Abstract
Conventional chemical crop protection with pesticides is increasingly seen as being critical, because of pesticide residues in food and the environment. Integrated alternative management strategies such as crop rotations and soil management might also involve the targeted use of certain mineral fertilizers with benefits for plant health. A key element required for healthy crops is nitrogen, which is applied at differing dosages in various chemical forms, all with distinct effects on crop physiology and plant growth. Here, we review classical and more recent evidence for the crop disease-protective effects of nitrogen and various chemical nitrogen forms. We conclude that simple general statements concerning disease-protective roles in agricultural environments remain elusive, although complex plant-soil microbial interaction networks are becoming increasingly understood. The health of modern varieties might be substantially improved by certain chemical nitrogen fertilizer forms, particularly when the disease-causing fungal species are known.
I. Introduction
The need to optimize crop yields and minimize crop losses from plant diseases is steadily becoming crucial because of the growth in the world’s population and its demand for food (Savary, Citation1995; Gilland, Citation2002; Parry et al., Citation2004; Dietzel et al., Citation2019; Savary et al., Citation2019). To date, such aims have been made possible through the extensive use of chemical crop protection products (Zhang, Citation2018; Sharma et al., Citation2019). However, pesticide residues are found in food and in the environment where they endanger biodiversity (Pogăcean and Gavrilescu, Citation2009; Jacquet et al., Citation2022). Furthermore, the development of new pesticides, in particular the discovery of novel active ingredients, is becoming increasingly difficult because of stricter regulations for their registration and the rapid spread and development of resistance (Boone et al., Citation2014; Kudsk and Mathiassen, Citation2020). Finally, pesticide use might threaten agricultural land itself, for example, through the impairment of soil fertility by altering soil food webs and the loss of important beneficial soil organisms (Pimentel et al., Citation1992; Damalas, Citation2009). For these reasons, a reduction in the use of pesticides is necessary. One of the greatest challenges of this century is the development of sustainable cropping systems capable of providing sufficient high-quality food for the world’s growing population (Giller et al., Citation2021; Zimmermann et al., Citation2021).
Plants are exposed to various biotic stresses and are attacked by insects, nematodes, fungi, bacteria and viruses, all of which can cause severe root and shoot damage. These detrimental organisms impair plant development and reduce crop yields. Among the major global cereal diseases, fungal diseases pose the most serious threats and widen the gap between actual and achievable yields (Goutam et al., Citation2015). Fungal pathogens are estimated to account for about 15–20% annual yield losses within the major plant crops (Figueroa et al., Citation2018; Różewicz et al., Citation2021). Therefore, fungal diseases represent the largest biotic challenge when it comes to maximizing yields with high product quality. A key component in addressing this challenge is early detection and the proper management of disease in the field. In this context, seasonal crop rotation is one of the most important agronomic factors used to restrict the occurrence of fungal diseases. Cereals are often grown in succession, which can increase the accumulation of fungal pathogens in the soil (Różewicz et al., Citation2021). Other determinant factors that strongly influence the occurrence of pathogens are seasonal climatic conditions and weather events. In general, favorable conditions for fungal pathogenic infection are high humidity and moderate temperatures. However, extreme climate events, such as drought and floods, have also been reported to increase the occurrence of fungal pathogens in natural and agricultural ecosystems (Francioli et al., Citation2020; Gao et al., Citation2020).
Notably, the fungal disease is also highly influenced by agronomic practices, plant nutritional status and mineral fertilization (Datnoff et al., Citation2007; Walters and Bingham, Citation2007). Nitrogen (N) is considered a key influencer of plant health, being indispensable for maximal crop yields and yield security. The addition of mineral N has been widely shown to alter plant physiology, growth and plant-associated microbiota (Hinzman et al., Citation1986; Francioli et al., Citation2016; Le Luo et al., Citation2020). The last-mentioned has profound effects on plant defenses, particularly against fungal diseases (Fernandes and Rossiello, Citation1995; Walters and Bingham, Citation2007; Geisseler and Scow, Citation2014; Ding et al., Citation2021). Additionally, various mineral N fertilizers are available and utilized in agriculture. Most commonly, ammonium nitrate (NH4NO3) and urea (CH4N2O) fertilizers are used but, less frequently, more specific fertilizers containing either ammonium or nitrate such as calcium nitrate (Ca(NO3)2) or “stabilized” ammonium sulfate (NH4SO4), combined with a nitrification inhibitor to impair the nitrification process) are also employed. All of these fertilizers primarily serve to ensure an adequate N supply to the plant, although a specific N fertilizer can also be chosen as an agronomic tool to influence the rhizosphere or soil pH and thus the availability of soil macro- and micronutrients (Fox and Hoffman, Citation1981). Additionally, a specific form of N for soil treatment can be selected with the aim of preventing certain pathogen threats (Huber and Watson, Citation1974; Mur et al., Citation2017; Sun et al., Citation2020).
This review summarizes the effects of N fertilization on fungal pathogen infection in wheat, maize and rice, as these are the most important monocotyledonous crops worldwide, being grown on about 40% of the world’s cropland (Oerke and Dehne, Citation2004; Skamnioti and Gurr, Citation2009). Results from studies of the N-form on plant disease have been reviewed earlier (Huber and Watson, Citation1974). Here, we additionally consider the influence of N fertilizer rates and form on fungal pathogens associated with cereal diseases. Furthermore, we summarize the way that the dosage and type of mineral N fertilization affect the microbiome associated with cereal crop roots. We conclude that the choice of N fertilizer rate and source in current cropping systems can improve the health of crops faced with attacks by fungal pathogens.
II. N fertilization alters the susceptibility of cereal plants to fungal diseases
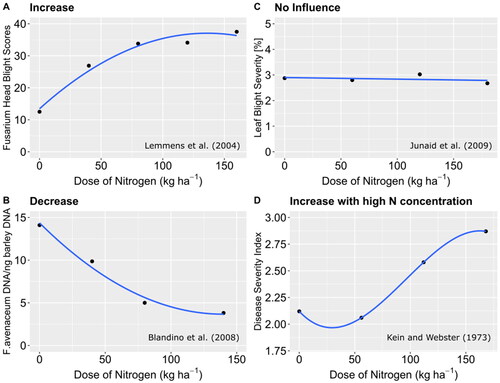
N fertilization is crucial for plant stress responses to fungal pathogens (Sun et al., Citation2020). In general, pathogen infection and disease severity depend on nitrogen dose with four different patterns being observed (). The most common pattern is that increased N fertilization is accompanied by more intense disease severity (). This, however, applies only up to a certain amount of N fertilization, because at very high N levels there is no further increase in disease severity. This pattern is common for stripe rust, leaf rust, fusarium head blight, powdery mildew, septoria leaf spot disease in wheat and rice blast disease (Simón et al., Citation2003; Fleitas et al., Citation2018a; Thapa et al., Citation2018; Gebrel et al., Citation2019; Luo et al., Citation2021). Pathogen infection may, however, also decrease with increasing amounts of N (). This has been reported for fusarium root rot in wheat and maize, a tan spot in wheat and aggregate sheath spot disease in rice (Linquist et al., Citation2008; Hofer et al., Citation2016; Fleitas et al., Citation2018b). Furthermore, increased N dose can have no effect on disease incidence () as observed, for example, in leaf blight disease in maize (Junaid et al., Citation2009). Finally, a rare pattern has been noted, for instance, for spot blotch and true eyespot disease in wheat and for stem rot in rice (Shipton, Citation1972; Keim and Webster, Citation1973; Colbach and Saur, Citation1998; Sharma et al., Citation2006; Singh and Singh, Citation2006; Martínez, Citation2021). In this cases, disease incidence is reduced or unaffected by N nutrition up to a medium level of N, whereas high levels of N fertilization increase disease severity ().
Figure 1. Common patterns of the effect of nitrogen fertilization on disease incidence. Individual literature sources are given as examples.
Here, we conducted a systematic review of the published literature according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) rules for the conduction of meta-analyses. Relevant studies were identified by searching the following data sources: Medline via PubMed, Scopus and Google Scholar. Search terms, including keywords and headings, were related to plant disease and N nutrition in wheat, rice and maize. The following criteria were applied to determine whether a particular report was included in the analysis. First, the article had to contain original research in the form of a field, greenhouse or climate chamber trial with wheat, rice or maize plants in soil or hydroponic systems. Second, the report had to include information on N application rates, preferably in units of kg N ha −1. N application rates that were expressed in kg N acre−1 were converted to kg N ha−1. Application rates in mg N per plant, mg N kg−1 soil, or mg N L−1 were, however, also considered. Third, the report had to provide at least one measure of disease intensity, such as disease incidence, disease severity, the area under the disease progress curve or disease intensity, expressed as an index or on an arbitrary scale. Fourth, control had to be included in the form of plants that were not fertilized with N or were fertilized with less N than the treatment being compared. In addition, studies comparing various chemical forms of N were included.
The meta-analysis included 83 published papers ranging from 1924 to 2022, most of which (48 studies) dealt with the effect of n fertilization on wheat diseases. Twenty and fifteen studies were found for rice and maize diseases, respectively. In total, 25 different diseases were covered, including 12 major diseases in wheat, 8 in rice and 5 in maize. Analysis of the effect of N nutrition on plant disease incidence revealed that, in 33 cases, N fertilization (applied as ammonium nitrate, urea, nitrate, ammonium or an unspecified N source) increased susceptibility to cereal diseases (). By contrast, N nutrition decreased pathogen infection in 15 cases. Several previous meta-analyses examining the general effect of N fertilization on plant disease incidence also stated that, in the majority of cases, N fertilization increased disease severity. However, decreased pathogen infection was also reported in a similar proportion as in this meta-analysis (Dordas, Citation2008; Veresoglou et al., Citation2013; Sun et al., Citation2020). In 16 cases, disease incidence was reported not to be changed by nitrogen. These diverse responses to N nutrition can be partially linked to (1) the various infection strategies that the pathogens use to invade the plant, (2) whether the causal pathogen is (hemi)biotrophic or necrotrophic or (3) whether the pathogen is a foliar or root-infecting type, as will be discussed in the subsequent sections in more detail.
Table 1. Number of published papers reporting the effects of various nitrogen forms on plant disease incidence.
Investigations of the role of the N-form on plant disease incidence revealed that ammonium nitrate is the most frequently studied N-form, followed by urea, in line with these chemical forms being the most frequently employed in practical farming. Studies with fertilizers containing either nitrate or ammonium were rare. Following ammonium nitrate fertilization, nearly twice as many disease increases were reported compared with disease decreases. With urea, over three times as many disease increases were described, indicating that N nutrition in the form of urea increases plant susceptibility to fungal diseases. N fertilization in the form of nitrate, ammonium or unspecified N increased the disease level in three cases each. In the same number of cases, ammonium fertilization was determined to decrease disease severity, whereas nitrate fertilization was reported to decrease the disease level only in a single case.
