Abstract
Objective
Konjac is a food mainly consumed in Asian countries with high fiber and low energy. Although glucomannan, a component of konjac, have been used for several clinical studies, there is few reports using konjac itself. This study examined the effects of the active consumption of konjac in patients with type 2 diabetes mellitus (T2DM).
Methods
The study included 26 Japanese patients with T2DM. Participants were recommended to take konjac at least once a day using free konjac products (various noodles, rice, and desserts) and plate konjac for 12 weeks.
Results
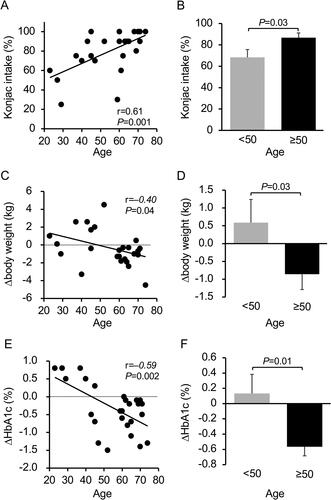
HbA1c and fasting plasma glucose levels significantly decreased from 8.3 ± 0.9% to 8.0 ± 0.8% and from 173.2 ± 44.4 to 152.8 ± 36.7 mg/dL, respectively. No significant changes were observed in body weight and insulin resistance indices, but the index for insulin secretion significantly increased. Serum high molecular weight adiponectin levels significantly increased. Plasma ghrelin, leptin and glucagon-like peptide-1 levels tended to decrease (p = 0.084), decrease (p = 0.057) and increase (p = 0.071), respectively. Actual konjac intake positively correlated with age (r = 0.61, p = 0.001). Body weight and HbA1c significantly decreased in patients aged ≥50 years than in those aged <50 years, and the changes significantly inversely correlated with age.
Conclusion
Active consumption of konjac and konjac products seems to be a useful dietary therapy with multifaceted action for T2DM. Further studies with greater sample size and long-term are needed to confirm these findings.
Introduction
Konjac is mainly consumed in Asian countries such as Japan and China. Using konjac yam as the raw material, konjac consists of approximately 97% water. It is extremely low in calories, containing only 5 kcal per 100 g (Citation1). Konjac is also rich in dietary fiber, containing approximately 2.2 g in a 100-g plate konjac. Konjac yam contains approximately 10% glucomannan, a water-soluble polysaccharide. Glucomannan has the largest molecular weight among all natural polysaccharides, and its aqueous solution has extremely high viscosity. When in contact with water, glucomannan swells to approximately 200 times or more of its original volume. Since glucomannan is hardly broken down by human digestive enzymes, it retains a high molecular weight while passing through the stomach and small intestine to reach the large intestine (Citation2). Therefore, glucomannan reportedly affects the absorption of nutrients in the small intestine and intestinal flora in the large intestine, thereby when adding glucomannan to usual diet, there are various reports of suppressing blood cholesterol elevation, improving bowel movements, lowering postprandial blood glucose levels, increasing weight loss, and having antiallergic activity (Citation3–12).
When konjac glucomannan is added as an ingredient in foods like sausage, yogurt, or mozzarella cheese, the final product has been reported to have lower energy content than the same product without konjac glucomannan (Citation13–15). For example, when half of pork backfat (usually used in normal sausage) was replaced with konjac glucomannan, the appearance, color, and flavor were equivalent or superior to and the energy content was 32% lower than that of the full-fat product (Citation13). These foods are expected to be useful in diet therapy for obesity. However, we could not find any clinical studies that used this type of food as therapy.
Almost all previous studies focusing on the effects of konjac used protocols that provided participants with approximately 4 g of purified glucomannan per day in addition to regular meals (Citation7–9, Citation12, Citation16–18). There are only 3 previous reports that have used konjac or various foods based on konjac. The first one investigated the effect of food intake ad libitum after ingesting pasta containing konjac glucomannan in healthy subjects (Citation19). The others studied factors like glycemic control, body weight, and serum lipids after eating konjac-containing food (such as konjac noodle and konjac toast) for 45–60 days in patients with diabetes or dyslipidemia (Citation20, Citation21). Although these studies are similar to our investigation, these were done over 20 years ago and with limited evaluations. Although glucomannan is one of the components of konjac, it is insufficient to investigate the effects of konjac itself. Therefore, in this study, we decided to actively consume konjac itself but not glucomannan only. However, it may be difficult to eat konjac every day because it requires cooking and preparation. Therefore, we prepared a variety of konjac products (noodles, rice, and desserts) that can be easily eaten every day with minimal preparation. We examined the efficacy of active consumption of konjac and konjac products without glucomannan to determine its antidiabetic and anti-obesity effects as a part of diet therapy against diabetes.
