin D supplementation. J Clin Endocrinol Metab 2017;102(1):100–110. :
- Zhu JG, Ochalek JT, Kaufmann M, Jones G, Deluca HF. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc Natl Acad Sci U S A 2013;110(39):15650–15655.
- Miller WL. Genetic disorders of vitamin D biosynthesis and degradation. J Steroid Biochem Mol Biol 2017;165(Pt A):101–108.
- Ferrucci L, Perry JR, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, et al. Common variation in the beta-carotene 15,15'-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet 2009;84(2):123–133.
- D’Adamo CR, D’Urso A, Ryan KA, Yerges-Armstrong LM, Semba RD, Steinle NI, Mitchell BD, Shuldiner AR, McArdle PF. A common variant in the SETD7 gene predicts serum lycopene concentrations. Nutrients. 2016;8(2):82.
- Anderson OS, Sant KE, Dolinoy DC. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem 2012;23(8):853–859.
- Bassett SA, Barnett MP. The role of dietary histone deacetylases (HDACs) inhibitors in health and disease. Nutrients. 2014;6(10):4273–4301.
- Theodoratou E, Timofeeva M, Li X, Meng X, Ioannidis JPA. Nature, nurture, and cancer risks: genetic and nutritional contributions to cancer. Annu Rev Nutr. 2017;37293–320.
- Gill SR, Pop M, DeBoy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science 2006;312(5778):1355–1359.
- Knight R, Callewaert C, Marotz C, Hyde ER, Debelius JW, McDonald D, Sogin ML. The microbiome and human biology. Annu Rev Genomics Hum Genet. 2017;1865–86.
- Fulbright LE, Ellermann M, Arthur JC. The microbiome and the hallmarks of cancer. PLoS Pathog 2017;13(9):e1006480.
- Knights D, Costello EK, Knight R. Supervised classification of human microbiota. FEMS Microbiol Rev 2011;35(2):343–359.
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334(6052):105–108.
- Debelius J, Song SJ, Vazquez-Baeza Y, Xu ZZ, Gonzalez A, Knight R. Tiny microbes, enormous impacts: what matters in gut microbiome studies?. Genome Biol 2016;17(1):217.
- Davis SC, Yadav JS, Barrow SD, Robertson BK. Gut microbiome diversity influenced more by the Westernized dietary regime than the body mass index as assessed using effect size statistic. Microbiologyopen. 2017;6(4):e00476.
- Martinez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, Louk JA, Rose DJ, Kyureghian G, Peterson DA, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J 2013;7(2):269–280.
- Smits SA, Leach J, Sonnenburg ED, Gonzalez CG, Lichtman JS, Reid G, Knight R, Manjurano A, Changalucha J, Elias JE, et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017;357(6353):802–806. :
- Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529(7585):212–215.
- Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and "western-lifestyle" inflammatory diseases. Immunity. 2014;40(6):833–842.
- Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343d6617.
- Donohoe DR, Holley D, Collins LB, Montgomery SA, Whitmore AC, Hillhouse A, Curry KP, Renner SW, Greenwalt A, Ryan EP, et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014;4(12):1387–1397.
- World Cancer Research Fund/American Institute for Cancer Research: "Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective." [cited 2018 Mar 23]. Available from: http://www.aicr.org/continuous-update-project/.
- O’Keefe SJD, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, Posma JM, Kinross J, Wahl E, Ruder E, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun2015;6(1):6342.
- Jobin C. Precision medicine using microbiota. Science 2018;359(6371):32–34.
- Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018;359(6371):104–108.
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359(6371):91–97.
- Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018;359(6371):97–103.
- Bhatt AP, Redinbo MR, Bultman SJ. The role of the microbiome in cancer development and therapy. CA Cancer J Clin 2017;67(4):326–344.
- Song M, Wu K, Meyerhardt JA, Ogino S, Wang M, Fuchs CS, Giovannucci EL, Chan AT. Fiber intake and survival after colorectal cancer diagnosis. JAMA Oncol 2018;4(1):71–79.
- Daniel CR, Hoffman KL, Raju GS, Hanash SM, Hutchinson DS, Ajami NJ, Fowler RG, Browman GJ, Sood A, Scheet P, et al. Evidence of early colorectal cancer risk and prevention pathways in the fecal microbiome of colonoscopy patients: associations with diet and circulating adipocytokines. Cancer Res. 2017;77(13 Supplement):238.