III. Fungal lifestyle determines the impact of mineral N supply on fungal disease development in wheat and barley
Numerous studies have been conducted on the interaction between nitrogenous fertilizers and diseases in wheat (). Traditionally, fungal pathogens are classified according to their mode of nutrition and are divided into three categories: biotrophs, necrotrophs and hemibiotrophs (Cal et al., Citation2022). Biotrophic organisms are pathogens that require living plant tissue to survive and complete their life cycle. They create a long-term feeding relationship with the living cells of their hosts, rather than killing it, as part of the infection process (Fei and Liu, Citation2023). During plant infection, pathogens synthesize and secrete effector proteins, some of which are translocated to the plant cytosol, where they can alter the host response to the invading pathogen. After successful infection, the pathogen’s effector proteins facilitate suppression of the plant’s immune system and reprogram the infected tissue so that it becomes a source of nutrients needed by the pathogen for growth and development. Generally, these fungi grow between host cells, penetrate only a few cells and form nutrient-absorbing structures called haustoria (Koeck et al., Citation2011). Through their feeding activity, they create a nutrient sink at the site of infection so that the host is weakened, but not killed. This type of parasitism can result in severe economic losses in crops, primarily from the two major biotrophic plant pathogens of rust fungi (Basidiomycota) and powdery mildew (Ascomycota). Necrotrophic fungi infect living plants with the aim of killing the plant at or shortly after infection and extracting nutrients from its dead or dying tissues (Oliver and Ipcho, Citation2004; Shao et al., Citation2021). As efficient plant killers, they are notorious for causing significant losses in the field and in storage worldwide (Fones et al., Citation2020). Hemibiotrophic pathogens first invade living cells in an initial period of biotrophy, before switching to a necrotrophic lifestyle to obtain nutrients by killing host cells (Perfect and Green, Citation2001).
Table 2. Biotrophic, necrotrophic and hemibiotrophic fungal diseases in Triticum aestivum and Hordeum vulgare and the influence of mineral nitrogen fertilizer rate and form on disease infection in comparison with control.
Wheat, as one of the most important staple foods in the world, is the major source of calories and vegetable protein in the human diet (Curtis et al., Citation2002). Therefore, ensuring a reliable harvest is extremely important for satisfying the global population’s demand for food and for dealing with emerging challenges such as the decreasing availability of suitable farmland, climate change and a variety of unpredictable abiotic and biotic stressors (Figueroa et al., Citation2018). The global supply of wheat is particularly threatened by the emergence of pathogens because the genetic diversity of wheat declines in the pursuit of elite high-performance varieties. In particular, pathogenic fungi are a major liability when it comes to serious yield losses, and they are difficult to control in cases of beneficial infection conditions. Therefore, N fertilization, which might affect wheat plant infection, must be taken into consideration more closely, especially in the context of reducing pesticide use.
A. N fertilization increases the severity of biotrophic pathogens in wheat
The stripe (yellow) rust and leaf (brown) rust diseases of wheat are caused by fungal species affiliated with the genus Puccinia (phylum Basidiomycota). This genus is considered to harbor the most economically destructive biotrophic fungi worldwide (Lorrain et al., Citation2019). Indeed, members of this genus are serious pathogens on all major cereal crop species, except rice. Specifically, Puccinia. striiformis f. sp. tritici, P. triticina and P. graminis f. sp. tritici are three of the most widespread wheat pathogens and severely limit wheat production (Knott, Citation1989; Khan et al., Citation2013). As biotrophic pathogens, the wheat rust fungi derive their nutrients and energy from living cells, without killing their host. Rust fungi spread in the form of clonally produced dikaryotic uredinospores, which are dispersed over long distances by wind (Roelfs, Citation1989). For stripe (yellow) rust, N fertilization at 20-300 kg ha−1 is well known to favor disease infection (Huber and Watson, Citation1974; Darwinkel, Citation1980; Ash and Brown, Citation1991; Danial and Parlevliet, Citation1995; Neumann et al., Citation2004; Devadas et al., Citation2014; Gebrel et al., Citation2019). The N-form apparently plays a minor role, as opposed to the N rate, in influencing stripe rust infection (Darwinkel, Citation1980). The effect of N nutrition on stem rust was investigated by (Stakman, Citation1924), who found that the severity of stem rust increased under field conditions. Similarly, fertilization with ammonium nitrate also increased the disease severity of leaf (brown) rust in greenhouse experiments (Piening, Citation1972; Tiedemann, Citation1996). Nevertheless, fertilization with low doses of urea and calcium nitrate did not increase leaf rust disease severity (Singh, Citation2006; Bürling et al., Citation2011).
Another common biotrophic pathogen of wheat is powdery mildew, caused by Blumeria graminis f. sp. tritici (phylum Ascomycota), which is particularly important in highly productive areas with maritime or semi-continental climates (Cunfer, Citation2002). Compared with rust fungi, powdery mildew conidia do not spread over extensive distances, but the rate of evolution of the fungus is high (Duveiller et al., Citation2007). Fertilization with N, independent of the chemical N-form, has been shown to favor powdery mildew infection (Last, Citation1953; Bainbridge, Citation1974; Grybauskas et al., Citation1988; Jensen and Munk, Citation1997; Chen et al., Citation2007; Luo et al., Citation2021). At the same N dosage, ammonium fertilization is reported to increase wheat susceptibility to B. graminis infection compared with fertilization by nitrate (Maywald et al., Citation2022).
In general, N fertilization leads to an increase in the disease severity of biotrophic pathogens in wheat (). This can be attributed to a changed microclimate favorable to biotrophic pathogens resulting from altered plant structure, higher above-ground biomass and increased plant density, attributable to the increased tillering achieved by N fertilization (Maidl et al., 1998 Neumann et al., Citation2004; Devadas et al., Citation2014). In addition, N supplementation increases the greenness index, resulting in a greener leaf color and higher spectral reflectance of wheat plants, which might enhance the plant’s attractiveness to biotrophic pathogens (Hinzman et al., Citation1986; Walters and Bingham, Citation2007). Furthermore, higher leaf N concentrations and a delay in senescence increase the availability of N compounds for exploitation by pathogens (Walters and Bingham, Citation2007). In particular, biotrophic pathogens benefit from increased metabolite pools in host cells mediated by a high N status (Jensen and Munk, Citation1997; Fleitas et al., Citation2018b; Simón et al., Citation2020). In detail, the increase in organic N compounds, such as gamma-aminobutyric acid (GABA), in plant leaves leads to its increased utilization as a substrate by biotrophic pathogens at the site of infection (Dordas, Citation2008; Sun et al., Citation2020). Moreover, the phytohormone salicylic acid (SA) plays a critical role in inducing plant defence against biotrophic pathogens via acquired systemic resistance (SAR), a broad-spectrum systemic resistance after primary fungal infection (Devadas et al., Citation2002; Lefevere et al., Citation2020). In this context, N nutrition has been reported to have a negative effect on SA accumulation, resulting in enhanced pathogen infection (Yaeno and Iba, Citation2008).
Figure 2. Biotrophic and necrotrophic wheat diseases react differently depending upon the dose of nitrogen applied. The area under the disease progress curve (AUDPC) was taken as a disease measure for leaf rust and tan spot. Adapted from (Simón et al., Citation2020).
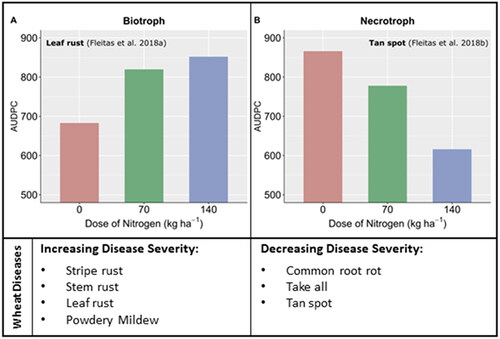
All in all, the increased susceptibility of wheat plants to biotrophic pathogens after N fertilization can be attributed to anatomical and biochemical changes initiated by increased N availability in the soil and later in the plant. Without the use of additional chemical or biological plant protection products, any yield increases achieved through N fertilization might potentially be negated by biotrophic pathogen infection. Thus, a suitable choice of N-form might lead to reductions in pathogenic infections under certain circumstances.
B. Disease severity of necrotrophic pathogens in wheat are reduced by N fertilization
Some of the most important and devastating diseases of wheat are caused by necrotrophic soilborne fungal pathogens (Kwak and Weller, Citation2013). Among them, the take-all disease of wheat caused by the ascomycete Gaeumannomyces graminis var. tritici (Ggt) is the most economically important necrotrophic fungus (Hornby, Citation1998; Freeman and Ward, Citation2004; Kwak and Weller, Citation2013). N fertilization in the form of ammonium and urea has been reported to decrease take-all disease, whereas fertilization with nitrate increases the disease severity (Smiley and Cook, Citation1973; Smiley, Citation1978; Brennan, Citation1992a, Citation1992b, Citation1993). This process has been mainly attributed to the direct inhibition of the ectotrophic hyphal growth of Ggt by a decrease in the pH of the wheat root surface because of the application of ammonium fertilizer. Additionally, Ggt suppression by ammonium and urea seems highly dependent on soil microbes, as the suppressive effect is removed by soil fumigation before Ggt inoculation (Smiley and Cook, Citation1973; Smiley, Citation1978). Although the mechanisms of such soil suppressiveness are not fully understood, Ggt-suppressive soils have often been reported as being characterized by a decrease in rhizosphere pH, together with the enrichment of populations of root-associated Pseudomonas spp. (Weller et al., Citation2002). In particular, Pseudomonas fluorescens strains can produce the antifungal polyketide 2,4-diacetylphlorogucinol, 2,4-DAPG (Ownley et al., Citation2003). However, recent research has observed the opposite effect of ammonium on take-all disease. In a greenhouse trial, ammonium fertilization increased the disease severity compared with nitrate fertilization (Maywald et al., Citation2022). Even though ammonium fertilization was able to lower the pH of the rhizosphere, no increase was observed in the abundance of Ggt-suppressing bacteria. Instead, ammonium fertilization significantly increased the abundance of plant pathogenic fungal taxa, including Ggt, in the wheat rhizosphere.
The necrotrophic pathogen Pyrenophora tritici-repentis (anamorph Drechslera tritici-repentis) is the causal pathogen of tan spot, which induces severe foliar necrosis and chlorosis in wheat plants (Hosford, Citation1982; Lamari and Bernier, Citation1991; Hane et al., Citation2007; Antoni et al., Citation2010). The fungal infection damages leaf tissues and reduces photosynthetic activity, which can result in significant yield losses of up to 50% (Rees et al., Citation1982; Guo et al., Citation2020). This disease can also cause red smudges on wheat kernels, which reduces wheat grain quality (Fernandez et al., Citation1996). N nutrition in the form of ammonium nitrate, calcium nitrate or urea decreased disease incidence in multiple trials (Huber, Citation1987; Krupinsky and Tanaka, Citation2001; Krupinsky et al., Citation2007; Fleitas et al., Citation2018b). However, (Bockus and Davis, Citation1993) observed no influence on disease severity when fertilizing plants with ammonium sulfate or calcium nitrate.