Materials and methods
Study participants
The study included patients with T2DM aged 20–75 years who were receiving outpatient treatment at the Department of Endocrinology, Metabolism, and Diabetes, the Faculty of Medicine, University of Miyazaki Hospital; Department of Internal Medicine, Koga General Hospital; or the Department of Internal Medicine, Miyazaki Prefectural Miyazaki Hospital and who had a body mass index (BMI) ≥22 kg/m2 and glycated hemoglobin A1c (HbA1c) of 7.0%–10% and who consented to participate in this study (Citation22). Exclusion criteria were patients who had the following conditions: advanced diabetic retinopathy, currently receiving dialysis, Meckel’s diverticulum following gastrectomy, risk of intestinal obstruction following konjac ingestion owing to a history of abdominal surgery, severe ketosis, diabetic coma or pre-coma, severe infection, before or after surgery, or serious trauma. We did not limit the type or number of antidiabetic drugs, including insulin, because diet therapy is the most important therapy in type 2 diabetes even if insulin therapy is performed (Citation23). Medical doctors or support staff recruited outpatients based on the inclusion and exclusion criteria. Patients who provided informed consent were included in this study. The predicted sample size using a paired t-test was 22 according to the statistical software (paired-t-test-sample-size, HHA Shop, Japan), which calculated the sample size using 0.8, 0.6, and 0.05 as detection power, standardized difference, and α value, respectively. Finally, 27 cases were included in this study.
Study protocol and ethical statement
This study was a single-arm, open-label trial. This study was approved by the Ethics Review Committee of each of the three hospitals and was registered in the University Hospital Medical Information Network Clinical Trials Registry as UMIN000033362 (https://rctportal.niph.go.jp/s/detail/um?trial_id=UMIN000033362). All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and the Helsinki Declaration of 1964, as revised in 2013. Patient registration was performed by the physicians participating in this study. After providing written consent for participation in this study, participants continued to receive outpatient treatment without any change to their antidiabetic drugs during 12 weeks. Novel exercise therapy was not recommended during the study. At baseline and week 12, the participants visited the study site without eating breakfast for physical measurements and blood testing. Height was measured at week 0, and body weight and blood pressure were measured at weeks 0, 4, 8, and 12. Blood pressure was measured using an automated sphygmomanometer (BP-203RVIII, OMRON, Kyoto, Japan). Height and body weight were measured using a stadiometer with an electronic scale (AD-6228P, A&D Co., Tokyo, Japan). At week 12, the participants’ appetite and satisfaction were assessed using a visual analog scale (VAS) questionnaire. At weeks 0, 4, and 8, the participants were provided individual nutritional guidance including appropriate daily energy intake and the method of the intake of konjac and konjac products by a nutritionist for around 45 min, and an interview about their intake of provided konjac and konjac products was also conducted. Nutritional guidance was provided based on the 2019 diabetes medical treatment guidelines established by the Japan Diabetes Society according to factors including standard body weight, physical activity, and occupation. The participants received free home delivery of 30 servings of konjac products consisting of two packages of 175-g plate konjac to be cooked, eight packages of konjac noodles with different flavors, eight packages of konjac rice, nine packages of seven types of seasoned konjac side dishes, and three packages of different konjac desserts (Moteki Food Engineering Co., Ltd., Gunma, Japan) once every 4 weeks for three times (). For example, 170 g of konjac pasta with garlic, chili peppers, and olive oil is 45 kcal. A seasoned 300-g konjac shaped ball is 36 kcal. When 2 go (about 300 g) rice is cooked with a package of konjac rice, the energy provided is 30% lower than that produced by rice without konjac. The study was conducted while instructing the participants to eat at least 1 konjac dish or konjac product per day for 12 weeks, with the goal of consuming at least 100 g of konjac each day. This study was conducted in three hospitals. All data were forwarded to the University of Miyazaki. Some data ordered to SRL Inc. were mailed to the University of Miyazaki directly. The height, body weight, blood pressure, and blood data measured in each hospital were forwarded to University of Miyazaki after anonymizing the personal information of the patients. One doctor (H. Ueno) of the University of Miyazaki managed all data of this study using a personal computer separated from the internet.
