Abstract
Background:
Lifestyle changes that emphasis on plant-based diets (PBD) are typically recommended for those at risk for type 2 diabetes mellitus (T2DM) to mitigate their cardo-metabolic risk. We examined the impact of the inclusion of eggs compared with their exclusion from PBD on diet quality among adults at risk for T2DM.
Methods:
This was a randomized, controlled, single-blind, crossover trial of 35 adults (mean age 60.7 years; 25 women, 10 men; 34 Caucasians, 1 African-American) at risk for T2DM (i.e., pre- diabetes or metabolic syndrome) assigned to one of two possible sequence permutations of two treatments (PBD with eggs and exclusively PBD), with a 4-week washout period. Participants received dietary counseling from a dietitian to exclude or to include 2 eggs daily in the context of PBD for a 6-week period. Diet quality was assessed using the Healthy Eating Index 2015 (HEI-2015) at baseline and 6 weeks.
Results:
Compared with the exclusion of eggs, the inclusion of eggs in the context of PBD improved the diet quality score for intake of total protein foods (1.0 ± 1.1 vs. −0.4 ± 1.0; p <.0001); seafood and plant proteins (0.2 ± 1.2 vs. −0.4 ± 1.1; p = 0.0338); and fatty acids (0.8 ± 2.5 vs. −0.7 ± 2.7; p = 0.0260). Overall diet quality score depreciated with the adoption of exclusively PBD without eggs (−3.1 ± 8.3; p = 0.0411), while it was unaffected with the adoption of a PBD with the inclusion of eggs (−0.6 ± 7.9; p = 0.6892).
Conclusions:
Eggs could be used as an adjuvant to enhance the diet quality among those at risk for T2DM who adopt plant-based dietary patterns.
Introduction
Pre-diabetes is a serious medical condition associated with elevated blood glucose that is higher than normal, but not high enough to be considered for a diagnosis of diabetes (Citation1). An estimated 88 million adults aged 18 years and older have pre-diabetes in the United States (U.S.), yet more than 84% of them are unaware (Citation1). Those with pre-diabetes are at increased risk for developing diabetes, cardiovascular disease (CVD), and stroke (Citation1, Citation2). Fifteen to 30% of individuals with pre-diabetes are likely to develop type 2 diabetes mellitus (T2DM) within 5 years (Citation3). Pre-diabetes is a major risk factor associated with metabolic syndrome. Insulin resistance and excess body weight are common in both pre-diabetes and metabolic syndrome (Citation4).
Metabolic syndrome—a cluster of risk factors that increase the risk of T2DM and CVD—affects about 35% of adults in the U.S (Citation5, Citation6). The risk factors for metabolic syndrome include hypertension, dyslipidemia, hyperglycemia, and excess body weight (especially due to excess central body fat) (Citation7). These risk factors represent an independent risk for developing T2DM, CVD, and stroke, as well as an increased risk of mortality (Citation2, Citation8). The risk of T2DM, CVD, and stroke increases with the number of metabolic risk factors (Citation2, Citation5, Citation9). Persons with metabolic syndrome, when compared with healthy persons, have a 5-fold increased risk for T2DM (Citation2). The combination of pre-diabetes and metabolic syndrome compared with healthy persons is associated with an even higher (i.e., 21-fold) risk for T2DM (Citation2).
Lifestyle changes could help control metabolic risk factors and reduce the risk for CVD and T2DM related complications among those at risk for T2DM (Citation10, Citation11). Dietary patterns that place emphasis on foods such as fruits, vegetables, whole grains, fat-free or low-fat dairy, protein-rich foods, oils and foods rich in mono- and polyunsaturated fatty acids while limiting sodium (salt), alcohol, added sugar, saturated and trans fatty acids are typically recommended for those at risk for T2DM to prevent the development of cardio-metabolic complications (Citation12). While plant-based diets (PBD) are recommended to improve cardio-metabolic risk factors in those at risk for T2DM (Citation13), nevertheless, without adequate planning, it can be difficult for those adopting PBD that exclude animal products to consume optimal amounts of protein and other nutrients (Citation14). Eggs are a nutrient-dense food with the highest quality of protein. Additionally, eggs are satiating, and therefore have the potential to regulate calorie intake and reduce body weight (Citation15). Therefore, the addition of eggs to PBD has the potential to improve diet quality by increasing intake of a variety of nutrients and ensuring the appropriate balance of healthful foods. In our previous report, the inclusion of eggs in PBD did not adversely affect cardio-metabolic health among persons at risk for T2DM, but improved their intake of some nutrients known to be depleted in exclusively PBD (Citation16). In this report we examined the impact of the inclusion of eggs in the context of a PBD on the dietary pattern among those at risk for T2DM. Specifically, we hypothesized that consumption of 2 eggs daily in the context of PBD for a 6-week period, compared with exclusively PBD, would improve the diet quality among those at risk for T2DM.