- Canavan N. A cure within: scientists unleashing the immune system to kill cancer. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2018.
- McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol2018;19(3):310–322.
- Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, Cohen P, Longo VD. Fasting and cancer treatment in humans: a case series report. Aging (Albany NY) 2009;1(12):988–1007.
- Dorff TB, Groshen S, Garcia A, Shah M, Tsao-Wei D, Pham H, Cheng CW, Brandhorst S, Cohen P, Wei M, et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer. 2016;16:360.
- Klement RJ, Sweeney RA. Impact of a ketogenic diet intervention during radiotherapy on body composition: I. Initial clinical experience with six prospectively studied patients. BMC Res Notes. 2016;9:143.
- Tan-Shalaby JL, Carrick J, Edinger K, Genovese D, Liman AD, Passero VA, Shah RB. Modified Atkins diet in advanced malignancies – final results of a safety and feasibility trial within the Veterans Affairs Pittsburgh Healthcare System. Nutr Metab (Lond). 2016;13:52.
- . National Institutes of Health: "Cancers: A 'Constellation' of Diseases." [cited 2018 Mar 23]. Available from: https://medlineplus.gov/magazine/issues/winter13/articles/winter13pg2-3.html.
- Seyfried TN, Flores RE, Poff AM, D'Agostino DP. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis. 2014;35(3):515–527.
- Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutr Metab (Lond) . 2010;7:77.
- Seyfried TN. Cancer as a mitochondrial metabolic disease. Front Cell Dev Biol. 2015;343.
- Seyfried TN. Cancer as a metabolic disease: on the origin, management, and prevention of cancer. Hoboken, NJ: John Wiley & Sons, 2012.
- Seyfried TN, Yu G, Maroon JC, D’Agostino DP. Press-pulse: a novel therapeutic strategy for the metabolic management of cancer. Nutr Metab (Lond) 2017;14(1):19.
- Allen BG, Bhatia SK, Anderson CM, Eichenberger-Gilmore JM, Sibenaller ZA, Mapuskar KA, Schoenfeld JD, Buatti JM, Spitz DR, Fath MA. Ketogenic diets as adjuvant cancer therapy: History and potential mechanism. Redox Biol. 2014;2:963–970.
- Hursting SD, Kari FW. The anti-carcinogenic effects of dietary restriction: mechanisms and future directions. Mutat Res 1999;443(1-2):235–249.
- Mukherjee P, Abate LE, Seyfried TN. Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clin Cancer Res 2004;10(16):5622–5629.
- Mukherjee P, El-Abbadi MM, Kasperzyk JL, Ranes MK, Seyfried TN. Dietary restriction reduces angiogenesis and growth in an orthotopic mouse brain tumour model. Br J Cancer 2002;86(10):1615–1621.
- Marsh J, Mukherjee P, Seyfried TN. Akt-dependent proapoptotic effects of dietary restriction on late-stage management of a phosphatase and tensin homologue/tuberous sclerosis complex 2-deficient mouse astrocytoma. Clin Cancer Res 2008;14(23):7751–7762.
- Meidenbauer JJ, Mukherjee P, Seyfried TN. The glucose ketone index calculator: a simple tool to monitor therapeutic efficacy for metabolic management of brain cancer. Nutr Metab (Lond) 2015;12:12:12.
- Iyikesici MS, Slocum AK, Slocum A, Berkarda FB, Kalamian M, Seyfried TN. Efficacy of metabolically supported chemotherapy combined with ketogenic diet, hyperthermia, and hyperbaric oxygen therapy for stage IV triple-negative breast cancer. Cureus. 2017;9(7):e1445.
- Toth C, Clemens Z. Halted progression of soft palate cancer in a patient treated with the paleolithic ketogenic diet alone: a 20-months follow-up. Am J Med Case Rep. 2016;4:288–292.
- Shelton LM, Huysentruyt LC, Seyfried TN. Glutamine targeting inhibits systemic metastasis in the VM-M3 murine tumor model. Int J Cancer 2010;127(10):2478–2485.
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781.
- Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, Fabian CJ, Gucalp A, Hershman DL, Hudson MM, et al. American Society of Clinical Oncology position statement on obesity and cancer. JCO. 2014;32(31):3568–3574.
- Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body Fatness and Cancer–Viewpoint of the IARC Working Group. N Engl J Med2016;375(8):794–798.
- Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis 2015;6(2):109–120.
- Chen X, Qian Y, Wu S. The Warburg effect: evolving interpretations of an established concept. Free Radic Biol Med. 2015;79:253–263.
- Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 2012;21(3):297–308.
- Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
- Eto H, Suga H, Matsumoto D, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast Reconstr Surg2009;124(4):1087–1097.
- Wang GX, Zhao XY, Lin JD. The brown fat secretome: metabolic functions beyond thermogenesis. Trends Endocrinol Metab 2015;26(5):231–237.
- O’Flanagan CH, Bowers LW, Allott EH, Hursting SD. Molecular and metabolic mechanisms underlying the obesity–cancer link. "Energy Balancer and Obesity (IARC Working Group Reports 10)." Lyon, France: International Agency for Research on Cancer, pp 95–104, 2017.
- Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 2014;15(4):6184–6223.
- Balaban S, Lee LS, Schreuder M, Hoy AJ. Obesity and cancer progression: is there a role of fatty acid metabolism? Biomed Res Int. 2015;2015:274585.
- Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4(3):329–346.
- Ho E, Beaver LM, Williams DE, Dashwood RH. Dietary factors and epigenetic regulation for prostate cancer prevention. Adv Nutr 2011;2(6):497–510.
- Dashwood RH, Ho E. Dietary histone deacetylase inhibitors: from cells to mice to man. Semin Cancer Biol 2007;17(5):363–369.
- Dashwood RH, Ho E. Dietary agents as histone deacetylase inhibitors: sulforaphane and structurally related isothiocyanates. Nutr Rev. 2008;66(Suppl 1):S36–S38.
- Dashwood RH, Myzak MC, Ho E. Dietary HDAC inhibitors: time to rethink weak ligands in cancer chemoprevention? Carcinogenesis. 2006;27(2):344–349.
- Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 2001;1(3):194–202.
- Marks PA, Richon VM, Breslow R, Rifkind RA. Histone deacetylase inhibitors as new cancer drugs. Curr Opin Oncol 2001;13(6):477–483.
- Eigl BJ, North S, Winquist E, Finch D, Wood L, Sridhar SS, Powers J, Good J, Sharma M, Squire JA, et al. A phase II study of the HDAC inhibitor SB939 in patients with castration resistant prostate cancer: NCIC clinical trials group study IND195. Invest New Drugs 2015;33(4):969–976. :
- Gao S, Li X, Zang J, Xu W, Zhang Y. Preclinical and clinical studies of chidamide (CS055/HBI-8000), an orally available subtype-selective HDAC inhibitor for cancer therapy. Anticancer Agents Med Chem 2017;17(6):802–812.
- Govindaraj C, Tan P, Walker P, Wei A, Spencer A, Plebanski M. Reducing TNF receptor 2+ regulatory T cells via the combined action of azacitidine and the HDAC inhibitor, panobinostat for clinical benefit in acute myeloid leukemia patients. Clin Cancer Res 2014;20(3):724–735.
- Razak AR, Hotte SJ, Siu LL, Chen EX, Hirte HW, Powers J, Walsh W, Stayner LA, Laughlin A, Novotny-Diermayr V, et al. Phase I clinical, pharmacokinetic and pharmacodynamic study of SB939, an oral histone deacetylase (HDAC) inhibitor, in patients with advanced solid tumours. Br J Cancer 2011;104(5):756–762.
- Shankar S, Srivastava RK. Histone deacetylase inhibitors: mechanisms and clinical significance in cancer: HDAC inhibitor-induced apoptosis. Adv Exp Med Biol. 2008;615:261–298.
- Srinivas NR. Clinical pharmacokinetics of panobinostat, a novel histone deacetylase (HDAC) inhibitor: review and perspectives. Xenobiotica 2017;47(4):354–368.
- Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett 2008;269(2):291–304.
- Clarke JD, Hsu A, Yu Z, Dashwood RH, Ho E. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol Nutr Food Res 2011;55(7):999–1009.
- Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis. 2006;27(4):811–819.
- Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp Biol Med (Maywood) . 2007;232(2):227–234.
- Atwell LL, Hsu A, Wong CP, Stevens JF, Bella D, Yu TW, Pereira CB, Lohr CV, Christensen JM, Dashwood RH, et al. Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol Nutr Food Res 2015;59(3):424–433. :
- Atwell LL, Zhang Z, Mori M, Farris PE, Vetto JT, Naik AM, Oh KY, Thuillier P, Ho E, Shannon J. Sulforaphane bioavailability and chemopreventive activity in women scheduled for breast biopsy. Cancer Prev Res (Phila). 2015;8(12):1184–1191.
- American Cancer Society: "Nutrition for children with cancer." [cited 2018 May 4]. Available from: https://www.cancer.org/treatment/children-and-cancer/when-your-child-has-cancer/nutrition.html.
- Schadler KL, Kleinerman ES, Chandra J. Diet and exercise interventions for pediatric cancer patients during therapy: tipping the scales for better outcomes. Pediatr Res 2018;83(1-1):50–56.
- Goh YI, Bollano E, Einarson TR, Koren G. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther 2007;81(5):685–691.
- Murry DJ, Riva L, Poplack DG. Impact of nutrition on pharmacokinetics of anti-neoplastic agents. Int J Cancer Suppl. 1998;1148–51.
- Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet 2000;39(3):215–231.
- Krishnaswamy K. Drug metabolism and pharmacokinetics in malnourished children. Clin Pharmacokinet. 1989;17 Suppl 1:68–88.
- Ladas EJ, Sacks N, Meacham L, Henry D, Enriquez L, Lowry G, Hawkes R, Dadd G, Rogers P. A multidisciplinary review of nutrition considerations in the pediatric oncology population: a perspective from children's oncology group. Nutr Clin Pract 2005;20(4):377–393.
- Bechard LJ, Adiv OE, Jasksic T, Collins JJ. Nutritional supportive care. In Piazzo PA and Poplack DG editors. Principles and Practice of Pediatric Oncology, 5th ed. Philadelphia: Lippincott Williams and Wilkins, 2006, pp 1330–1347.
- Brinksma A, Huizinga G, Sulkers E, Kamps W, Roodbol P, Tissing W. Malnutrition in childhood cancer patients: a review on its prevalence and possible causes. Crit Rev Oncol Hematol 2012;83(2):249–275.
- Co-Reyes E, Li R, Huh W, Chandra J. Malnutrition and obesity in pediatric oncology patients: causes, consequences, and interventions. Pediatr Blood Cancer 2012;59(7):1160–1167.
- Finney L, Vogt S, Fukai T, Glesne D. Copper and angiogenesis: unravelling a relationship key to cancer progression. Clin Exp Pharmacol Physiol 2009;36(1):88–94.
- MacDonald G, Nalvarte I, Smirnova T, Vecchi M, Aceto N, Dolemeyer A, Frei A, Lienhard S, Wyckoff J, Hess D, et al. Memo is a copper-dependent redox protein with an essential role in migration and metastasis. Sci Signal 2014;7(329):ra56.
- Brady DC, Crowe MS, Turski ML, Hobbs GA, Yao X, Chaikuad A, Knapp S, Xiao K, Campbell SL, Thiele DJ, Counter CM. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature. 2014;509(7501):492–496.
- Ishida S, Andreux P, Poitry-Yamate C, Auwerx J, Hanahan D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc Natl Acad Sci U S A 2013;110(48):19507–19512.
- Brem SS, Zagzag D, Tsanaclis AM, Gately S, Elkouby MP, Brien SE. Inhibition of angiogenesis and tumor growth in the brain. Suppression of endothelial cell turnover by penicillamine and the depletion of copper, an angiogenic cofactor. Am J Pathol 1990;137(5):1121–1142.
- Li S, Zhang J, Yang H, Wu C, Dang X, Liu Y. Copper depletion inhibits CoCl2-induced aggressive phenotype of MCF-7 cells via downregulation of HIF-1 and inhibition of Snail/Twist-mediated epithelial-mesenchymal transition. Sci Rep. 2015;5:12410.