In contrast to the N effect on biotrophic pathogens, N supply can also enhance plant defenses against pathogens. This has been noted for important necrotrophic wheat pathogens. The decline in disease severity with increasing N rates is attributed to the finding that higher N availability strengthens plants, which are then able to defend themselves more effectively (Simón et al., Citation2020). In addition, fertilization with N can suppress soilborne necrotrophic diseases by affecting soil pH and the microbiota associated with plant roots (Maywald et al., Citation2022). Indeed, the rhizosphere microbiome has an important role in the suppression of soilborne diseases and in improving plant resistance against soilborne pathogens (Berendsen et al., Citation2012; Ruijven et al., Citation2020).
C. Hemibiotrophic pathogens in wheat respond individually to N fertilization
Hemibiotrophic pathogens are characterized as having a biotrophic phase during primary infection and a necrotrophic phase, in which enzymes are secreted to degrade host cell walls, together with a build-up of mycotoxins (Goswami and Kistler, Citation2004; Kazan et al., Citation2012; Castiblanco et al., Citation2018). The Ascomycota Mycosphaerella graminicola (anamorph Zymoseptoria tritici) causes one of the most economically devastating foliar diseases of wheat in Europe, namely septoria leaf blotch (Orton et al., Citation2011). Temperate climates and rain-fed environments favor the development of this disease. Characteristic symptoms are the necrotic leaf blotches containing variable densities of pycnidia, the asexual sporocarps that harbor the conidia (Cohen and Eyal, Citation1993; Kema et al., Citation1996; Duncan and Howard, Citation2000). N fertilization in the form of ammonium nitrate or urea has been shown to increase the severity of septoria leaf blotch (Gheorgies, Citation1974; Prew et al., Citation1983; Broscious, Citation1985; Howard et al., Citation1994; Leitch and Jenkins, Citation1995; Simón et al., Citation2002; Simón et al., Citation2003). This effect has been suggested to be mainly attributable to a denser leaf stand that results from the increased N supply and that promotes spore germination by extending periods of leaf wetness following rain events (Simón et al., Citation2003). Higher N applications also improve the nutritional status of host tissues, thereby resulting in an increased incidence of eptoria leaf blotch (Leitch and Jenkins, Citation1995). In general, the pattern by which N inputs increase disease severity is more reminiscent of that of biotrophic fungi. This can be correlated with the life cycle of Zymoseptoria tritici, which is characterized by a prolonged and asymptotic biotrophic phase, during which it grows slowly and protects itself from the host’s defenses, before eliciting a strong necrotic response (Sánchez-Vallet et al., Citation2015).
The category of hemibiotrophic wheat pathogens also includes various species of the genus Fusarium, which are mainly filamentous fungi that are widely distributed in the soil and are associated with plants. Although most of the species affiliated with this genus are saprobes, some Fusarium species are related to important diseases in crops, causing great economic concern in agriculture (Wu, Citation2007; Hollingsworth et al., Citation2008). For example, F. culmorum is a ubiquitous soilborne fungus that can cause foot rot, root rot and head blight in wheat and barley (Scherm et al., Citation2013). It causes significant yield and quality losses and leads to contamination of the grain with mycotoxins (Motallebi et al., Citation2017). With regard to N fertilization, ammonium nitrate application at 80–140 kg N ha−1 has been shown to reduce disease severity compared with the unfertilized control. Being a soilborne fungus with a mainly necrotrophic phase and a life cycle that is not yet fully understood, F. culmorum is sometimes considered a necrotroph (Petti et al., Citation2012). Giberella zeae (ana. Fusarium graminearum) and Giberella avenacea (ana. Fusarium avenaceum) are two of 16 known fungal species that cause fusarium head blight (FHB), also known as scab (O'Donnell et al., Citation2004). Because of the formation of various mycotoxins such as deoxynivalenol and zearalenone, which are toxic to humans and animals, they are among the most devastating fungal diseases in wheat, barley and maize (Darwish et al., Citation2014; Dweba et al., Citation2017). F. graminearum, the predominant species causing FHB, was not affected by an ammonium nitrate application of 140 kg ha−1 (Fauzi and Paulitz, Citation1994). Contradictory results were found by (Lemmens et al., Citation2004) who demonstrated that ammonium nitrate and ammonium nitrate urea solution increased the disease severity of F. graminerarum and F. avenaceum. This trend was attributed to the change in crop characteristics, particularly the increase in plant density and alteration of the microclimate of the crop stand at increased N supply. As a result, plants remained moist for an extended period, which favored the formation of macroconidia and ascospores, resulting in enhanced infection potential. Additionally, higher N input extended the anthesis and flowering of wheat, developmental phases in which the plant is most susceptible to fungal infection. FHB infection severity has been reported to be positively correlated with a prolonged duration of flowering (Aufhammer et al., Citation1999).
Another important hemibiotrophic fungal pathogen that is responsible for a variety of cereal and forage grass diseases is the Ascomycete Tapesia yallundae (ana. Pseudocercosporella herpotrichoides). During the autumn, this fungus infects wheat seedlings via the coleoptile and spreads through successive leaf sheaths in winter, until eyespot lesions form on the stem possibly leading to progressive stem weakening in the spring (Higgins et al., Citation1986; Bierman et al., Citation2002). Substantial yield losses have been associated with eyespot, particularly in the UK (Fitt et al., Citation1988; Hunter, Citation1989). Eyespot disease is a monocyclic disease that survives on infected stem residues during the period between harvests, with conidia being considered the main inoculum (Lucas et al., Citation2000). Fertilization with a mixture of urea and ammonium nitrate had no effect on eyespot disease severity at low N fertilization rates of 15–60 kg ha−1 (Shipton, Citation1972). However, fertilization with various N sources at high N fertilizer rates of 200–380 kg ha−1 increased disease severity (Colbach and Saur, Citation1998). Unlike other foot diseases, an increase in eyespot could not be linked to the N content of the plants. However, plant tissue was concluded to be more susceptible to plant diseases at high N levels because of faster plant growth and increased tillering (Colbach and Saur, Citation1998). The differing results found by (Shipton, Citation1972) and (Colbach and Saur, Citation1998) can be explained on the basis of the location and climate being the most important factors in the development of the disease. When the environmental conditions were unfavorable for disease development, the incidence of eyespot remained low, even when all other factors were favorable, i.e. early sowing, high plant density and high N availability.
The fungus Cochliobolus sativus (ana. Bipolaris sorokiniana) is known to attack all parts of wheat plants, causing common root rot in basal parts, spot blotch on leaves, black nodes and breakage of stems, blighting of heads and black point or smudge of seed (Raemaekers and Tinline, Citation1981). Common root rot in wheat has been reported to decrease with increasing N fertilization (Chaurasia and Duveiller, 2006; Baba, Citation2019). In contrast to these findings, fertilization at 90 kg ha−1 of urea has been shown not to influence the occurrence of common root rot disease (Sisterna and Sarandón, Citation1996). C. sativus is the causal agent of wheat spot blotch, which is considered the most important disease of wheat in the eastern regions of South Asia (Duveiller et al., Citation2005; Joshi et al., Citation2007). Whereas N fertilization at 120 kg ha−1 had no effect on spot blotch severity, a mixture of urea and di-ammonium phosphate at 150 and 180 kg ha−1 was reported to increase disease severity (Maity and Sanyal, Citation2002; Sharma et al., Citation2006; Singh and Singh, Citation2006).
The way in which N nutrition affects the severity of the disease strongly depends on the respective hemibiotrophic pathogen. For example, Z. tritici, with its more biotrophic character, behaves similarly to other biotrophic pathogens resulting in increased disease severity with additional N fertilization. On the other hand, the life cycle of F. culmorum is characterized by a prolonged necrotrophic phase and, in agreement with findings on other necrotrophic wheat pathogenic fungi, N fertilization has been reported to reduce the disease severity of these fungi. However, controversial results have been found for many hemibiotrophic fungi and their N-associated effects on plant health. Such responses appear to be pathogen-specific and seem to depend on environmental conditions and the detailed life cycle of the individual fungal pathogen (Gilbert and Webb, Citation2007; Simón et al., Citation2020).
Thus, overall, disease progression is concluded to be influenced by N fertilization, and dependencies on the different biotrophic or necrotrophic lifestyles have been observed. Whereas the disease severity of biotrophic pathogens increases with N fertilization, a decrease has been observed for necrotrophs. In contrast, general conclusions for hemibiotrophic fungi are more difficult to draw, because their characteristics depend on the current stage of their life cycle and the infection process of the fungus (Ruijven et al., Citation2020). In general, the application of excess N has been shown to increase disease incidence in almost all fungal diseases studied (Huber and Watson, Citation1974; Sun et al., Citation2020).
IV. N fertilization and its influence on disease severity of fungal pathogens in rice
Tropical and sub-tropical areas in which rice is grown often provide ideal environmental conditions for rice-infecting pathogens. Collectively, these pose a significant threat to rice production. Annual estimated yield losses in rice of up to 10% have been reported to be attributable to a combination of fungal diseases (Savary et al., Citation2000). Modern agricultural development has transformed the diverse traditional rice cropping system into a monoculture system based on only a few fertilizer-responsive high-yielding varieties. Although most modern varieties have a built-in resistance to multiple diseases, genetic uniformity inevitably predisposes the cultivation system to disease attack and can lead to serious disease-related yield losses under certain circumstances (Khush and Virk, Citation2002). For this reason, the impact of N fertilization on fungal pathogens in rice needs to be closely examined in order to achieve both high yields and plant health.
A. N fertilization increases susceptibility to biotrophic pathogens in rice
The ascosmycetes Sarocladium oryzae, the causal agent of sheath rot of rice, can cause yield losses of 3–85%, depending on the severity of the disease (Chakravarty and Biswas, Citation1978; Ayyadurai et al., Citation2005). Since no completely resistant variety to this pathogen exists, the control of rice sheath rot relies almost exclusively on the use of chemical pesticides (Saravanakumar et al., Citation2009). The fungus causes characteristic grey-brown lesions on the uppermost flag leaf surrounding the panicle. This disease results in young panicles either remaining in the leaf sheath or partially breaking open. The broken panicles form chaffy or partially filled, discolored and shriveled grains (Saravanakumar et al., Citation2009). Although S. oryzae is seed-borne and seed-transmitted, the fungus also survives as mycelium in infected plant debris, weed hosts and soil (Ayyadurai et al., Citation2005; Saravanakumar et al., Citation2009; Chien and Huang, Citation2013). In terms of N fertilization and sheath rot disease, one study has reported that urea application of 100–300 kg N ha−1 increases the disease severity of sheath rot (Bhaskar et al., Citation2001).