Methods
Study design
This was a randomized, single-blind, controlled, crossover trial designed with 2 treatment assignments (PBD plus eggs, and PBD with no eggs) to assess the effects over a 6-week period of each treatment assignment on diet quality among individuals at risk for T2DM. After a 4-week run-period of an ad libitum PBD, participants were randomized to 1 of 2 possible sequence permutations and then underwent repeated dietary assessments following inclusion of 2 eggs per day in their otherwise ad libitum PBD, or ad libitum PBD without eggs over a 6-week period, with a 4-week washout period between treatment assignments. The participants maintained their plant-based dietary patterns during the 4-week washout period. This study was conducted in a community hospital in the Lower Naugatuck Valley of Connecticut, USA. The study was approved by the Griffin Hospital Institutional Review Board (IRB) and also registered on the clinicaltrials.gov website (NCT04316429) before initiating recruitment and screening.
Recruitment and screening
Participants were recruited from the Lower Naugatuck Valley of Connecticut through a variety of methods such as flyers, social media, and newspaper advertisements. Potential participants who responded were prescreened by the study coordinator for eligibility through a structured telephone interview using established inclusion and exclusion criteria as stipulated in the study protocol. Those who met preliminary eligibility criteria and agreed to participate were invited to undergo clinical eligibility screening, and were asked to read and sign a written consent form that was approved by the IRB. All participants were informed of the option of discontinuing participation at any time during the study.
The clinical screening consisted of a physical examination that included assessment of body weight, height, waist circumference and blood pressure measures obtained by experienced study personnel with calibrated equipment. Blood samples were collected from the potential participants to assess their serum fasting lipids profile (total, HDL, LDL cholesterol, and triglycerides), glycated hemoglobin and fasting plasma glucose. The screening laboratory assays were performed at the Griffin Hospital laboratory. Findings of the physical examination together with blood test were used to determine whether or not a potential candidate met criteria for pre-diabetes or metabolic syndrome.
Participants
We enrolled 35 participants who were at risk for T2DM, and who met the eligibility criteria. The inclusion criteria included: male or postmenopausal female age 25–75 years; nonsmoker; and at risk for T2DM as defined by fasting blood glucose >100mg/dL and <126mg/dL or glycated hemoglobin 5.7–6.4% or meeting the metabolic syndrome criteria as defined by the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) (Citation7).
Exclusion criteria included: allergy to eggs; anticipated inability to complete the study protocol for any reason; current eating disorder; unstable use of lipid-lowering or antihypertensive medications (i.e., change in dose during the 3 months prior to enrollment); regular use of high doses of vitamin E (>400IU/day) or vitamin C (>500mg/day); fish oil, flaxseed oil, omega-3 fatty acid and/or fiber supplement, unless willing to discontinue supplementation for the study duration; use of insulin, glucose-sensitizing medication; unstable use of antidepressant medications (i.e., change in dose during the three months prior to enrollment); diagnosed diabetes; diagnosed sleep apnea; established CVD (including symptomatic coronary artery disease, myocardial infarction, peripheral vascular disease, congestive heart failure, carotid stenosis); coagulopathy, known bleeding diathesis, or history of clinically significant hemorrhage; current use of warfarin; substance abuse (chronic alcoholism, other chemical dependency); any unstable medical condition (e.g., cancer, AIDS, tuberculosis, psychotic disorder) that would limit the ability of a subject to participate fully in the study; and the use of hormone replacement therapy.
Randomization and blinding
The study participants were randomized to 1 of 2 sequence permutations (PBD with eggs and PBD with no eggs) using simple randomization technique by the Principal Investigator (PI) with SAS software for Windows version 9.4 (SAS Institute, Cary, NC). Each sequence permutation consisted of a 6-week treatment period, followed by a 4-week washout period, followed by an alternative 6-week treatment period. The study coordinator enrolled the participants and assigned them to 1 of the 2 sequence permutations generated with SAS by the PI. The PI and study personnel assessing the clinical outcome measures were blinded to the treatment assignments during the course of the study. Participants were categorized as receiving either sequence A or B. Only the study coordinator knew the sequence allocations that each study participant received. Treatment assignment of the study participants were unveiled by the study coordinator after the statistical analyses have been completed.
Procedures
Inclusion of eggs in the context of PBD
The study’s registered dietitian met with the participants and provided counseling advice to adopt PBD centered on the U.S. Department of Agriculture healthy vegetarian meal plan, with modifications to exclude dairy products from animal sources (Citation17). Supplemental resources (e.g., educational materials, recipes, etc.) for adopting and maintaining a PBD were also provided. The dietitian provided instructions to the participants during the counseling to include 2 eggs per day for 6 weeks as part of their otherwise PBD while instructing them on foods to displace from their diets to preserve an isocaloric condition relative to an exclusively PBD. Dietary intake of the participants was assessed at baseline and subsequently at 6 weeks.
PBD without eggs
The dietitian provided counseling and sample meal plans to the participants to adopt exclusively PBD as described above in the egg inclusion phase. The dietitian counseled them to exclude any products such as mayonnaise or baked goods that contained eggs as a known ingredient, and provided a handout with suggestions for various egg substitutes that they could use while cooking and baking. The participants consumed an exclusively PBD for 6 weeks, and their dietary intake was evaluated at baseline and 6 weeks.
Compliance
Participants’ reported dietary intake and their egg consumption logs were used to assess their compliance to the study interventions. Good compliance for adopting PBD was defined as the absence of meat and animal source dairy products from 3 consecutive 24-hour food recalls within each of the 2 dietary phases of the study, the absence of eggs within the egg exclusion phase of the study, and the consumption of at least 80% of the total number of eggs recommended for the 6-week period while in the egg inclusion phase.