- Lowndes SA, Sheldon HV, Cai S, Taylor JM, Harris AL. Copper chelator ATN-224 inhibits endothelial function by multiple mechanisms. Microvasc Res 2009;77(3):314–326.
- Xiao Q, Ge G. Lysyl oxidase, extracellular matrix remodeling and cancer metastasis. Cancer Microenviron 2012;5(3):261–273.
- Cox TR, Bird D, Baker AM, Barker HE, Ho MW, Lang G, Erler JT. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res 2013;73(6):1721–1732.
- Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nat Rev Cancer 2012;12(8):540–552.
- Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440(7088):1222–1226.
- Redman BG, Esper P, Pan Q, Dunn RL, Hussain HK, Chenevert T, Brewer GJ, Merajver SD. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin Cancer Res. 2003;9(5):1666–1672.
- Henja GF, Merajver SD, Pan Q, Brewer GJ, Henja GF, Merajver SD, Zalupski MM. A pilot trial of the anti-angiogenic copper lowering agent tetrathiomolybdate in combination with irinotecan, 5-flurouracil and leucovorin for metastatic colorectal cancer. Invest New Drugs. 2009;27(2):159–165.
- Brewer GJ, Dick RD, Grover DK, LeClaire V, Tseng M, Wicha M, Peinta K, Redman BG, Jahan T, Sondak VK, et al. Treatement of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin Cancer Res. 2000;6(1):1–10.
- Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C, De Carvalho M, Mesri EA, Robins DM, Dick RD, et al. Copper deficiency induced by tetrathiomolybdate supresses tumor growth and angiogenesis. Cancer Res. 2002;62(17):4854–4859.
- Jain S, Cohen J, Ward MM, Kornhauser N, Chuang E, Cigler T, Moore A, Donovan D, Lam C, Cobham MV, et al. Tetrathiomolybdate-associated copper depletion decreases circulating endothelial progenitor cells in women with breast cancer at high risk of relapse. Ann Oncol. 2013;24(6):1491–1498.
- Chan N, Willis A, Kornhauser N, Ward MM, Lee SB, Nackos E, Seo BR, Chuang E, Cigler T, Moore A, et al. Influencing the tumor microenvironment: a phase II study of copper depletion using tetrathiomolybdate in patients with breast cancer at high risk for recurrence and in preclinical models of lung metastases. Clin Cancer Res 2017;23(3):666–676. :
- Sahota S, Kornhauser N, Willis A, Ward MM, Cigler T, Moore A, Andreopoulou E, Fitzpatrick V, Schneider SE, Wiener A, et al. A phase II study of copper-depletion using tetrathiomolybdate (TM) in patients (pts) with breast cancer (BC) at high risk for recurrence: Updated results. J Clin Oncol 2017;35(15_suppl):2557-2557
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol 2009;19(2):73–78.
- Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 2004;79(3):362–371.
- Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor?. Arch Biochem Biophys 2012;523(1):123–133.
- Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014;14(5):342–357.
- Hobaus J, Thie U, Hummel DM, Kallay E. Role of calcium, vitamin D, and the extrarenal vitamin D hydroxylases in carcinogenesis. Anticancer Agents Med Chem 2013;13(1):20–35.
- Hsu JY, Feldman D, McNeal JE, Peehl DM. Reduced 1αhydroxylase activity in human prostate cancer cells correlates with decreased susceptibility to 25-dihydroxyvitamin D3 induced growth inhibition. Cancer Res. 2001;61:2852–2856.
- Garland FC, Garland CF, Gorham ED, Young JF. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med. 1990;19(6):614–622.
- Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, Jansen EH, Slimani N, Byrnes G, Rinaldi S, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations:a nested case-control study. BMJ. 2010;340:b5500.
- Lowe LC, Guy M, Mansi JL, Peckitt C, Bliss J, Wilson RG, Colston KW. Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer. 2005;41(8):1164–1169.
- Goodwin P, Ennis M, Pritchard K, Koo J, Hood N, Lunenfeld S. Vitamin D deficiency is common at breast cancer diagnosis and is associated with a significantly higher risk of distant recurrence and death in a prospective cohort study of T1-3, N0-1, M0 BC (Abstract). JCO. 2008;26(15_suppl):511.