Another important rice disease is the so-called rice false smut, which is caused by the Ascomycota Villosiclava virens (ana. Ustilaginoidea virens). This fungus transforms individual grains of the panicle into greenish-black smut balls, which are often covered by sclerotinia and may fall to the ground, leaving powdery chlamydospores (Guo et al., Citation2012). Damage caused by false smut includes yield reductions and contamination of grains and panicles with ustiloxins, which are antimitotic cyclic peptides toxic to humans and animals (Koiso et al., Citation1994; Tanaka et al., Citation2008; Guo et al., Citation2012). N fertilization with urea or unspecified N of 99–247 kg N ha−1 showed increased infection by rice false smut (Brooks et al., Citation2010, Citation2011; Rani et al., Citation2015). This was attributed to increased N uptake by the plant leading to a thinning of the cell walls, which thereby facilitates penetration of the pathogen into the host plant (Rani et al., Citation2015).
In general, N fertilization increases the disease severity of biotrophic pathogens in rice. This is probably based on similar reasons to those described for wheat in chapter 3.1. N fertilization not only creates a microclimate conducive to fungal infections by promoting plant growth, but also increases the pool of metabolites that can be used by the plant pathogens. Additionally, histochemical staining has revealed that high N application decreases lignin deposition in the secondary cell wall of rice plants compared with low N treatments. This is accompanied by a down-regulation of the genes involved in lignin biosynthesis, leading to a lack of lignin in secondary cell walls and a weakening of mechanical tissue structure, both of which favor foliar pathogen penetration (Zhang et al., Citation2017).
B. N fertilization alters the severity of necrotrophic pathogens in rice
Rice sheath spot disease, an important and widespread rice disease, is caused by the Basidiomycota Rhizoctonia oryzae-sativae (ana. Ceratobasidium oryzae-sativae). Disease symptoms include oval-shaped lesions on leaves and sheaths (Miller and Webster, Citation2001). Sclerotia, which persist in plant debris after harvest, form between leaf sheaths or within the leaf sheath tissue of rice (Nakata and Kawamura, Citation1939; Guo et al., Citation2006). (Linquist et al., Citation2008) determined that fertilization with 50–200 kg N ha−1 ammonium sulfate reduced disease severity, compared with no N fertilization. They hypothesized that the vertical movement of R. oryzae-sativae up the rice stem is slow and that higher N rates allow the plant to grow faster than the spread of rice sheath spot disease up the stem, thereby suppressing the severity of rice sheath spot disease (Linquist et al., Citation2008). This hypothesis is supported by findings that semi-dwarf varieties are more susceptible to rice sheath spot disease than tall varieties (Gunnell, Citation1984). Taller varieties have faster growth rates than semi-dwarf varieties, possibly allowing them to resist the spread of sheath spot disease better.
Another necrotrophic rice pathogen is the Ascomycota Cochliobolus miyabeanus (ana. Bipolaris oryzae), which causes brown spot disease, one of the most severe fungal diseases of rice (Dasgupta, Citation1984; Scheffer, Citation1997; Kim et al., Citation2014). Symptoms of this disease include small circular dark brown or purplish spots on the leaves (Waller, Citation1987; Kim et al., Citation2014). (Gangopadhyay and Chattopadhyay, Citation1975) reported that N fertilization with ammonium nitrate reduced brown spot disease at a dose of 20–40 kg N ha−1, but opposing results were observed at a higher dose (50–60 kg N ha−1) and during N deficiency (0–10 kg N ha−1). They also found that N fertilization correlated with leaf silicon content, which was highest at 20–40 kg N ha−1 and decreased at higher and lower N fertilization rates. Silicon has been reported to induce resistance to the brown spot fungus C. miyabeanus by preventing the pathogen from hijacking the ethylene signaling pathway of rice (van Bockhaven et al., Citation2015). Another study has shown that ammonium nitrate fertilization reduces disease severity regardless of N concentration (Carvalho et al., Citation2010).
Sheath blight disease occurs in all rice production areas and is caused by Thanatephorus cucumeris (ana. Rhizoctonia solani) (Srinivasachary et al., Citation2011). The main symptoms of sheath blight are greenish-grey lesions with irregular purplish-brown margins on the leaf sheaths, at the base of the plants or near the waterline in irrigated rice fields. The sheath rot lesions can spread rapidly on the plant and the corresponding leaf blade wilts and die within a few days (Taheri and Tarighi, Citation2011). In multiple trials involving the use of urea or an unspecified source of N as fertilizer, a N supply of 67–320 kg N ha−1 has been commonly reported to increase disease severity (Savary, Citation1995; Slaton et al., Citation2003; Tang et al., Citation2007; Li et al., Citation2012; Wu et al., Citation2012; Wu et al., Citation2015). These studies suggest that the N supply increases sheath blight intensity primarily indirectly by promoting plant growth and increasing plant stand density, which in turn leads to favorable infection conditions through increased moisture within the plant stand.
Overall, necrotrophic fungi are a serious problem for rice production. Disease severity of most necrotrophic fungal diseases is reduced by N fertilization, similar to the observations on the necrotrophic fungal diseases of wheat. In rice, this has been attributed to the increased growth and higher plant N content, which helps the plant to protect itself against the invasion and spread of the disease within the plant. Sheath blight is an exception, as N fertilization increases disease susceptibility; better growth and elevated N concentrations in this case is detrimental.
C. Hemibiotrophic pathogens respond individually to N fertilization in rice
Rice blast disease, caused by Magnaporte oryzae (ana. Pyricularia oryzae), is the most important disease affecting rice production worldwide (Debona et al., Citation2012). The rice blast fungus attacks rice plants at all stages of development and can affect leaves, stems, nodes and panicles. The fungal hyphae branch through the plant tissue and lead to the disease lesions symptomatic of rice blast (Wilson and Talbot, Citation2009). N fertilization in the form of ammonium nitrate or urea is known to increase the severity of rice blast, regardless of N concentration (Long et al., Citation2000; Ballini et al., Citation2013; Huang et al., Citation2017; Thapa et al., Citation2018). Interestingly, (Ballini et al., Citation2013) have found, by means of cytological studies, that, despite higher infection, the penetration rate is not affected by high N. Instead, the fungus grows faster inside the plant under high N. The increase in visible necrosis under these conditions has been suggested to be attributable to a mechanical side effect of this increased growth. A more recent study by (Huang et al., Citation2017) has revealed that N application does not significantly affect plant physiology in terms of total N and the metabolome. On the one hand, increased disease incidence has been attributed to increased nutrient availability to the fungus, as some amino acids, such as glutamine and alanine, and other metabolites occur at higher concentrations in N-fertilized plants. On the other hand, defence-related genes such as PR genes and genes involved in the biosynthesis of antimicrobial secondary metabolites have been found to be more highly expressed during infection under increased N fertilization. Nevertheless, the host seems unable to counteract a concomitant increase in fungal pathogenicity, resulting in increased susceptibility.
Stem rot is caused by the fungus Magnaporthe salvinia (ana. Sclerotium oryzae) is another serious rice disease. Sclerotia produced by the fungus serve as the primary inoculum by floating on water and infecting rice stems at the waterline (Bockus, Citation1979; Cintas and Webster, Citation2001). They then develop within infected rice plant tissues and remain in crop residues (Webster et al., Citation1981). (Keim and Webster, Citation1973) reported that an ammonium sulfate fertilizer rate of 56 kg N ha−1 had no effect on the disease severity of stem rot in rice, whereas a N fertilizer rate of 112–224 kg N ha−1 significantly increased it. In another study, fertilization with 140 kg N ha−1 did not increase disease incidence in plant tillers, whereas stem rot severity increased slightly (Martínez, Citation2021). Only excessive N fertilization above the required level for a maximum yield was concluded to promote stem rot severity (Martínez, Citation2021).
Sheath rot of rice, also known as bakanae, Japanese for “foolish seedling,” is caused by the Ascomycota Gibberella fujikuroi (ana. Fusaium moniliforme) (Sun and Snyder, Citation1981; Desjardins et al., Citation1997). It occurs mainly in Asia and can be detected by the yellowing and abnormal growth of infected rice seedlings. In older plants, the roots, crowns, stems, leaf sheaths and panicles can all be infected. The disease is seed-borne and is mainly transmitted by seeds (Webster and Gunnell, Citation1992; Desjardins et al., Citation1997). In terms of N supply, urea supplied in the range of 62–125 kg N ha−1 has been reported to increase disease severity (Grewal and Kang, Citation1990; Singh and Kaur, Citation2005).
In summary, N fertilization increases the susceptibility of rice plants to hemibiotrophic pathogens. The reasons for this are not yet well understood and appear to be pathogen-specific. However, N-fertilized rice plants appear to be more susceptible to hemibiotrophic pathogens because of increased nutrient availability to the fungus ().
Table 3. Biotrophic, necrotrophic and hemibiotrophic fungal diseases in Oryza sativa and the influence of mineral nitrogen fertilizer rate and form on disease infection in comparison with control.
V. N nutrition and fungal pathogens in maize
Maize (Zea mays ssp. mays) is a staple crop used worldwide as human food, animal feed, bio-fuel and bioproducts (Edgerton, Citation2009). Maize is grown in both in temperate and tropical regions and is one of the most important food crops in the world (Thompson and Raizada, Citation2018). Because of its global importance, maize diseases, which can cause severe yield losses, can threaten the world’s food supply and farmers’ livelihoods. Nevertheless, only a few fungal maize pathogens seriously threaten maize cultivation. Therefore, biotrophic, necrotrophic and hemibiotrophic fungi are discussed here in one chapter rather than separately for wheat and rice. Problematic fungal pathogens are mainly those that directly attack the grain. Many of these fungi form mycotoxins as toxic metabolic products (Miller, Citation2008). Mycotoxins are secondary metabolites produced by the fungal species that colonize cereal crops and that contaminate them with these toxic compounds, either in the field or post-harvest (Leite et al., Citation2021). In maize, increased contamination with mycotoxins has been observed in recent years and reference values for animal nutrition are often exceeded.
Basiodiomycetes and biotrophic pathogen Ustilago maydis is the causal agent of common maize smut. This fungus causes distinct symptoms on all above-ground parts of its host maize plant (Brefort et al., Citation2009). Maize seedlings are infected at the trifoliate stage and symptoms appear around a week later in the form of tumor formation. In these structures, the fungal hyphae multiply and differentiate into spores (Doehlemann et al., Citation2008). N fertilization in the form of ammonium nitrate or urea increases disease severity at application rates of 74–341 kg N ha−1 (Kostandi and Soliman, Citation1991; Kostandi et al., Citation1997; Kostandi and Soliman, Citation1997; Aydogdu and Boyraz, Citation2011; Szulc et al., Citation2014), although some studies involving application rates of 50–150 kg N ha−1 have shown no effects on disease severity (Kostandi et al., Citation1997; Szulc et al., Citation2014). Thus, disease severity can be concluded to increase in most cases with ascending N doses. (Aydogdu and Boyraz, Citation2011) have attributed this to the higher plant N content, which increases the size of the plant cells and reduces the thickness of its cell walls. Additionally, N fertilization results in a prolonged vegetative period and delayed plant maturity (Agrios, Citation2008). Over time, these effects make the plant more susceptible to pathogens that attack the relevant tissues. Nevertheless, N supply does not seem to lead necessarily to an increase in disease severity, and disease development under N fertilization seems to be highly dependent on external conditions.