Outcome measures
The primary outcome measure of the study was endothelial function measured as flow-mediated dilatation. The primary outcome measure and some of the secondary outcome measures (including lipid panel, blood pressure, glycemic control, anthropometry, and nutrient intake) have been reported in our previous report (Citation16). Diet quality as assessed by the Healthy Eating Index (HEI) 2015, which we are reporting in this report, was also one of the secondary outcome measures.
Diet quality
We tracked variation in dietary patterns over the course of the study by asking study participants to provide information on the foods and beverages that they consumed during a 3-day period (i.e., 2 weekdays and 1 weekend day) at each of the 4 assessment visits (i.e., 2 baseline visits for the 2 phases and two 6-week visits for the 2 dietary treatment phases). For each 3-day period, participants completed 3 consecutive 24-hour recalls using a web-based Automated Self-Administered 24-Hour Recall (ASA24) (http://riskfactor.cancer.gov/tools/instruments/asa24/), which guided them through the process of completing the recall data. ASA24 utilized information of the food and beverage intake reported to generate information regarding intake of nutrients and food groups on its output file. The averages of the 3 food records were computed and were used as estimates of dietary intake at each time point.
The participants’ food groups and nutrients generated from the ASA24 output files were used to populate the HEI-2015 algorithm to compute scores of the various components of HEI-2015 and overall diet quality. HEI-2015 measures diet quality, independent of quantity, which is used to assess compliance with the 2015–2020 U.S. Dietary Guidelines for Americans and monitor changes in dietary patterns. HEI is a scoring metric that could be used to determine the quality of a given dietary pattern, set of foods, or menu. HEI-2015 gives emphasis to a variety of food groups, nutrient density, and improvement in food and beverage choices within calorie needs. The HEI-2015 consists of 13 components that sum up to a total maximum score of 100 points. Higher HEI score indicates better diet quality. All of the key 2015–2020 Dietary Guidelines food choice recommendations that relate to diet quality are reflected in the HEI-2015s 13 components. Nine of the components [i.e., total fruits (i.e., includes 100% fruit juice); whole fruits (i.e., includes all forms except juice); total vegetables (i.e., includes legumes (beans and peas); greens and beans (i.e., includes legumes (beans and peas); whole grains; dairy (i.e., includes all milk products, such as fluid milk, yogurt, and cheese, and fortified soy beverages); total proteins foods (i.e., includes legumes (beans and peas); sea foods and plant proteins ((i.e., includes seafood, nuts, seeds, soy products (other than beverages), and legumes (beans and peas)); and fatty acids (ratio of poly- and monounsaturated fatty acids (PUFAs and MUFAs) to saturated fatty acids (SFAs) focus on adequacy (i.e., dietary components to increase), while the remaining 4 components (i.e., refined grains; sodium; added sugars; and saturated fats) focus on moderation (i.e., dietary components to decrease). The individual components are scored on a density basis out of 1,000 calories, with the exception of fatty acids which is a ratio of unsaturated to saturated fatty acids. Reported intakes between minimum and maximum standards for each component of the HEI-2015 are scored proportionately. The performance of the HEI-2015 has been evaluated through an assessment of its construct validity, reliability and criterion validity (Citation18). Higher HEI-2015 scores have been associated with lower risk of mortality of all-cause, CVD and cancer (Citation18). We analyzed the 13 components of the HEI-2015 of our participants’ self-reported dietary intake to assess the diet quality of PBD plus eggs compared with exclusively PBD to test our study hypothesis.
Assessment of safety and adverse events
Participants reported any adverse events experienced during the study to the study coordinator. Events reported to the study coordinator were all presented to the PI, who informed the IRB.
Statistical analysis
Generalized linear models were used to compare the HEI-2015 components pre-post scores between the consumption of PBD plus eggs versus an exclusively PBD. Paired student t-tests were used to assess difference from baseline to endpoints for each treatment assignment. Regression models were used to control for covariates (i.e., age, gender, race compliance, and treatment sequence). Descriptive and exploratory analyses to test for normality of our data of all measured outcomes were carried out before embarking on modeling or hypothesis testing procedures. As indicated, logarithm transformation of data analytic techniques were employed. All analyses at endpoints were based on intention-to-treat principle. SAS software for Windows version 9.4 (SAS Institute, Cary, NC) was used to carry out all statistical analyses. P-values of < 0.05 were considered statistically significant. Data are presented as mean ± standard deviation except otherwise stated. The sample size estimations were based on the study’s primary outcome measure, endothelial function measured as flow-mediated vasodilatation. The sample size estimate allowed for 20% attrition and noncompliance and provided at least 80% power to detect a minimal difference of 2.5% in endothelial function between egg-included PBD and egg-excluded PBD, with maximum allowable type I error of 5% (Citation16).