Study endpoints
The primary endpoint was change in the HbA1c level. Secondary endpoints were body weight, BMI, and insulin resistance (fasting serum insulin and homeostasis model assessment insulin resistance [HOMA-R]); however, five patients on insulin therapy were excluded. Other endpoints included changes in fasting serum C-peptide reactivity (CPR), CPR index (CPI), secretory units of islets in transplantation (SUIT), and homeostasis model assessment of beta-cell function (HOMA2-%B) as indices of insulin secretion ability; changes in blood test results associated with glycolipid metabolism and hepatic/renal function; changes in blood test results associated with appetite, inflammation, glucose metabolism, and obesity (ghrelin, des-acyl ghrelin, active glucagon-like peptide-1 [GLP-1], active glucose-dependent insulinotropic polypeptide [GIP], glucagon, high molecular weight adiponectin [HMW-Adipo], and high-sensitivity CRP [hs-CRP]); appetite; and satisfaction.
Blood sample processing
Blood glucose levels were measured using a hexokinase assay; IRI, HMW-adiponectin, and CPR levels were measured using a chemiluminescent enzyme immunoassay (SRL Inc., Tokyo, Japan). To measure blood active GLP-1, active GIP, and glucagon levels, blood was collected using P-800 blood collection tubes (Japan Becton, Dickinson and Company, Tokyo, Japan), and the tubes were centrifuged at 1,468 g for 15 min at 4 °C to isolate the plasma. The samples for glucagon measurements were immediately placed in a freezer at −30 °C, and glucagon levels were measured using a sandwich enzyme-linked immunosorbent assay (ELISA) using N- and C-terminal specific antibodies (Mercodia AB, Uppsala, Sweden). Samples for active GLP-1 and active GIP measurements were extracted using the OASIS HLB (Nihon Waters, Tokyo, Japan). Active GLP-1 and active GIP levels were measured using an active GLP-1 ELISA kit (Merck, Darmstadt, Germany) and active GIP ELISA kit (Yanaihara Inc., Shizuoka, Japan), respectively. To measure ghrelin and des-acyl ghrelin levels, blood was collected directly into tubes containing aprotinin; the tubes were immediately centrifuged for plasma isolation at 4 °C. The isolated plasma was treated with one-tenth of its volume of 1 N HCl, and the tubes were rocked gently. These samples were then used to measure plasma ghrelin and des-acyl ghrelin levels with an automated enzyme immunoassay (AIA-600II, Tosoh Corp., Tokyo, Japan) as described elsewhere (Citation24).
SUIT, an index of insulin secretion ability in response to postprandial glucose elevation, was calculated using the following formula: 1500 × fasting CPR/(fasting plasma glucose − 61.7) (Citation25, Citation26). CPI, an index of insulin resistance, was calculated using the following formula: 20/(fasting CPR × fasting plasma glucose) (Citation27). HOMA2-%B, an index of insulin secretion ability, was calculated using a software (HOMA2 Calculator, University of Oxford, UK) (Citation28, Citation29).
Statistical analysis
All results are presented as the mean ± standard deviation. Statistical analyses were performed using appropriate parametric and non-parametric methods. Changes in continuous measures of laboratory test values before and 12 weeks after treatment were analyzed using paired t-tests. Non-parametric methods were used for non-normally distributed values. JMP Pro 15 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses.
Results
Twenty-seven patients participated in this study. No patients were excluded based on the exclusion criteria. Although 27 patients were initially enrolled, one dropped out midway during the study. Finally, 26 patients with data collected both before and after the intervention were included in the analysis. The details are presented in . Many of the patients (88.5%) were obese, and more than half of them had concomitant hypertension or dyslipidemia. Most patients were receiving at least two antidiabetic drugs; many patients used metformin, dipeptidyl peptidase-4 inhibitors, and sodium glucose transporter 2 inhibitors.