The necrotrophic fungus Petromyces flavus (ana. Aspergillus flavus), which is the causal agent of maize ear rot, is highly problematic in maize cultivation because of the formation of aflatoxin B1 during infection (Blandino et al., Citation2008). Aflatoxin B1 is the most abundant toxin found in food and is one of the most potent genotoxic and carcinogenic aflatoxins (Sétamou et al., Citation1997). N fertilization with various N sources results in a reduction in disease incidence, with a lower aflatoxin formation being observed at fertilizer rates ranging from 26 to 400 kg N ha−1 (Anderson et al., Citation1975; Jones and Duncan, Citation1981; Blandino et al., Citation2008; Mutiga et al., Citation2017). Plant stress associated with reduced fertilization, resulting in lower levels of total N in leaf and grain tissues, has been concluded to increase the incidence of aflatoxin contamination. Furthermore, the altered nutrient status of inadequately N-fertilized maize plants has been suggested to provide a better substrate for aflatoxin formation.
Another important necrotrophic pathogen of maize is the Ascomycota Cochliobolus heterostrophus (ana. Bipolaris maydis), which causes leaf blight. It is an important foliar disease of maize and is common in warm and humid areas around the world (Chen et al., Citation2018b). Three races of B. maydis are known, namely races O, C and T. Symptoms vary depending on the race of the infectious agent, although all cause various types of leaf lesions (Leonard, Citation1977). N fertilization does not affect leaf blight severity when fertilizer rates range from 60 to 180 kg N ha−1 (Junaid et al., Citation2009).
The fungal genus Gibberella (Ascomycota) includes several species of hemibiotrophic maize pathogens, such as the important Gibberella zeae (ana. Fusarium graminerarum), the causal agent of ear rot of maize (Bujold et al., Citation2001). G. zeae infection is highly problematic because of the production of mycotoxin during the infection process. As in wheat, the mycotoxins produced by Fusarium species in infected maize are deoxynivalenol and zearalenone (Ramirez et al., Citation2006). Another member of the Gibberella genus, Gibberella moniliformis (ana. Fusarium verticillioides), is also of concern in maize production. This fungus causes rots of the ear and stalk (Bacon et al., Citation2001). Additionally, G. moniliformis produces mycotoxins, such as fumonisins, which are a family of polyketide mycotoxins that are also produced by other Fusarium species (Munkvold and Desjardins, Citation1997). Fertilization with ammonium nitrate and urea at a rate of 0–400 kg N ha−1 shows an increase, a decrease or even no effect on disease severity (Reid et al., Citation2001; Blandino et al., Citation2008; Szulc et al., Citation2014; Abiodun et al., Citation2015; Abd-Rabboh et al., Citation2020). Variable results have been found not only between different studies but also within a study conducted at the same location over several years (Blandino et al., Citation2008). This indicates that external influences, such as the infection pressure and weather conditions have a greater impact on Fusarium sp. disease severity and mycotoxin production than N fertilization. In contrast, (Abiodun et al., Citation2015) have reported an increase in disease with the application of urea fertilizer and suggest that a higher N fertilizer rate can increase the incidence and severity of fungal diseases in maize. An interaction of plant vegetative development, plant microclimate and nutrient availability is necessary for pathogen multiplication. Furthermore, fertilization with urea promotes microorganisms such as Fusarium or Macrophomina, which are known to hydrolyze urea into carbon dioxide and ammonia (Ahmad, Citation1996). The increase in carbon dioxide also has an indirect effect on fungal growth, as it raises the temperature around the roots of the maize plant, thereby increasing the soluble sugar content, which can then be used as a substrate by phytopathogenic fungi such as Fusarium sp. (Abiodun et al., Citation2015).
Another Ascomycota hemibiotrophic pathogen, Mycosphaerella sp. (ana. Cercospora zeae-maydis) causes an important leaf disease of maize, known as grey leaf spot. The individual lesions of grey leaf spots are long and narrow and typically brown in color. Under favorable conditions, the number of lesions increases rapidly resulting in extensive necrosis of the entire leaf tissue (Beckman and Payne, Citation1982). Grey leaf spot disease severity increases following 60 and 120 kg N ha−1 N fertilization in the form of ammonium nitrate (Caldwell et al., Citation2002).
Toxin-producing fungal pathogens are of particular interest, as maize can withstand the natural infection pressure of most fungal diseases quite well, without major yield losses. Several factors, such as soil type, rate and form of fertilizer applied, weather conditions and crop management (tillage or nontillage practices, use of fungicides), seem to influence the effect of fertilization on the expression of mycotoxin-producing fungal pathogens such as Gibberella zeae and Petromyces flavus. In maize infected by Gibberella zeae, which mainly produces the mycotoxins deoxynivalenol and zearalenone, an increased N supply seems to have a favorable effect on disease progression, whereas Petromyces flavus, which produces the mycotoxin aflatoxin, is instead suppressed by N fertilization ().
Table 4. Biotrophic, necrotrophic and hemibiotrophic fungal diseases in Zea mays and the influence of mineral nitrogen fertilizer rate and form on disease infection in comparison with control.
VI. How does the chemical N-form affect plant diseases severity: nitrate vs. ammonium?
As elaborated in the previous chapters, the form of N influences the severity of several plant diseases. The form of N that can be considered beneficial or detrimental depends on many factors and is not the same for all host-parasite associations (Huber and Watson, Citation1974). To improve our understanding of these relationships, we need to comprehend exactly the way in which the major plant-available N-forms, namely nitrate and ammonium, affect various soil factors and plant defence mechanisms if we are to control specific diseases (Menzies, Citation1970; Huber, Citation1990).
For example, nitrate fertilization is known to promote cation uptake by the plant. In this context, high calcium concentrations have been found in shoot tissues and are needed, for example, in the pectin fractions of the cell wall and to help the plant to defend itself against pathogens. Additionally, Ca2+ sensors and their target proteins are part of defence-signaling pathways that interact downstream with effectors that modulate numerous biochemical and cellular functions in pathogen defence responses (Huber and Haneklaus, Citation2007; Maywald et al., Citation2022). Furthermore, nitrate supply is associated with increased nitric oxide (NO) formation, which plays an important role in many aspects of plant defence (Mur et al., Citation2017). For example, NO plays a role in the accumulation of phytoalexin biosynthesis and cell death during the hypersensitive response (Noritake et al., Citation1996; Durner et al., Citation1998; Mur et al., Citation2005). In the context of nitrate-mediated resistance, metabolite profiling also suggests the importance of polyamine production. For example, increased polyamine content in barley has been found to enhance resistance to powdery mildew when plants are fertilized with nitrate (Cowley and Walters, Citation2002a, Citation2002b; Gupta et al., Citation2013). Mechanistically, polyamines might increase resistance by acting as substrates for a NO-forming complex (Yamasaki and Cohen, Citation2006). Polyamines are also known to contribute to reactive oxygen species production and cell death (Cona et al., Citation2006) and to strengthen the cell wall through conjugation with hydroxycinnamic acid (Walters, Citation2003; Gupta et al., Citation2013).
Ammonium nutrition also possesses the ability to suppress certain diseases, such as take-all disease in wheat. This has been attributed to an acidification of the rhizosphere, which increases the availability of micronutrients, e.g. manganese (Huber and Haneklaus, Citation2007). As a cofactor of superoxide dismutase, manganese participates in the plant’s defence against oxidative stress. In addition, the lowering of the pH in the rhizosphere has been reported to lead to the suppression of certain pathogens in the soil by enriching disease-suppressing microorganisms associated with the roots. In particular, the suppression of take-all disease is attributed to Pseudomonas fluorescens strains, which can produce the antifungal polyketide 2,4-diacetylphlorogucinol (2,4-DAPG; (Raaijmakers and Weller, Citation1998; Raaijmakers et al., Citation1999; Weller et al., Citation2002; Ownley et al., Citation2003). However, ammonium nutrition is reported to increase the content of apoplastic sugar and amino acids and gamma-aminobutyric acid, thereby increasing the availability of nutrients to the invading pathogen (Gupta et al., Citation2013; Mur et al., Citation2017).
VII. Influence of N fertilization on soil microbiota: next-generation sequencing as a means of revealing soilborne pathogenic fungi?