Results
Participants recruited and included in the analysis
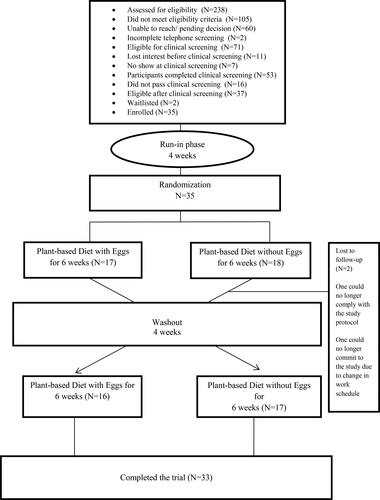
The study participants were recruited from February 24th, 2020 through July 31st, 2020. A total of 238 potential participants responded to our advertisements and were assessed through telephone screening for eligibility into the study. Of those screened by telephone, 105 did not meet the study inclusion criteria; 60 were unable to be reached while awaiting decision of their eligibility for clinical screening; 2 did not complete the screening; and 71 were considered eligible for clinical screening, of whom 7 did not show up and 11 lost interest before clinical screening. Fifty three potential participants were screened, with 37 meeting the study inclusion/exclusion criteria. Thirty-five participants (10 men and 25 women) were enrolled into the study and were subsequently assigned to 1 of 2 possible sequence permutations (PBD with eggs and PBD with no eggs). Two participants dropped out of the study while in the egg-excluded phase: one was unable to maintain adherence to PBD, and the other was unable to continue participation in the study due to modification in work schedule that made it impossible to come in for assessments. The study flow diagram of the participants is presented in .
All participants were Caucasians, except one who was African-American. The study participants were predominantly female. The mean age of the study participants was 60.7 years. Their average overall diet quality score was 73.4 ± 8.9 at baseline. Demographic characteristics and the diet quality scores at baseline of the study participants are presented in .
Table 1. Demographic characteristics and diet quality scores at baseline in adults at risk for T2DM.
Efficacy endpoint
Compared with exclusively PBD, PBD with eggs improved the diet quality scores for the intake of total protein foods (1.0 ± 1.1 vs. −0.4 ± 1.0; p <.0001); seafood and plant proteins (0.2 ± 1.2 vs. −0.4 ± 1.1; p = 0.0338); and fatty acids (0.8 ± 2.5 vs. −0.7 ± 2.7; p = 0.0260). The diet quality score for dairy intake decreased with the inclusion of eggs in PBD compared with exclusively PBD (−1.0 ± 2.6 vs. 0.8 ± 1.6; p = 0.0017). Adopting a plant-based dietary pattern with the inclusion of eggs, compared with exclusively PBD, did not significantly (p = 0.1759 to 0.9238) affect the diet quality scores of total vegetables; greens and beans; total fruit; whole fruit; whole grains; sodium; refined grains; added sugars; and saturated fats. While the overall diet quality score was not affected by the inclusion of eggs when adopting a plant-based dietary pattern (−0.6 ± 7.9; p = 0.6892), the exclusion of eggs from PBD led to a depreciation (−3.1 ± 8.3; p = 0.0411) in the score (see ).
Table 2. Change in diet quality scores from baseline to 6 weeks in adults at risk for T2DM with the inclusion of eggs in PBD compared with their exclusion.
Adverse events
A study participant reported of an allergic type reaction (red eyes) about 2 to 3 weeks after including eggs in her otherwise PBD. Another reported feeling nauseated and having an upset stomach while consuming eggs in the context of a PBD.
Discussion
Our data suggest that the consumption of eggs in the context of ad libitum PBD, compared with ad libitum exclusively PBD, improved the diet quality scores for the intake of total protein foods; seafood and plant proteins; and fatty acids. Adopting PBD with the inclusion of eggs compared with exclusively PBD did not affect the diet quality scores for total vegetables; greens and beans; total fruit; whole fruit; whole grains; sodium; refined grains; added sugars; and saturated fats. The overall diet quality score depreciated while the participants were adopting exclusively PBD, but not while including eggs in their otherwise PBD. These findings support our study hypothesis that the consumption of 2 eggs daily in the context of PBD for a 6-week period, compared with exclusively PBD, would improve the diet quality among those at risk for T2DM. Although the inclusion of eggs in the context of PBD compared with their exclusion significantly increased reported intake of dietary cholesterol, it still led to improved diet quality and did not adversely affect endothelial function and lipid panel in these participants at risk for T2DM (Citation16).
Similar to our previous study (Citation19), the inclusion of eggs in habitual diets led to an improvement in the intake of the quality of total protein foods. The consumption of high-quality protein foods such as plant-based proteins has been associated with the prevention of the onset of T2DM in patients with coronary artery disease (Citation20–22). Conversely, the consumption of lower quality protein foods such as highly processed meats and red meat has been associated with higher risk of the development of T2DM (Citation22–25). The improvement of the intake of the quality of total protein foods with the inclusion of eggs in PBD in this study could be attributed directly to the eggs. In addition to being rich in vitamins and minerals, eggs contain the highest quality protein. While some observational studies (Citation26, Citation27) have identified associations between egg consumption and greater risk of T2DM, experimental trials (Citation28, Citation29) in contrast have shown that eggs may improve insulin sensitivity and some cardio-metabolic risk factors in individuals with T2DM.