Table 1. Baseline characteristics of subjects.
Regarding the primary endpoint, HbA1c levels significantly decreased. ΔHbA1c was not influenced by the type of antidiabetic drugs, according to multiple regression analysis (data not shown). Fasting plasma glucose levels also significantly decreased (). The body weight and blood pressure remained unchanged. No significant changes were observed in fasting insulin levels, CPR concentrations, or indices of insulin resistance, namely HOMA-R and CPI. Regarding the indices of insulin secretion ability, HOMA2-%B significantly increased, while SUIT tended to increase (p = 0.085). No significant changes were observed in LDL-cholesterol or HDL-cholesterol levels, while triglyceride levels tended to decrease (p = 0.060). No significant changes were observed in AST or ALT levels, but γ-GTP levels were significantly decreased. No significant changes were observed in serum creatinine, eGFR, uric acid, urinary albumin, or hs-CRP levels. Leptin levels tended to decline (p = 0.057), while HMW-Adipo levels increased significantly (p = 0.037). Plasma ghrelin concentrations tended to decline (p = 0.08), but plasma des-acyl ghrelin and glucagon concentrations did not change significantly. Active GLP-1 levels tended to increase (p = 0.071), while active GIP levels remained unchanged.
Table 2. Changes in the parameters after 12-week konjac intake.
Regarding konjac intake, there was a strong positive correlation with age (r = 0.61, p = 0.001) (). The intake rate was 87 ± 18% in patients aged ≥50 years (n = 17) and 68 ± 22% in patients aged <50 years (n = 9), showing a significant difference (p = 0.031) (). Body weight and HbA1c significantly decreased in patients aged ≥50 years than in those aged <50 years, and changes in both variables significantly inversely correlated with age (). Between the two age groups, no differences were observed with respect to changes in fasting plasma glucose, serum lipid, AST, γ-GTP, uric acid, hs-CRP, HMW-Adipo, leptin, ghrelin, des-acyl ghrelin, glucagon, GLP-1, or GIP levels. However, changes in ALT tended to be lower in patients aged ≥50 years (–7.7 ± 13.5 vs. 4.1 ± 17.7 U/L, p = 0.06).
Regarding the results of the VAS questionnaire about appetite and satisfaction at week 12, the appetite score was 2.7 ± 4.1 (on a scale of 0 [unchanged] to 10 [markedly decreased]), and appetite remained unchanged in two-thirds of participants. The satisfaction score was high at 7.3 ± 2.6 (on a scale of 0 [dissatisfied] to 10 [satisfied]). There were no significant factors that could explain the high satisfaction levels. Although there was no significant correlation between the actual intake of konjac products and changes in appetite or body weight, patients with higher satisfaction had higher intake (r = 0.59, p = 0.006), and improvement in HbA1c was greater in patients with higher konjac intake (r = −0.52, p = 0.009).
When analyzed by sex, there were no differences in major variables such as body weight or HbA1c, but SUIT (an index of insulin secretion ability) increased significantly more in men than in women (18.9 ± 21.8 vs. −0.1 ± 19.0, p = 0.04). Additionally, uric acid levels were significantly higher in men at baseline (7.0 ± 1.3 vs. 5.0 ± 1.2, p = 0.0006); however, they were significantly lower after konjac intake only in men (−0.7 ± 0.8 vs. 0.2 ± 0.7, p = 0.006).
No adverse events occurred during the study period, and bowel movements improved in some participants.
Discussion
In this study, the active consumption of konjac and konjac products, but not glucomannan alone, as part of dietary therapy for T2DM led to significant improvement in blood glucose control and a significant increase in HMW-Adipo, along with a tendency for improvement in hypertriglyceridemia and insulin secretion ability. In addition, plasma appetite-increasing ghrelin concentrations tended to decrease after konjac intake, while plasma appetite-suppressing active GLP-1 concentrations tended to increase. This clinical study is novel because it used a protocol with konjac and konjac products but not glucomannan. Our results indicated that diet therapy using konjac and konjac products can be considered to be effective as an optional treatment method for type 2 diabetes. Furthermore, the changes of HMW-Adipo and appetite regulating peptides after the intervention were also novel. However, the intake rate was lower among younger participants even though konjac and konjac products were provided free of charge, resulting in no improvement in body weight or blood glucose control. As an extremely low-calorie high-fiber food, konjac is clearly useful for treating obesity and its related conditions, including T2DM. However, as demonstrated in this study, middle-aged and elderly people are more familiar with konjac and are thereby more likely to benefit from it. Therefore, it is important to devise optimal methods for continuous konjac intake based on the characteristics of individual patients.