In the last decade, novel molecular approaches have revolutionized the study of soil microbiota. DNA metabarcoding approaches, which include the amplification of specific taxonomically-informative gene markers via PCR with their subsequent sequencing by using next-generation sequencing (NGS) techniques (Taberlet et al., Citation2012; Francioli et al., Citation2021), have allowed us to improve our knowledge of soil bacteria and fungi in agriculture, highlighting their crucial role in cropping systems (Trivedi et al., Citation2016; Manoharan et al., Citation2017; Schmidt et al., Citation2019). In particular, DNA metabarcoding techniques have revealed that the plant rhizosphere (the plant-root interface) is inhabited by a myriad of different microbes (Torsvik et al., Citation2002; Delmont et al., Citation2014) and that such microbial networks are associated with the establishment of essential ecosystem services that crucially serve plant growth by alleviating the effects of pathogens and that interact with (a)biotic stresses (Syed Ab Rahman et al., Citation2018). However, microbial communities are highly dynamic in soil, as their composition and structure are deeply influenced by environmental factors affecting the soil-plant system. Indeed, short- and long-term N fertilization has been widely reported to have a strong effect on soil microbial community assemblage (Semenov et al., Citation2020; Windisch et al., 2020; Meier et al., Citation2021), with several studies demonstrating that particular N fertilization treatments can select, promote or reduce specific groups of beneficial or detrimental soil microorganisms (Francioli et al., Citation2016; Kavamura et al., Citation2018; Sommermann et al., Citation2022). For instance, fungal pathogen richness and diversity decreased in wheat and barley cropping systems treated with ammonium nitrate (regardless of N dosage) compared with unfertilized and organically fertilized systems (Soonvald et al., Citation2020). Likewise, ammonium nitrate input significantly reduced the relative abundance of the potential plant saprotrophs or pathogenic Stachybotrys, Acrocalymma, Achroiostachys, Arachnomyces, Setophoma and Periconia in a wheat cropping system (Zhai et al., Citation2022). An opposite trend was observed in a wheat system under long-term N fertilization in which urea addition for more than 20 years increased significantly the abundance of fungal taxa affiliated to the pathogenic genera Fusarium, regardless of the urea dosage applied (Chen et al., Citation2019). Similarly, the application of urea (120 kg N ha−1) over 30-years was also reported to increase plant pathogenic fungi in wheat cultivations compared with the unfertilized and organic fertilized treatments (Wang et al., Citation2018b). Conversely, a long-term fertilization study (37 years) revealed that repeated urea applications decreased the abundance of putative wheat pathogenic fungi Cochliobolus sativus and Dendryphion sp., whereas it increased beneficial fungal taxa affiliated with the phylum Glomeromycota (Wang et al., Citation2019). In maize cropping systems, urea ammonium nitrate (UAN) at a dosage of 112 kg N ha−1 showed reduced pathogenic Fusarium spp., whereas Mycosphaerella abundance was increased compared with that following organic fertilization (Wattenburger et al., Citation2019). In another long-term fertilization trial seeded with corn, a high dosage of UAN was shown to favor fungal taxa with plant pathogenic lifestyles, such as Microdochium, Periconia, Dactylonectria and Pseudopithomyces (Tosi et al., Citation2021). Urea application (180 kg N ha−1) led to a strong increase in the relative abundances of putative maize pathogenic genera such as Fusarium, Gibberella (the teleomorph of some Fusarium species) and Mycosphaerella in a 7-year maize fertilization experiment (Semenov et al., Citation2022). Likewise, in a longer-term fertilization experiment (35 years) with wheat-maize crop rotation, urea fertilization (150 kg N ha−1) enriched the soil with putative plant pathogenic fungi, such as Fusarium, Gibberella and Leptosphaerulina spp. (Ding et al., Citation2017). At a lower dosage (75 kg N ha−1) and in a similar agricultural context, urea was also found to increase the abundance of potential plant pathogenic fungi commonly found in cereal cropping systems (Ma et al., Citation2018a). In a 10-year fertilization experiment, urea fertilization (391 kg ha−1) increased the abundance of rice pathogens when compared with the unfertilized treatment (Wang et al., Citation2018a). An analogous trend was observed in a rice field receiving urea (103.5 kg N ha−1) for 34 years (Nie et al., Citation2018). Interestingly, a 5-year experiment showed that rice fields receiving urea application of 150 kg N ha−1 were characterized by a significant reduction of fungal plant pathogens, whereas under conditions of higher N fertilization (Urea, 300 kg N ha−1), enrichment of these detrimental fungal taxa occurred (Dong et al., Citation2021). In short-term experiments, ammonium, nitrate or cyanamide had minor effects on bacterial rhizosphere microbiomes, although ammonium-fertilized wheat was more susceptible to soilborne take-all and powdery mildew leaf disease. Fertilizer type-specific changes were, however, observed in the fungal microbiome depending on the chemical N-form (Maywald et al., Citation2022).
Although DNA metabarcoding represents a powerful approach for exploring the microbial biodiversity of environmental samples, we should mention that such methodology has several limitations, especially in assigning the attribute “fungal pathogen” to the fungal taxa detected. Indeed, the pathogenicity of a particular fungal taxon may depend on the host-fungus interaction and the environmental context (Gilbert and Parker, Citation2016) and, thus, the presence of a pathogenic fungal species does not necessarily imply pathogenicity. In short, the identification of a well-described active pathogenic fungal species by using DNA metabarcoding approaches may not be sufficient to infer pathogenic effects on plants, such effects having to be assessed by specific in vivo testing (Ruijven et al., Citation2020; Ampt et al., Citation2022). Therefore, new techniques have provided a novel in-depth view of well-known agricultural soilborne fungal pathogens across various agroecosystems (Cobo-Díaz et al., Citation2019a; Cobo-Díaz et al., Citation2019b; Kerdraon et al., Citation2019; Milazzo et al., Citation2021; Chen et al., Citation2022), we have to be careful when predicting the ecological presence of the fungal taxa in such production systems (Tedersoo et al., 2014; Nguyen et al., Citation2016). Accordingly, if those fungi that are actually acting as pathogens in agriculture, and specifically those in cereal farming, are to be understood, then considerably more studies are required in which the fungi are isolated, taxonomically characterized and tested for pathogenicity on the plant species present in the respective agricultural context under various (a)biotic conditions (e.g. Lofgren et al., Citation2018; Rojas et al., Citation2020; Maywald et al., Citation2022).
Nevertheless, these novel DNA-based approaches have provided important insights into the complex interactions that occur in the rhizosphere between soil microbes and plants and highlight the crucial roles played by several bacterial and fungal taxa in protecting plants from biotic stresses. Thus, published research have shown that, among the total diversity associated with plants, only a few microbes are indeed pathogenic, with most of them showing positive interactions and promoting plant survival and fitness (van Elsas et al., Citation2012; Mendes et al., Citation2013; Philippot et al., Citation2013). Plant microbiomes can directly act as antagonists of plant pathogens by producing antibiotics or secondary metabolites (Tyc et al., Citation2017; Liu and Brettell, Citation2019), modulating the physiology of pathogens (Chen et al., Citation2018a) and competing for nutrients and space (Raaijmakers and Mazzola, Citation2012; Caravaca et al., Citation2015). In addition, microbial communities can also prime plant defence genes, resulting in induced systemic resistance (Berendsen et al., Citation2012). Among the microbes that have been described as beneficial for plant growth, arbuscular mycorrhizal fungi (AMF) represent a group of fungi highly important for plant fitness and productivity in natural and agroecosystems. In addition to their crucial role in plant nutrition, whereby they can remarkably improve N and phosphorous uptake by plants (Clark and Zeto, Citation2000; Hodge and Fitter, Citation2010), AMF have been repeatedly documented to contribute to the plant’s ability to defend itself against pathogens and pests (Campos-Soriano et al., Citation2012; Veresoglou and Rillig, Citation2012; Murrell et al., Citation2015; Song et al., Citation2015; Pérez-de-Luque et al., Citation2017). In response to colonization by AMF, plants develop an enhanced defensive strategy known as mycorrhiza-induced resistance (MIR) (Cameron et al., Citation2013). AMF might suppress plant diseases and aid plants in pest warfare through induced systemic resistance (ISR) (Pozo et al., Citation2009; Jung et al., Citation2012). Furthermore, MIR shares features of pathogen-induced SAR and nonpathogenic rhizobacterium-ISR (Nishad et al., Citation2020). However, N fertilization alters significantly the composition and diversity of AMF, with distinct patterns in community assemblies being promoted by various types of N fertilizers and their dosage (Santos et al., Citation2006; Avio et al., Citation2013; Savolainen and Kytöviita, Citation2022). Several studies have found that the addition of N decreases the richness, diversity and abundance of AMF (Antoninka et al., Citation2011; Williams et al., Citation2017; Jach-Smith and Jackson, Citation2018; Ma et al., Citation2018b), whereas other research has detected no effects (Eom et al., Citation1999; van Diepen et al., Citation2011; Mueller and Bohannan, Citation2015) or positive effects instead (Jefwa et al., Citation2006; Porras-Alfaro et al., Citation2007; Zheng et al., Citation2014; Thirkell et al., Citation2019). These discrepancies in the effects of N input on AM fungal communities might be attributable to the differences in the host plants investigated, soil resource availability (N and P) and experimental variables (the form, amount and duration of N addition) (Han et al., Citation2020). Furthermore, cereal crops have been demonstrated to be mainly unresponsive to AMF when grown in soil with phosphorous concentrations in the range typically encountered in fertilized arable land (Kahiluoto et al., Citation2001; Verbruggen et al., Citation2013; Sarabia et al., Citation2017). Although these findings indicate that an optimal balance exists between fertilizer application and AMF-derived plant benefits, further research is needed to determine those agricultural management approaches that mitigate the reduction of root colonization by AMF under mineral fertilization.
In addition to AM fungi, other soil microbes have been reported to have a determinant role in eliciting plant defence and/or antagonistic capabilities against important cereal diseases. As mentioned above, Pseudomonas fluorescens strains have been found to suppress take-all disease induced by Gaeumannomyces graminis f. sp. tritici in wheat by producing the antifungal polyketide 2,4-diacetylphlorogucinol (Raaijmakers and Weller, Citation1998; Raaijmakers et al., Citation1999; Ownley et al., Citation2003). P. fluorescens can also suppress soilborne pathogens such as Meloidogyne incognita and Fusarium oxysporum by DAPG production (Meyer et al., Citation2016). Intriguingly, N deficiency seems to decrease root colonization by P. fluorescens, whereas ammonium nutrition increases root colonization by P. fluorescens when compared with nitrate treatment (Marschner et al., Citation1999). Strains of Bacillus subtilis are further examples of beneficial microbes with antagonist capabilities toward relevant agricultural fungal pathogens, e.g. against F. graminearum, the most common causal agent of Fusarium head blight, Fusarium foot rot and Fusarium root rot on cereals crops, by destroying the cellular structure of the pathogenic fungus, with such antifungal activity possibly being associated with the co-production of chitinase, fengycins and surfactins (Zhao et al., Citation2014; Zalila-Kolsi et al., Citation2016). B. subtilis has also been reported as a biocontrol agent against the fungal pathogen Rhizoctonia cerealis in wheat (Yi et al., Citation2022) and against Fusarium oxysporum, F. solani, F. verticillioides, Rhizoctonia solani, Phytophthora infestans and Penicillium italicum in maize (Cavaglieri et al., Citation2005; Ekundayo et al., Citation2011). N fertilization with ammonium nitrate and urea has been observed to increase the antagonistic activity of B. subtilis on crop pathogens in both greenhouse and field experiments and such beneficial effects increase with higher N dosage (Kayin et al., Citation2015; Santos et al., 2021; Pholthaweechai and Pengnoo, Citation2022).
Overall, molecular and novel DNA-based analyses are increasingly demonstrating the huge potential of soil microbes as biocontrol agents in combination with optimized N fertilization against several plant diseases in cereal cropping systems. Further exploration of the role of agricultural practices, such as chemical N fertilizer, on rhizosphere microbial community dynamics is therefore imperative if we are to improve cereal crop resistance to fungal pathogens.
VIII. Concluding remarks
Mineral nutrients, especially N, are applied to meet the potential needs of crops for their efficient production in an economical manner but without negative environmental consequences. However, nutrient requirements and uptake depend on various factors, such as the stage of plant growth, the availability of nutrients in the soil, the timing of application, microbial activity and the overall health of the crop. Because high N uptake promotes disease severity in most cases, irreversible N deficiencies need to be minimized without promoting pathogen activity through excessive N applications. Overall, the complex relationship between plant pathogens and the soil, rhizosphere microorganisms, environmental factors and the host is dynamic. Its variability negates the usefulness of generalizing the effect of N nutrition on pathogen occurrence. Indeed, it is noteworthy to mention that is quite difficult to draw precise conclusions on the specific effect of a particular N form on the interactions between cereal crops and fungal pathogens in agroecosystems, since these complex plant-microbiota interactions are influenced by a wide array of factors, such as cultivation practices, crop genotype, ecosystem type, topography and climate. Nonetheless, this review provides a basis for changing current agricultural N fertilization practices to reduce the severity of diseases by targeting and adapting applications to the pests that are problematic at a particular field site. The form in which N is applied also represents a promising opportunity for targeting N fertilization more specifically to individual pathogens, although few studies have examined the various types of N-containing fertilizer under comparable experimental conditions. In this regard, we need further investigations into the influence of the N form on pathogen infection and a better understanding of the interactions between the most severe cereal pathogens and plant defence mechanisms under various environmental conditions. Thus, the more selective application of N fertilizer has the great potential of enabling reductions to be made in the use of synthetic chemical pesticides, but without sacrificing yield and quality.