Likewise, we observed improvement of the quality of the intake of seafood and plant protein foods with the inclusion of eggs in PBD compared with exclusively PBD. In a previous study by Patel et al. (Citation30), the consumption of one or more portions compared with less than one portion of overall, white or oily fish per week was associated with reduced risk of developing T2DM. In another study (Citation31), while lean fish, shellfish and overall fish intake were not associated with the incidence of T2DM, fatty fish intake was associated with the prevention of T2DM. In addition, Nkondjock and Receveur (Citation32) demonstrated that higher fish and seafood intake were associated with reduced risk of T2DM in a population with a high prevalence of obesity. Similarly, Nanri et al. (Citation33) demonstrated that in a population with higher fish and seafood consumption, fish intake was associated with lower risk of T2DM in men but not in women. Furthermore, Villegas et al. (Citation34) demonstrated an inverse association between fish and shellfish intake and T2DM in women. Conversely, a meta-analysis by Zhou et al. (Citation35) showed a weak association between T2DM and the intake of both fish and omega-3 fatty acids. In the Women’s Health Study by Djoussé et al. (Citation36), the intake of marine long-chain omega-3 fatty acids was associated with the incidence of T2DM, especially among those consuming more than 2 servings of fish per day.
Also in our study, we observed an improvement in fat quality intake with the inclusion of eggs in otherwise PBD compared with exclusively PBD. A study by Harding et al. (Citation37) showed that increasing the dietary ratio of PUFAs to SFAs was associated with lower risk of T2DM. In addition, Vessby et al. (Citation38) demonstrated that decreasing dietary SFAs and increasing MUFAs improved insulin sensitivity among healthy participants. Furthermore, in a meta-analysis by Imamura et al. (Citation39), substituting carbohydrates and SFAs with PUFAs in the diets was associated with improvement in glycemic control, insulin sensitivity and insulin secretion capacity. Additionally, in another meta-analysis, Qian et al. (Citation40) demonstrated that consuming MUFA-enriched diets was associated with improved metabolic risk factors in patients with T2DM. However, a meta-analysis by Brown et al. (Citation41) suggests that increased omega-3, omega-6 and PUFA intake has little or no effects on the prevention and management of T2DM.
Interestingly, we observed a reduction in the diet quality of dairy intake with the inclusion of eggs in the PBD compared with their exclusion. Dietary patterns with increased dairy intake have been associated with reduced risk of developing T2DM (Citation42). Choi et al. (Citation43) also demonstrated that dietary patterns characterized by high dairy intake, especially low-fat dairy, are inversely associated with the development of T2DM in men. Additionally, Fumeron et al. (Citation44) also demonstrated that the consumption of dairy products reduced risk factors for T2DM. Again, Diaz-Lopez et al. (Citation45) demonstrated that higher consumption of dairy products, especially yogurt, among elderly Spanish population with high cardiovascular risk adopting healthy dietary pattern was inversely associated with the development of T2DM. Furthermore, Babio et al. (Citation46) demonstrated that low-fat dairy products, yogurt (independent of the level of fat content) and low-fat milk were associated inversely with the metabolic syndrome among persons at higher risk for cardiovascular disease. Our participants must have displaced some dairy products from their diets while including eggs in their diets and also improving the intake of other healthful food choices.
We did not observe any meaningful improvement in intake of total vegetables; greens and beans; total fruit; whole fruit; and whole grains intake or reduction in sodium; refined grains; added sugars and saturated fat intake with the inclusion of eggs in the context of PBD compared with their exclusion. Improvements in the intake of these parameters have been associated with glycemic control and therefore a reduced risk of developing T2DM (Citation23, Citation31, Citation47–58). We did not observe any meaningful improvements in our own study, likely due to the small sample size.
Several limitations have to be considered as we interpret these data. First, the sample size was small, with just 35 participants enrolled, which may have precluded us from seeing statistically significant differences. This could be also considered as a strength of our study because despite the small sample size, we were still able to detect some statistically significant findings. The study’s crossover design reduced our data variation and therefore improved the study’s statistical power. Second, our study participants were predominantly Caucasians and female; therefore, this limits our ability to extrapolate our findings to other racial and ethnic groups. Third, this study relied on self-report by the participants regarding their dietary intake, which can introduce measurement and recall biases by under- or over-estimating intake. However, the dietary data of the study participants were captured using a reliable, validated dietary tool (i.e., ASA-24) that provided guidance to the participants in providing estimates of their portion sizes, which reduced the likelihood of under- or over-estimating their dietary intake. In addition, the study dietitian provided participants with guidance on how to enter their dietary data and later reviewed these data before statistical analyses. Fourth, the study participants were not monitored on a daily basis and were not administered a supervised diet. Therefore, the veracity of the dietary intake data depended on the honesty of the participants. However, this could also be viewed as a strength of the study because it provided a more realistic scenario and potentially improved external validity. Fifth, another source of limitation may stem from the inherent day-to-day variability of the participants’ diets. Nonetheless, by averaging the reported 3-day (i.e., 2 weekdays and 1 weekend day) foods and beverages consumed by each participant to some extend adjusted for the day-to-day variation to arrive at scores that are closer to the participants’ usual intake.
Conclusion
Our data suggest that the inclusion of eggs in otherwise PBD compared with exclusively PBD improved the intake of the quality of total protein foods; seafood and plant proteins; and fatty acids. In addition, overall diet quality deteriorated among our participants while adopting exclusively PBD. The inclusion of eggs while adopting plant-based dietary patterns did not affect the intake of total vegetables; greens and beans; total fruit; whole fruit; whole grains; sodium; refined grains; added sugars; and saturated fats. Therefore, including eggs in PBD enhanced diet quality without adversely affecting endothelial function and lipid panel. While PBD can play an important role in preventing T2DM development, precautions should be taken when adopting PBD to ensure consumption of an appropriate balance of healthful foods to enhance diet quality. Eggs are nutrient-dense, have the highest quality protein, and are rich in healthful fatty acids; and they could be considered as an adjuvant to improve diet quality in those at risk for T2DM adopting plant-based dietary pattern. A larger study is warranted to elucidate these findings and to inform the USDA Dietary Guidelines Committee to consider reclassifying eggs, especially among those at risk for T2DM who plan to adopt plant-based dietary patterns.