Hypoadiponectinemia is observed in patients with diabetes, hypertension, and coronary artery disease, and their blood levels are correlated with indices of insulin sensitivity and vascular endothelial dysfunction (Citation30). Adiponectin has also been shown to possess anti-arteriosclerotic and antidiabetic effects. The fact that blood adiponectin levels increased after konjac consumption in this study is considered favorable for the long-term prognosis of diabetes patients. Two studies have investigated changes in adiponectin levels in mice that were administered glucomannan; however, to the best of our knowledge, this study is the first to report the outcomes in humans. Administering glucomannan to obese mice fed a high-fat diet decreased blood adiponectin concentrations, but markedly increased adiponectin levels in adipose tissues (Citation31). In another study, adipose tissues in obese mice fed a high-fat diet clearly showed decreased expression of adiponectin and increased expression of leptin compared with those in normal mice. However, single or concomitant administration of bacterial cellulose or konjac glucomannan significantly increased the expression of adiponectin and significantly decreased the expression of leptin, restoring them to the same levels as those in normal mice (Citation32). Although this study examined only its blood levels, it is desirable to verify the effects of konjac intake on adiponectin in a larger number of patients or for a longer period in the future.
Most clinical studies to date have used purified glucomannan at approximately 4 g/day, not konjac itself. Although glucomannan is a particularly important component, konjac contains a larger amount of water and clearly has a greater volume of food than glucomannan. Eating konjac also requires chewing, which is important to achieve satiety. Once ingested, konjac takes up a certain volume in the gastrointestinal tract. For example, the grain-shaped konjac product used in this study was mixed with rice. Once cooked, it is almost indistinguishable from regular rice. The volume and appearance of each konjac grain were similar to those of the rice grain (Supplementary Figure 2). The taste and texture were nearly identical to those of rice grains cooked alone. In fact, some participants purchased the product after the study to maintain their konjac intake. While glucomannan is easier to consume, eating konjac is considered more beneficial, as described earlier. The decreased fasting ghrelin concentration and increased active GLP-1 concentration observed in this study may have affected the gastrointestinal tract, possibly contributing to the sensation of satiety and favorable blood glucose control.
In a crossover study conducted among young healthy participants who consumed regular pasta only (442 kcal), a combination of half regular pasta and half konjac glucomannan pasta (259 kcal), and konjac glucomannan pasta only (77 kcal) on different days, each followed by the consumption of cookies until they became full, the calories consumed from the cookies were almost the same in all three groups (Citation19). In other words, the total energy intake was lower in pasta containing a higher proportion of glucomannan, but the same level of satiety could be achieved as with regular pasta. One reason for this is that high fiber intake elevates satiety and lowers energy intake (Citation33, Citation34). Furthermore, eating highly viscous food reduces subsequent food intake, which may have played a part (Citation35). After consumption of food derived from konjac in addition to the usual diet for 65 days, fasting blood glucose and glycosylated hemoglobin were significantly reduced in 72 diabetic patients. Body weight was reduced in 80.4% of participants and was reduced by 2.2 kg on average, but there was no description of significance test for this result (Citation20). In our study, patients with a high intake of konjac and konjac products also had high satisfaction and improved HbA1c.