Additional information
Funding
References
- Abd-Rabboh, A. M. K., Ghazy, N. A., Awad, M. M., and Farahat, G. A. 2020. Effect of nitrogen fertilizer and foliar spraying with humic acid on productivity of maize, soybean and ear rot disease of maize. J. Plant Prod. 11:1045–1054. doi:10.21608/jpp.2020.122663
- Abiodun, M., Nafiu, A., and Osunlaja, S. 2015. Different rates of urea as nitrogen fertilizer affect root and stalk rot diseases of maize in South West Nigeria. IJPSS. 7:55–66. doi:10.9734/IJPSS/2015/13208
- Agrios, G. N. 2008. Plant Pathology. Elsevier Academic Press, Amsterdam.
- Ahmad, N., Ed. 1996. Nitrogen Economy in Tropical Soils. Springer Netherlands, Dordrecht.
- Ampt, E. A., Francioli, D., van Ruijven, J., Gomes, S. I. F., Maciá‐Vicente, J. G., Termorshuizen, A. J., Bakker, L. M., and Mommer, L. 2022. Deciphering the interactions between plant species and their main fungal root pathogens in mixed grassland communities. J. Ecol. 110:3039–3052. doi:10.1111/1365-2745.14012
- Anderson, H., Nehring, E., and Wichser, W. 1975. Aflatoxin contamination of corn in the field. J. Agric. Food Chem. 23:775–782. doi:10.1021/jf60200a014
- Antoni, E. A., Rybak, K., Tucker, M. P., Hane, J. K., Solomon, P. S., Drenth, A., Shankar, M., and Oliver, R. P. 2010. Ubiquity of ToxA and absence of ToxB in Australian populations of Pyrenophora tritici-repentis. Austral. Plant Pathol. 39:63–68. doi:10.1071/AP09056
- Antoninka, A., Reich, P. B., and Johnson, N. C. 2011. Seven years of carbon dioxide enrichment, nitrogen fertilization and plant diversity influence arbuscular mycorrhizal fungi in a grassland ecosystem. New Phytol. 192:200–214. doi:10.1111/j.1469-8137.2011.03776.x
- Ash, G. J., and Brown, J. F. 1991. Effect of nitrogen nutrition of the host on the epidemiology of Puccinia Striiformis F.sp. Tritici and crop yield in wheat. Austral. Plant Pathol. 20:108–114. doi:10.1071/APP9910108
- Aufhammer, W., Hermann, W., and Kubler, E. 1999. Fusarium (F. graminearum) infection of ears and toxin concentration of grains of winter wheat, triticale and rye depending on cultivars and production intensity. Pflanzenbauwissenschaften 3:32–39.
- Avio, L., Castaldini, M., Fabiani, A., Bedini, S., Sbrana, C., Turrini, A., and Giovannetti, M. 2013. Impact of nitrogen fertilization and soil tillage on arbuscular mycorrhizal fungal communities in a Mediterranean agroecosystem. Soil Biol. Biochem. 67:285–294. doi:10.1016/j.soilbio.2013.09.005
- Aydogdu, M., and Boyraz, N. 2011. Effects of nitrogen and organic fertilization on corn smut (Ustilago maydis (DC) Corda). African J. Agric. Res. 6:4539–4543.
- Ayyadurai, N., Kirubakaran, S. I., Srisha, S., and Sakthivel, N. 2005. Biological and molecular variability of Sarocladium oryzae, the sheath rot pathogen of rice (Oryza sativa L.). Curr. Microbiol. 50:319–323. doi:10.1007/s00284-005-4509-6
- Baba, S. H. A. 2019. Molecular response of wheat to Bipolaris sorokiniana under nitrogen stress, Newcastle University, pp 1–215.
- Bacon, C. W., Yates, I. E., Hinton, D. M., and Meredith, F. 2001. Biological control of Fusarium moniliforme in maize. Environ. Health Perspect. 109:325–332. doi:10.2307/3435026
- Bainbridge, A. 1974. Effect of nitrogen nutrition of the host on barley powdery mildew. Plant Pathol. 23: 160–161. doi:10.1111/j.1365-3059.1974.tb01842.x
- Ballini, E., Nguyen, T. T., and Morel, J.-B. 2013. Diversity and genetics of nitrogen-induced susceptibility to the blast fungus in rice and wheat. Rice 6: 13. doi:10.1186/1939-8433-6-32
- Beckman, P. M., and Payne, G. A. 1982. External growth, penetration, and development of Cercospora zeae-maydis in corn leaves. Phytopathology 72:810–815. doi:10.1094/Phyto-72-810
- Berendsen, R. L., Pieterse, C. M. J., and Bakker, P. A. H. M. 2012. The rhizosphere microbiome and plant health. Trends Plant Sci. 17: 478–486. doi:10.1016/j.tplants.2012.04.001
- Bhaskar, C. V., Rao, G. R., and Reddy, K. B. 2001. Effect of nitrogen and potassium nutrition on sheath rot incidence and phenol content in rice (Oryza sativa L.). Indian J.Plant Physiol. 6: 254–257.
- Bierman, S. M., Fitt, B. D. L., van den Bosch, F., Bateman, G. L., Jenkyn, J. F., and Welham, S. J. 2002. Changes in populations of the eyespot fungi Tapesia yallundae and T. acuformis under different fungicide regimes in successive crops of winter wheat, 1984-2000. Plant Pathol. 51: 191–201. doi:10.1046/j.1365-3059.2002.00673.x
- Blandino, M., Reyneri, A., and Vanara, F. 2008. Influence of nitrogen fertilization on mycotoxin contamination of maize kernels. Crop Prot. 27: 222–230. doi:10.1016/j.cropro.2007.05.008
- Bockus, W. W. 1979. Decline in Numbers and Inoculum Potential of Sclerotium oryzae in Field Soil. Phytopathology 69: 389–392. doi:10.1094/Phyto-69-389
- Bockus, W. W., and Davis, M. A. 1993. Effect of Nitrogen Fertilizers on Severity of Tan Spot of Winter Wheat. Plant Dis. 77: 508–510. doi:10.1094/PD-77-0508
- Boone, M. D., Bishop, C. A., Boswell, L. A., Brodman, R. D., Burger, J., Davidson, C., Gochfeld, M., Hoverman, J. T., Neuman-Lee, L. A., Relyea, R. A., Rohr, J. R., Salice, C., Semlitsch, R. D., Sparling, D., and Weir, S. 2014. Pesticide Regulation amid the Influence of Industry. BioScience 64: 917–922. doi:10.1093/biosci/biu138
- Brefort, T., Doehlemann, G., Mendoza-Mendoza, A., Reissmann, S., Djamei, A., and Kahmann, R. 2009. Ustilago maydis as a pathogen. Annu. Rev. Phytopathol. 47: 423–445. doi:10.1146/annurev-phyto-080508-081923
- Brennan, R. F. 1992a. Effect of superphosphate and nitrogen on yield and take-all of wheat. Fertilizer Res. 31: 43–49. doi:10.1007/BF01064226
- Brennan, R. F. 1992b. The role of manganese and nitrogen nutrition in the susceptibility of wheat plants to take-all in Western Australia. Fertilizer Res. 31: 35–41. doi:10.1007/BF01064225
- Brennan, R. F. 1993. Effect of ammonium chloride, ammonium sulphate, and sodium nitrate on take‐all and grain yield of wheat grown on soils in South‐western Australia. J. Plant Nutr. 16: 349–358. doi:10.1080/01904169309364536
- Brooks, S. A., Anders, M. M., and Yeater, K. M. 2010. Effect of furrow irrigation on the severity of false smut in susceptible rice varieties. Plant Dis. 94: 570–574. doi:10.1094/PDIS-94-5-0570
- Brooks, S. A., Anders, M. M., and Yeater, K. M. 2011. Influences from long-term crop rotation, soil tillage, and fertility on the severity of rice grain smuts. Plant Dis. 95: 990–996. doi:10.1094/PDIS-09-10-0689
- Broscious, S. C. 1985. Influence of winter wheat management practices on the severity of powdery mildew and septoria blotch in Pennsylvania. Phytopathology 75: 538–542. doi:10.1094/Phyto-75-538
- Bujold, I., Paulitz, T. C., and Carisse, O. 2001. Effect of Microsphaeropsis sp. on the production of perithecia and ascospores of Gibberella zeae. Plant Dis. 85: 977–984. doi:10.1094/PDIS.2001.85.9.977
- Bürling, K., Hunsche, M., and Noga, G. 2011. Use of blue-green and chlorophyll fluorescence measurements for differentiation between nitrogen deficiency and pathogen infection in winter wheat. J. Plant Physiol. 168: 1641–1648. doi:10.1016/j.jplph.2011.03.016
- Cal, A. d., Melgarejo, P., and Del Jimenez-Gasco, M. M. 2022. Editorial: necrotrophic fungal plant pathogens. Front. Plant Sci. 13: 839674. doi:10.3389/fpls.2022.839674
- Caldwell, P. M., Ward, J. M. J., Miles, N., and Laing, M. D. 2002. Assessment of the effects of fertilizer applications on gray leaf spot and yield in maize. Plant Dis. 86: 859–866. doi:10.1094/PDIS.2002.86.8.859
- Cameron, D. D., Neal, A. L., van Wees, S. C. M., and Ton, J. 2013. Mycorrhiza-induced resistance: more than the sum of its parts? Trends Plant Sci. 18: 539–545. doi:10.1016/j.tplants.2013.06.004
- Campos-Soriano, L., García-Martínez, J., and San Segundo, B. 2012. The arbuscular mycorrhizal symbiosis promotes the systemic induction of regulatory defence-related genes in rice leaves and confers resistance to pathogen infection. Mol. Plant Pathol. 13: 579–592. doi:10.1111/j.1364-3703.2011.00773.x
- Caravaca, F., Maboreke, H., Kurth, F., Herrmann, S., Tarkka, M. T., and Ruess, L. 2015. Synergists and antagonists in the rhizosphere modulate microbial communities and growth of Quercus robur L. Soil Biol. Biochem. 82: 65–73. doi:10.1016/j.soilbio.2014.12.004
- Carvalho, M. P., Rodrigues, F. A., Silveira, P. R., Andrade, C. C. L., Baroni, J. C. P., Paye, H. S., and Loureiro Junior, J. E. 2010. Rice resistance to brown spot mediated by nitrogen and potassium. J Phytopathol 158: 160–166. doi:10.1111/j.1439-0434.2009.01593.x
- Castiblanco, V., Castillo, H. E., and Miedaner, T. 2018. Candidate genes for aggressiveness in a natural Fusarium culmorum population greatly differ between wheat and rye head blight. JOF. 4: 14. doi:10.3390/jof4010014
- Cavaglieri, L., Orlando, J., Rodríguez, M. I., Chulze, S., and Etcheverry, M. 2005. Biocontrol of Bacillus subtilis against Fusarium verticillioides in vitro and at the maize root level. Res. Microbiol. 156: 748–754. doi:10.1016/j.resmic.2005.03.001
- Chakravarty, D. K., and Biswas, S. 1978. Estimation of yield loss in rice affected by sheath rot. Plant Dis. Rep. 62: 226–227.