Funding sources
This study was conducted with funding from the Centers for Disease Control and Prevention and the American Egg Board/Egg Nutrition Center.
Author contributions
VYN: Conceptualization; Funding acquisition; Investigation; Methodology; Data curation; Supervision; Formal data analysis; Writing—original draft; Writing—review & editing. GCMK: Data interpretation, Writing—review & editing. JAT: Investigation; Validation; Visualization; Writing—review & editing. RGA: Project administration; Data curation; Resources. FMK: Data interpretation, Writing—review & editing. NMK: Data interpretation, Writing—review & editing. BC: Funding acquisition; Investigation; Administration. OA: Formal data analysis; Writing—original draft; Writing—review & editing. All authors reviewed and commented on subsequent drafts of the manuscript
Acknowledgments
The authors wish to acknowledge the American Egg Board/Egg Nutrition Center for providing funding for this study. We have received permission from those named in the acknowledgement.
Disclosure statement
The authors have nothing to disclose.
References
- Centers for Disease Control and Prevention. Prediabetes—your chance to prevent type 2 diabetes. 2020. [cited 2021 Nov 11]. https://www.cdc.gov/diabetes/basics/prediabetes.html.
- Lorenzo C, Williams K, Hunt KJ, Haffner SM. The National Cholesterol Education Program—Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30(1):8–13. doi:10.2337/dc06-1414.
- Centers for Disease Control and Prevention. National Diabetes Statistics Report 2020. Estimates of diabetes and its burden in the United States [cited 2021 Nov 11]. https://www.cdc.gov/diabetes/data/statistics-report/index.html.
- Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59(7):635–43. doi:10.1016/j.jacc.2011.08.080.
- Saely CH, Rein P, Drexel H. The metabolic syndrome and risk of cardiovascular disease and diabetes: experiences with the new diagnostic criteria from the International Diabetes Federation. Horm Metab Res. 2007;39(9):642–50. doi:10.1055/s-2007-985822.
- Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA. 2020;323(24):2526–8. doi:10.1001/jama.2020.4501.
- Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231–7. doi:10.1242/dmm.001180.
- Wild SH, Smith FB, Lee AJ, Fowkes FG. Criteria for previously undiagnosed diabetes and risk of mortality: 15-year follow-up of the Edinburgh Artery Study cohort. Diabet Med. 2005;22(4):490–6. doi:10.1111/j.1464-5491.2004.01433.x.
- Alshammary AF, Alharbi KK, Alshehri NJ, Vennu V, Ali Khan I. Metabolic syndrome and coronary artery disease risk: a meta-analysis of observational studies. Int J Environ Res Public Health. 2021;18(4):1773. doi:10.3390/ijerph18041773.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi:10.1056/NEJMoa012512.
- Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866–75. doi:10.1016/S2213-8587(15)00291-0.
- American Diabetes Association. What is the American Diabetes Association (ADA) diet? 2019 [cited 2021 Nov 11]. https://healthjade.net/what-is-the-american-diabetes-association-ada-diet/.
- Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB, et al. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13(6):e1002039. doi:10.1371/journal.pmed.1002039.
- Turner-McGrievy G. Nutrient adequacy of vegetarian diets. J Am Diet Assoc. 2010;110(10):1450–3. doi:10.1016/j.jada.2010.08.015.
- Vander Wal JS, Marth JM, Khosla P, Jen KL, Dhurandhar NV. Short-term effect of eggs on satiety in overweight and obese subjects. J Am Coll Nutr. 2005;24(6):510–5. doi:10.1080/07315724.2005.10719497.
- Njike VY, Treu JA, Kela GCM, Ayettey RG, Comerford BP, Siddiqui WT. Egg consumption in the context of plant-based diets and cardiometabolic risk factors in adults at risk of type 2 diabetes. J Nutr. 2021;nxab283. doi:10.1093/jn/nxab283.
- U.S. Department of Agriculture. 2015-2020 dietary guidelines. 2020 [cited 2021 Nov 11]. https://health.gov/our-work/food-nutrition/previous-dietary-guidelines/2015.
- Reedy J, Lerman JL, Krebs-Smith SM, Kirkpatrick SI, Pannucci TE, Wilson MM, Subar AF, Kahle LL, Tooze JA. Evaluation of the Healthy Eating Index-2015 [published correction appears in J Acad Nutr Diet. 2019 Aug 20]. J Acad Nutr Diet. 2018;118(9):1622–33. doi:10.1016/j.jand.2018.05.019.
- Njike VY, Annam R, Costales VC, Yarandi N, Katz DL. Which foods are displaced in the diets of adults with type 2 diabetes with the inclusion of eggs in their diets? A randomized, controlled, crossover trial. BMJ Open Diabetes Res Care. 2017;5(1):e000411. doi:10.1136/bmjdrc-2017-000411.