In healthy individuals who consumed three types of konjac glucomannan with different molecular weights followed by a test food in a crossover fashion, the greater the molecular weight of konjac glucomannan consumed, the smaller the postprandial increase in blood glucose and insulin levels, the greater the postprandial increase in GLP-1 levels, and the greater the postprandial decrease in ghrelin levels (Citation36). However, fasting glucose, insulin, GLP-1, and ghrelin levels were comparable to those in the control group. Our study did not investigate postprandial values. However, fasting plasma glucose levels significantly decreased, no difference was noted in insulin or C-peptide levels, active GLP-1 levels tended to increase, and leptin levels tended to decrease. This may be because the participants were patients with T2DM. However, in a double-blind study using glucomannan at 3.6 g/day or placebo for 28 days in 22 participants with T2DM and dyslipidemia, fasting blood glucose levels significantly decreased by 23.2%, and total cholesterol and LDL-cholesterol levels also significantly decreased. However, triglyceride, HDL cholesterol, and postprandial blood glucose levels and body weight remained unchanged (Citation4). Increased plasma GLP-1 concentration is beneficial for glucose metabolism and weight control, and further investigation is needed to include postprandial responses.
This study had several limitations. The first limitation was the small sample size. The second was the relatively short study period of 12 weeks. If konjac/konjac products were used for a longer duration, the effects may have accumulated to further improve blood glucose control. However, the participants may have also become tired of eating konjac/konjac products, causing a decline in their intake. Third, there was no control group. To increase the level of evidence, a two-group comparative study involving a control group should be conducted with a larger sample size.
Conclusions
This study found that active consumption of konjac and konjac products improved blood glucose control and increased blood HMW-adipo levels in Japanese patients with T2DM. All foods used in this study are available in Japan and China, where konjac is commonly consumed. Even in European countries and the United States, konjac pasta and desserts are considered likely to be accepted. In the future, we would like to examine the effects of konjac and konjac products on diabetes and obesity in a larger number of patients across various countries over a longer period.
Authors’ contribution
HU designed research; HU, MA, TS, TN, EE, YU, and TU conducted research; NH, KS, MN, NU, EK, AU, TN, ES, and YY conducted nutrition guidance and checked the intake situation of konjac/konjac products; HU and MN analyzed data; HU and MN drafted and revised the manuscript. All authors read and approved the final manuscript.
20210709-Supple_Fig.pdf
Download PDF (244.7 KB)Acknowledgments
The authors thank Itsuki Morinaga and Sumie Tajiri for measuring GLP-1, GIP, ghrelin, and des-acyl ghrelin.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chua M, Baldwin TC, Hocking TJ, Chan K. Traditional uses and potential health benefits of Amorphophallus konjac K. Koch ex N.E.Br. J Ethnopharmacol. 2010;128(2):268–78. doi:10.1016/j.jep.2010.01.021.
- Englyst HN, Cummings JH. Digestion of the polysaccharides of some cereal foods in the human small intestine. Am J Clin Nutr. 1985;42(5):778–87. doi:10.1093/ajcn/42.5.778.
- Bessell E, Maunder A, Lauche R, Adams J, Sainsbury A, Fuller NR. Efficacy of dietary supplements containing isolated organic compounds for weight loss: a systematic review and meta-analysis of randomised placebo-controlled trials. Int J Obes. 2021;45(8):1631–43. doi:10.1038/s41366-021-00839-w.
- Chen HL, Sheu WH, Tai TS, Liaw YP, Chen YC. Konjac supplement alleviated hypercholesterolemia and hyperglycemia in type 2 diabetic subjects–a randomized double-blind trial. J Am Coll Nutr. 2003;22(1):36–42. doi:10.1080/07315724.2003.10719273.
- Devaraj RD, Reddy CK, Xu B. Health-promoting effects of konjac glucomannan and its practical applications: a critical review. Int J Biol Macromol. 2019;126:273–81. doi:10.1016/j.ijbiomac.2018.12.203.
- Tester RF, Al-Ghazzewi FH. Beneficial health characteristics of native and hydrolysed konjac (Amorphophallus konjac) glucomannan. J Sci Food Agric. 2016;96(10):3283–91. doi:10.1002/jsfa.7571.
- Kaats GR, Bagchi D, Preuss HG. Konjac glucomannan dietary supplementation causes significant fat loss in compliant overweight adults. J Am Coll Nutr. 2015:1–7. doi:10.1080/07315724.2015.1009194.
- Arvill A, Bodin L. Effect of short-term ingestion of konjac glucomannan on serum cholesterol in healthy men. Am J Clin Nutr. 1995;61(3):585–9. doi:10.1093/ajcn/61.3.585.