- Motchnik PA, Frei B, Ames BN. Measurement of antioxidants in human blood plasma. Methods Enzymol. 1994;234:269–279.
- Krinsky N. Carotenoids and cancer: basic research studies. In Frei B editor. Natural Antioxidants in Human Health and Disease. San Diego, CA: Academic Press, 1994, pp 239–262.
- Bertram JS. Dietary carotenoids, connexins and cancer: what is the connection? Biochem Soc Trans 2004;32(Pt 6):985–989.
- Bendich A. Carotenoids and the immune response. J Nutr 1989;119(1):112–115.
- Krinsky NI. Actions of carotenoids in biological systems. Annu Rev Nutr. 1993;13561–587.
- Shultz TD, Chew BP, Seaman WR, Luedecke LO. Inhibitory effect of conjugated dienoic derivatives of linoleic acid and beta-carotene on the in vitro growth of human cancer cells. Cancer Lett 1992;63(2):125–133.
- Sporn MB, Roberts AB. Role of retinoids in differentiation and carcinogenesis. Cancer Res 1983;43(7):3034–3040.
- Ferlay J, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D, Forman D, Bray F. "GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11." Lyon, France: International Agency for Research on Cancer, 2013.
- Zhang X, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, van den Brandt PA, Buring JE, Gapstur SM, Giles GG, Giovannucci E, et al. Carotenoid intakes and risk of breast cancer defined by estrogen receptor and progesterone receptor status: a pooled analysis of 18 prospective cohort studies. Am J Clin Nutr 2012;95(3):713–725. :
- Micozzi MS, Beecher GR, Taylor PR, Khachik F. Carotenoid analyses of selected raw and cooked foods associated with a lower risk for cancer. J Natl Cancer Inst 1990;82(4):282–285.
- Mangels AR, Holden JM, Beecher GR, Forman MR, Lanza E. Carotenoid content of fruits and vegetables: an evaluation of analytic data. J Am Diet Assoc 1993;93(3):284–296.
- Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, Dorgan JF, Franke AA, Gao YT, Goodman MT, et al. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst 2012;104(24):1905–1916. :
- Maillard V, Kuriki K, Lefebvre B, Boutron-Ruault MC, Lenoir GM, Joulin V, Clavel-Chapelon F, Chajes V. Serum carotenoid, tocopherol and retinol concentrations and breast cancer risk in the E3N-EPIC study. Int J Cancer 2010;127(5):1188–1196.
- Kabat GC, Kim M, Adams-Campbell LL, Caan BJ, Chlebowski RT, Neuhouser ML, Shikany JM, Rohan TE. Longitudinal study of serum carotenoid, retinol, and tocopherol concentrations in relation to breast cancer risk among postmenopausal women. Am J Clin Nutr 2009;90(1):162–169.
- Bakker MF, Peeters PH, Klaasen VM, Bueno-de-Mesquita HB, Jansen EH, Ros MM, Travier N, Olsen A, Tjonneland A, Overvad K, et al. Plasma carotenoids, vitamin C, tocopherols, and retinol and the risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr 2016;103(2):454–464. :
- Wang Y, Gapstur SM, Gaudet MM, Furtado JD, Campos H, McCullough ML. Plasma carotenoids and breast cancer risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Causes Control 2015;26(9):1233–1244.
- Eliassen AH, Liao X, Rosner B, Tamimi RM, Tworoger SS, Hankinson SE. Plasma carotenoids and risk of breast cancer over 20 y of follow-up. Am J Clin Nutr 2015;101(6):1197–1205.
- Rock CL, Flatt SW, Natarajan L, Thomson CA, Bardwell WA, Newman VA, Hollenbach KA, Jones L, Caan BJ, Pierce JP. Plasma carotenoids and recurrence-free survival in women with a history of breast cancer. J Clin Oncol 2005;23(27):6631–6638.
- Rock CL, Natarajan L, Pu M, Thomson CA, Flatt SW, Caan BJ, Gold EB, Al-Delaimy WK, Newman VA, Hajek RA, et al. Longitudinal biological exposure to carotenoids is associated with breast cancer-free survival in the Women's Healthy Eating and Living Study. Cancer Epidemiol Biomarkers Prev 2009;18(2):486–494.
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334(18):1150–1155.