- Villegas R, Gao Y-T, Yang G, Li H-L, Elasy TA, Zheng W, Shu XO. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Health Study. Am J Clin Nutr. 2008;87(1):162–7. doi:10.1093/ajcn/87.1.162.
- de la Cruz-Ares S, Gutiérrez-Mariscal FM, Alcalá-Díaz JF, Quintana-Navarro GM, Podadera-Herreros A, Cardelo MP, Torres-Peña JD, Arenas-de Larriva AP, Pérez-Martínez P, Delgado-Lista J, et al. Quality and quantity of protein intake influence incidence of type 2 diabetes mellitus in coronary heart disease patients: from the CORDIOPREV study. Nutrients. 2021;13(4):1217. doi:10.3390/nu13041217.
- Malik VS, Li Y, Tobias DK, Pan A, Hu FB. Dietary protein intake and risk of type 2 diabetes in US men and women. Am J Epidemiol. 2016;183(8):715–28. doi:10.1093/aje/kwv268.
- Kim Y, Keogh JB, Clifton PM. Differential effects of red meat/refined grain diet and dairy/chicken/nuts/whole grain diet on glucose, insulin and triglyceride in a randomized crossover study. Nutrients. 2016;8(11):687. doi:10.3390/nu81106877.
- Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94(4):1088–96. doi:10.3945/ajcn.111.018978.
- Pan A, Sun Q, Bernstein AM, Manson JE, Willett WC, Hu FB. Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus: three cohorts of US men and women. JAMA Intern Med. 2013;173(14):1328–35. doi:10.1001/jamainternmed.2013.6633.
- Wallin A, Forouhi NG, Wolk A, Larsson SC. Egg consumption and risk of type 2 diabetes: a prospective study and dose-response meta-analysis. Diabetologia. 2016;59(6):1204–13. doi:10.1007/s00125-016-3923-6.
- Radzevičienė L, Ostrauskas R. Egg consumption and the risk of type 2 diabetes mellitus: a case-control study. Public Health Nutr. 2012;15(8):1437–41. doi:10.1017/S1368980012000614.
- Njike VY, Ayettey RG, Rajebi H, Treu JA, Katz DL. Egg ingestion in adults with type 2 diabetes: effects on glycemic control, anthropometry, and diet quality—a randomized, controlled, crossover trial. BMJ Open Diabetes Res Care. 2016;4(1):e000281. doi:10.1136/bmjdrc-2016-000281
- Becerra-Tomás N, Díaz-López A, Rosique-Esteban N, et al. Legume consumption is inversely associated with type 2 diabetes incidence in adults: a prospective assessment from the PREDIMED study. Clin Nutr. 2018;37(3):906–13. doi:10.1016/j.clnu.2017.03.015.
- Patel PS, Sharp SJ, Luben RN, et al. Association between type of dietary fish and seafood intake and the risk of incident type 2 diabetes: the European prospective investigation of cancer (EPIC)-Norfolk cohort study. Diabetes Care. 2009;32(10):1857–63. doi:10.2337/dc09-0116.
- Patel PS, Forouhi NG, Kuijsten A, et al. The prospective association between total and type of fish intake and type 2 diabetes in 8 European countries: EPIC-InterAct study. Am J Clin Nutr. 2012;95(6):1445–53. doi:10.3945/ajcn.111.029314.
- Nkondjock A, Receveur O. Fish-seafood consumption, obesity, and risk of type 2 diabetes: an ecological study. Diabetes Metab. 2003;29(6):635–42. doi:10.1016/S1262-3636(07)70080-0.
- Nanri A, Mizoue T, Noda M, et al. Fish intake and type 2 diabetes in Japanese men and women: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr. 2011;94(3):884–91. doi:10.3945/ajcn.111.012252.
- Villegas R, Xiang YB, Elasy T, et al. Fish, shellfish, and long-chain n-3 fatty acid consumption and risk of incident type 2 diabetes in middle-aged Chinese men and women. Am J Clin Nutr. 2011;94(2):543–51. doi:10.3945/ajcn.111.013193.
- Zhou Y, Tian C, Jia C. Association of fish and n-3 fatty acid intake with the risk of type 2 diabetes: a meta-analysis of prospective studies. Br J Nutr. 2012;108(3):408–17. doi:10.1017/S0007114512002036.
- Djoussé L, Gaziano JM, Buring JE, Lee IM. Dietary omega-3 fatty acids and fish consumption and risk of type 2 diabetes. Am J Clin Nutr. 2011;93(1):143–50. doi:10.3945/ajcn.110.005603.
- Harding A-H, Day NE, Khaw K-T, Bingham S, Luben R, Welsh A, Wareham NJ. Dietary fat and the risk of clinical type 2 diabetes: the European prospective investigation of Cancer-Norfolk study. Am J Epidemiol. 2004;159(1):73–82. doi:10.1093/aje/kwh004.
- Vessby B, Uusitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, Nälsén C, Berglund L, Louheranta A, Rasmussen BM, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia. 2001;44(3):312–9. doi:10.1007/s001250051620.
- Imamura F, Micha R, Wu JHY, de Oliveira Otto MC, Otite FO, Abioye AI, Mozaffarian D. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016;13(7):e1002087. doi:10.1371/journal.pmed.1002087.