- Chen HL, Cheng HC, Wu WT, Liu YJ, Liu SY. Supplementation of konjac glucomannan into a low-fiber Chinese diet promoted bowel movement and improved colonic ecology in constipated adults: a placebo-controlled, diet-controlled trial. J Am Coll Nutr. 2008;27(1):102–8. doi:10.1080/07315724.2008.10719681.
- Doi K. Effect of konjac fibre (glucomannan) on glucose and lipids. Eur J Clin Nutr. 1995;49(Suppl 3):S190–S7.
- Ho HVT, Jovanovski E, Zurbau A, Blanco Mejia S, Sievenpiper JL, Au-Yeung F, Jenkins AL, Duvnjak L, Leiter L, Vuksan V. A systematic review and meta-analysis of randomized controlled trials of the effect of konjac glucomannan, a viscous soluble fiber, on LDL cholesterol and the new lipid targets non-HDL cholesterol and apolipoprotein B. Am J Clin Nutr. 2017;105(5):1239–47. doi:10.3945/ajcn.116.142158.
- Vuksan V, Sievenpiper JL, Xu Z, Wong EY, Jenkins AL, Beljan-Zdravkovic U, Leiter LA, Josse RG, Stavro MP. Konjac-Mannan and American ginsing: emerging alternative therapies for type 2 diabetes mellitus. J Am Coll Nutr. 2001;20(5 Suppl):370S–80S. discussion 381S-383S. doi:10.1080/07315724.2001.10719170.
- Sorapukdee S, Jansa S, Tangwatcharin P. Partial replacement of pork backfat with konjac gel in Northeastern Thai fermented sausage (Sai Krok E-san). Asian-Australas J Anim Sci. 2019;32(11):1763–75. doi:10.5713/ajas.18.0811.
- Dai S, Corke H, Shah NP. Utilization of konjac glucomannan as a fat replacer in low-fat and skimmed yogurt. J Dairy Sci. 2016;99(9):7063–74. doi:10.3168/jds.2016-11131.
- Dai S, Jiang F, Corke H, Shah NP. Physicochemical and textural properties of mozzarella cheese made with konjac glucomannan as a fat replacer. Food Res Int. 2018;107:691–9. doi:10.1016/j.foodres.2018.02.069.
- Onakpoya I, Posadzki P, Ernst E. The efficacy of glucomannan supplementation in overweight and obesity: a systematic review and meta-analysis of randomized clinical trials. J Am Coll Nutr. 2014;33(1):70–8. doi:10.1080/07315724.2014.870013.
- Vuksan V, Sievenpiper JL, Owen R, Swilley JA, Spadafora P, Jenkins DJ, Vidgen E, Brighenti F, Josse RG, Leiter LA, et al. Beneficial effects of viscous dietary fiber from Konjac-mannan in subjects with the insulin resistance syndrome: results of a controlled metabolic trial. Diabetes Care. 2000;23(1):9–14. doi:10.2337/diacare.23.1.9.
- Keithley JK, Swanson B, Mikolaitis SL, DeMeo M, Zeller JM, Fogg L, Adamji J. Safety and efficacy of glucomannan for weight loss in overweight and moderately obese adults. J Obes. 2013;2013:610908. doi:10.1155/2013/610908.
- Au-Yeung F, Jovanovski E, Jenkins AL, Zurbau A, Ho HVT, Vuksan V. The effects of gelled konjac glucomannan fibre on appetite and energy intake in healthy individuals: a randomised cross-over trial. Br J Nutr. 2018;119(1):109–16. doi:10.1017/S0007114517003233.
- Huang CY, Zhang MY, Peng SS, Hong JR, Wang X, Jiang HJ, Zhang FL, Bai YX, Liang JZ, Yu YR, et al. Effect of Konjac food on blood glucose level in patients with diabetes. Biomed Environ Sci. 1990;3(2):123–31.
- Zhang MY, Huang CY, Wang X, Hong JR, Peng SS. The effect of foods containing refined Konjac meal on human lipid metabolism. Biomed Environ Sci. 1990;3(1):99–105.