- Qian F, Korat AA, Malik V, Hu FB. Metabolic effects of monounsaturated fatty acid-enriched diets compared with carbohydrate or polyunsaturated fatty acid-enriched diets in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2016;39(8):1448–57. doi:10.2337/dc16-0513.
- Brown TJ, Brainard J, Song F, Wang X, Abdelhamid A, Hooper L, PUFAH Group. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019;366:l4697. doi:10.1136/bmj.l4697.
- Pereira MA, Jacobs DR, Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA study. JAMA. 2002;287(16):2081–9. doi:10.1001/jama.287.16.2081.
- Choi HK, Willett WC, Stampfer MJ, Rimm E, Hu FB. Dairy consumption and risk of type 2 diabetes mellitus in men: a prospective study. Arch Intern Med. 2005;165(9):997–1003. doi:10.1001/archinte.165.9.997.
- Fumeron F, Lamri A, Emery N, Bellili N, Jaziri R, Porchay-Baldérelli I, Lantieri O, Balkau B, Marre M, et al. Dairy products and the metabolic syndrome in a prospective study, DESIR. J Am Coll Nutr. 2011;30(5 Suppl 1):454S–63S. doi:10.1080/07315724.2011.10719990.
- Díaz-López A, Bulló M, Martínez-González MA, Corella D, Estruch R, Fitó M, Gómez-Gracia E, Fiol M, García de la Corte FJ, Ros E, et al. Dairy product consumption and risk of type 2 diabetes in an elderly Spanish Mediterranean population at high cardiovascular risk. Eur J Nutr. 2016;55(1):349–60. doi:10.1007/s00394-015-0855-8.
- Babio N, Becerra-Tomás N, Martínez-González MÁ, Corella D, Estruch R, Ros E, Sayón-Orea C, Fitó M, Serra-Majem L, Arós F, et al. Consumption of yogurt, low-fat milk, and other low-fat dairy products is associated with lower risk of metabolic syndrome incidence in an elderly Mediterranean population. J Nutr. 2015;145(10):2308–16. doi:10.3945/jn.115.214593.
- Horikawa C, Yoshimura Y, Kamada C, Tanaka S, Tanaka S, Hanyu O, Araki A, Ito H, Tanaka A, Ohashi Y, et al. Dietary sodium intake and incidence of diabetes complications in Japanese patients with type 2 diabetes: analysis of the Japan Diabetes Complications Study (JDCS). J Clin Endocrinol Metab. 2014;99(10):3635–43. doi:10.1210/jc.2013-4315.
- Kalogeropoulos AP, Georgiopoulou VV, Murphy RA, Newman AB, Bauer DC, Harris TB, Yang Z, Applegate WB, Kritchevsky SB. Dietary sodium content, mortality, and risk for cardiovascular events in older adults: the Health, Aging, and Body Composition (Health ABC) Study. JAMA Intern Med. 2015;175(3):410–9. doi:10.1001/jamainternmed.2014.6278.
- Due A, Larsen TM, Mu H, Hermansen K, Stender S, Astrup A. Comparison of 3 ad libitum diets for weight-loss maintenance, risk of cardiovascular disease, and diabetes: a 6-mo randomized, controlled trial. Am J Clin Nutr. 2008;88(5):1232–41. doi:10.3945/ajcn.2007.25695.
- Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48(1):44–51. doi:10.1016/j.plipres.2008.10.002.
- Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain [published correction appears in J Nutr. 2013 Sep;143(9):1524]. J Nutr. 2012;142(7):1304–13. doi:10.3945/jn.111.155325.
- Wu Y, Zhang D, Jiang X, Jiang W. Fruit and vegetable consumption and risk of type 2 diabetes mellitus: a dose-response meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. 2015;25(2):140–7. doi:10.1016/j.numecd.2014.10.004.
- Aune D, Norat T, Romundstad P, Vatten LJ. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol. 2013;28(11):845–58. doi:10.1007/s10654-013-9852-5.
- Sun Q, Spiegelman D, van Dam RM, et al. White rice, brown rice, and risk of type 2 diabetes in US men and women [published correction appears in Arch Intern Med. 2010 Sep 13;170(16):1479]. Arch Intern Med. 2010;170(11):961–9. doi:10.1001/archinternmed.2010.109.
- Pan A, Malik VS, Schulze MB, Manson JE, Willett WC, Hu FB. Plain-water intake and risk of type 2 diabetes in young and middle-aged women. Am J Clin Nutr. 2012;95(6):1454–60. doi:10.3945/ajcn.111.032698.
- Fagherazzi G, Vilier A, S, Sartorelli D, Lajous M, Balkau B, Clavel-Chapelon F. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidemiologique aupres des femmes de la Mutuelle Generale de l’Education Nationale-European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr. 2013;97(3):517–23. doi:10.3945/ajcn.112.050997.
- Wu JHY, Micha R, Imamura F, Pan A, Biggs ML, Ajaz O, Djousse L, B. Hu F, Mozaffarian D. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr. 2012;107(S2):S214–S227. doi:10.1017/S0007114512001602.
- Kim Y, Keogh JB, Clifton PM. Consumption of red and processed meat and refined grains for 4weeks decreases insulin sensitivity in insulin-resistant adults: a randomized crossover study. Metabolism. 2017;68:173–83. doi:10.1016/j.metabol.2016.12.011.