- Ichihara K, Shima K, Nonaka K, Tarui S. Dietary therapy and insulin secretory response to glucose in adult-onset non-obese diabetic subjects. Endocrinol Jpn. 1975;22(5):399–408. doi:10.1507/endocrj1954.22.399.
- Brown A, Dornhorst A, McGowan B, et al. Low-energy total diet replacement intervention in patients with type 2 diabetes mellitus and obesity treated with insulin: a randomized trial. BMJ Open Diabetes Res Care. 2020;8:e001012. doi:10.1136/bmjdrc-2019-001012.
- Tsuchimochi W, Ueno H, Yamashita E, Tsubouchi C, Sakoda H, Nakamura S, Nakazato M. Teneligliptin improves glycemic control with the reduction of postprandial insulin requirement in Japanese diabetic patients. Endocr J. 2015;62(1):13–20. doi:10.1507/endocrj.EJ14-0393.
- Iwata M, Matsushita Y, Fukuda K, Wakura T, Okabe K, Koshimizu Y, Fukushima Y, Kobashi C, Yamazaki Y, Honoki H, et al. Secretory units of islets in transplantation index is a useful predictor of insulin requirement in Japanese type 2 diabetic patients. J Diabetes Invest. 2014;5(5):570–80. doi:10.1111/jdi.12181.
- Yamada Y, Fukuda K, Fujimoto S, Hosokawa M, Tsukiyama K, Nagashima K, Fukushima M, Suzuki H, Toyoda K, Sassa M, et al. SUIT, secretory units of islets in transplantation: An index for therapeutic management of islet transplanted patients and its application to type 2 diabetes. Diabetes Res Clin Pract. 2006;74(3):222–6. doi:10.1016/j.diabres.2006.03.030.
- Okura T, Nakamura R, Fujioka Y, Kawamoto-Kitao S, Ito Y, Matsumoto K, Shoji K, Sumi K, Matsuzawa K, Izawa S, et al. CPR-IR is an insulin resistance index that is minimally affected by hepatic insulin clearance-A preliminary research. PLoS One. 2018;13(5):e0197663. doi:10.1371/journal.pone.0197663.
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. doi:10.2337/diacare.27.6.1487.
- https://www.dtu.ox.ac.uk/homacalculator/.
- Maeda N, Funahashi T, Matsuzawa Y, Shimomura I. Adiponectin, a unique adipocyte-derived factor beyond hormones. Atherosclerosis. 2020;292:1–9. doi:10.1016/j.atherosclerosis.2019.10.021.
- Vazquez-Velasco M, Gonzalez-Torres L, Mendez MT, et al. Glucomannan and glucomannan plus spirulina-enriched squid-surimi added to high saturated diet affect glycemia, plasma and adipose leptin and adiponectin levels in growing Fa/Fa rats. Nutr Hosp. 2015;32:2718–24.
- Zhai X, Lin D, Zhao Y, Li W, Yang X. Enhanced anti-obesity effects of bacterial cellulose combined with konjac glucomannan in high-fat diet-fed C57BL/6J mice. Food Funct. 2018;9(10):5260–72. doi:10.1039/c8fo01211c.
- Wanders AJ, van den Borne JJGC, de Graaf C, Hulshof T, Jonathan MC, Kristensen M, Mars M, Schols HA, Feskens EJM. Effects of dietary fibre on subjective appetite, energy intake and body weight: a systematic review of randomized controlled trials. Obes Rev. 2011;12(9):724–39.
- Clark MJ, Slavin JL. The effect of fiber on satiety and food intake: a systematic review. J Am Coll Nutr. 2013;32(3):200–11. doi:10.1080/07315724.2013.791194.
- Vuksan V, Panahi S, Lyon M, Rogovik AL, Jenkins AL, Leiter LA. Viscosity of fiber preloads affects food intake in adolescents. Nutr Metab Cardiovasc Dis. 2009;19(7):498–503. doi:10.1016/j.numecd.2008.09.006.
- Shang L, Wang Y, Ren Y, Ai T, Zhou P, Hu L, Wang L, Li J, Li B. In vitro gastric emptying characteristics of konjac glucomannan with different viscosity and its effects on appetite regulation. Food Funct. 2020;11(9):7596–610. doi:10.1039/d0fo01104e.