- Chaurasia, P., and Duveiller, E. 2009. Management of leaf blight (Bipolaris sorokiniana) disease of wheat with cultural practices. Nepal Agric. Res. J. 7: 63–69. doi:10.3126/narj.v7i0.1870
- Chen, W., Radford, D., and Hambleton, S. 2022. Towards improved detection and identification of rust fungal pathogens in environmental samples using a metabarcoding approach. Phytopathology 112: 535–548. doi:10.1094/PHYTO-01-21-0020-R
- Chen, Y., Wang, J., Yang, N., Wen, Z., Sun, X., Chai, Y., and Ma, Z. 2018a. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat. Commun. 9: 3429. doi:10.1038/s41467-018-05683-7
- Chen, Y., Zhang, F., Tang, L., Zheng, Y., Li, Y., Christie, P., and Li, L. 2007. Wheat powdery mildew and foliar N concentrations as influenced by N fertilization and belowground interactions with intercropped faba bean. Plant Soil 291: 1–13. doi:10.1007/s11104-006-9161-9
- Chen, Y.-L., Mao, X.-W., Wang, J.-X., Wu, L.-Y., Zhou, M.-G., and Hou, Y.-P. 2018b. Activity of the dinitroaniline fungicide fluazinam against Bipolaris maydis. Pestic. Biochem. Physiol. 148: 8–15. doi:10.1016/j.pestbp.2018.03.005
- Chen, S., Waghmode, T. R., Sun, R., Kuramae, E. E., Hu, C., and Liu, B. 2019. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 7: 136. doi:10.1186/s40168-019-0750-2.
- Chien, C. C., and Huang, C. H. 2013. Relation between sheath rot and the sterility of rice plant. J. Agric. Res. China 28: 7–16.
- Cintas, N. A., and Webster, R. K. 2001. Effects of rice straw management on Sclerotium oryzae inoculum, stem rot severity, and yield of rice in California. Plant Dis. 85: 1140–1144. doi:10.1094/PDIS.2001.85.11.1140
- Clark, R. B., and Zeto, S. K. 2000. Mineral acquisition by arbuscular mycorrhizal plants. J. Plant Nutr. 23: 867–902. doi:10.1080/01904160009382068
- Cobo-Díaz, J. F., Baroncelli, R., Le Floch, G., and Picot, A. 2019a. A novel metabarcoding approach to investigate Fusarium species composition in soil and plant samples. FEMS Microbiol. Ecol. 95: 1–13. doi:10.1093/femsec/fiz084
- Cobo-Díaz, J. F., Baroncelli, R., Le Floch, G., and Picot, A. 2019b. Combined metabarcoding and co-occurrence network analysis to profile the bacterial, fungal and fusarium communities and their interactions in maize stalks. Front. Microbiol. 10: 261. doi:10.3389/fmicb.2019.00261
- Cohen, L., and Eyal, Z. 1993. The histology of processes associated with the infection of resistant and susceptible wheat cultivars with Septoria tritici. Plant Pathol. 42: 737–743. doi:10.1111/j.1365-3059.1993.tb01560.x
- Colbach, N., and Saur, L. 1998. Influence of crop management on eyespot development and infection cycles of winter wheat. Eur. J. Plant Pathol. 104: 37–48. doi:10.1023/A:1008673925979
- Cona, A., Rea, G., Angelini, R., Federico, R., and Tavladoraki, P. 2006. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 11: 80–88. doi:10.1016/j.tplants.2005.12.009
- Cowley, T., and Walters, D. R. 2002a. Polyamine metabolism in an incompatible interaction between barley and the powdery mildew fungus, Blumeria graminis f. sp. hordei. J. Phytopathol. 150: 581–586. doi:10.1046/j.1439-0434.2002.00816.x
- Cowley, T., and Walters, D. R. 2002b. Polyamine metabolism in barley reacting hypersensitively to the powdery mildew fungus Blumeria graminis f. sp. hordei. Plant, Cell Environ. 25: 461–468. doi:10.1046/j.0016-8025.2001.00819.x
- Cunfer, B. M. 2002. Powdery mildew. In Bread Wheat: Improvement and Production. Food and Agriculture Organization of the United Nations, Italy, Rome, pp 317–330.
- Curtis, B. C., Rajaram, S., and Macpherson, H. 2002. Bread Wheat: Improvement and Production. Food and Agriculture Organization of the United Nations, Italy, Rome.
- Damalas, C. A. 2009. Understanding benefits and risks of pesticide use. Sci. Res. Essay 4: 945–949.
- Danial, D. L., and Parlevliet, J. E. 1995. Effects of nitrogen fertilization on disease severity and infection type of yellow rust on wheat genotypes varying in quantitative resistance. J. Phytopathol. 143: 679–681. doi:10.1111/j.1439-0434.1995.tb00222.x
- Darwinkel, A. 1980. Grain production of winter wheat in relation to nitrogen and diseases, 1: relationship between nitrogen dressing and yellow rust infection. Zeitschrift fur Acker- und Pflanzenbau 149: 299–308.
- Darwish, W. S., Ikenaka, Y., Nakayama, S. M. M., and Ishizuka, M. 2014. An overview on mycotoxin contamination of foods in Africa. J. Vet. Med. Sci. 76: 789–797. doi:10.1292/jvms.13-0563
- Dasgupta, M. K. 1984. The Bengal famine, 1943 and the brown spot of rice–an inquiry into their relations. Hist. Agric. 2: 1–18.
- Datnoff, L. E., Elmer, W. H. and Huber, D. M., Eds. 2007. Mineral Nutrition and Plant Disease. APS Press American Phytopathological Soc, St. Paul, MN.
- Debona, D., Rodrigues, F. Á., Rios, J. A., and Nascimento, K. J. T. 2012. Biochemical changes in the leaves of wheat plants infected by Pyricularia oryzae. Phytopathology 102: 1121–1129. doi:10.1094/PHYTO-06-12-0125-R
- Delmont, T. O., Francioli, D., Jacquesson, S., Laoudi, S., Mathieu, A., Nesme, J., Ceccherini, M. T., Nannipieri, P., Simonet, P., and Vogel, T. M. 2014. Microbial community development and unseen diversity recovery in inoculated sterile soil. Biol. Fertil. Soils 50: 1069–1076. doi:10.1007/s00374-014-0925-8
- Desjardins, A. E., Plattner, R. D., and Nelson, P. E. 1997. Production of fumonisin B (inf1) and moniliformin by Gibberella fujikuroi from rice from various geographic areas. Appl. Environ. Microbiol. 63: 1838–1842. doi:10.1128/aem.63.5.1838-1842.1997
- Devadas, R., Simpfendorfer, S., Backhouse, D., and Lamb, D. W. 2014. Effect of stripe rust on the yield response of wheat to nitrogen. Crop J. 2: 201–206. doi:10.1016/j.cj.2014.05.002
- Devadas, S. K., Enyedi, A., and Raina, R. 2002. The Arabidopsis hrl1 mutation reveals novel overlapping roles for salicylic acid, jasmonic acid and ethylene signalling in cell death and defence against pathogens. Plant J. 30: 467–480. doi:10.1046/j.1365-313x.2002.01300.x
- Dietzel, K., Valle, D., Fierer, N., U'Ren, J. M., and Barberán, A. 2019. Geographical distribution of fungal plant pathogens in dust across the United States. Front. Ecol. Evol. 7: 304. doi:10.3389/fevo.2019.00304
- Ding, J., Jiang, X., Guan, D., Zhao, B., Ma, M., Zhou, B., Cao, F., Yang, X., Li, L., and Li, J. 2017. Influence of inorganic fertilizer and organic manure application on fungal communities in a long-term field experiment of Chinese Mollisols. Appl. Soil Ecol. 111: 114–122. doi:10.1016/j.apsoil.2016.12.003
- Ding, S., Shao, X., Li, J., Ahammed, G. J., Yao, Y., Ding, J., Hu, Z., Yu, J., and Shi, K. 2021. Nitrogen forms and metabolism affect plant defence to foliar and root pathogens in tomato. Plant. Cell Environ. 44: 1596–1610. doi:10.1111/pce.14019
- Doehlemann, G., Wahl, R., Vranes, M., de Vries, R.P., Kämper, J., and Kahmann, R. 2008. Establishment of compatibility in the Ustilago maydis/maize pathosystem. J. Plant Physiol. 165: 29–40. doi:10.1016/j.jplph.2007.05.016
- Dong, H., Fan, S., Sun, H., Chen, C., Wang, A., Jiang, L., and Ma, D. 2021. Rhizosphere-associated microbiomes of rice (Oryza sativa L.) under the effect of increased nitrogen fertilization. Front. Microbiol. 12: 730506. doi:10.3389/fmicb.2021.730506
- Dordas, C. 2008. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Dev. 28: 33–46. doi:10.1051/agro:2007051
- Duncan, K. E., and Howard, R. J. 2000. Cytological analysis of wheat infection by the leaf blotch pathogen Mycosphaerella graminicola. Mycol. Res. 104: 1074–1082. doi:10.1017/S0953756299002294
- Durner, J., Wendehenne, D., and Klessig, D. F. 1998. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. U S A. 95: 10328–10333. doi:10.1073/pnas.95.17.10328
- Duveiller, E., Kandel, Y. R., Sharma, R. C., and Shrestha, S. M. 2005. Epidemiology of foliar blights (spot blotch and tan spot) of wheat in the plains bordering the Himalayas. Phytopathology 95: 248–256. doi:10.1094/PHYTO-95-0248
- Duveiller, E., Singh, R. P., and Nicol, J. M. 2007. The challenges of maintaining wheat productivity: pests, diseases, and potential epidemics. Euphytica 157: 417–430. doi:10.1007/s10681-007-9380-z
- Dweba, C. C., Figlan, S., Shimelis, H. A., Motaung, T. E., Sydenham, S., Mwadzingeni, L., and Tsilo, T. J. 2017. Fusarium head blight of wheat: pathogenesis and control strategies. Crop Prot. 91: 114–122. doi:10.1016/j.cropro.2016.10.002
- Edgerton, M. D. 2009. Increasing crop productivity to meet global needs for feed, food, and fuel. Plant Physiol. 149: 7–13. doi:10.1104/pp.108.130195
- Ekundayo, E. A., Adetuyi, F. C., and Ekundayo, F. O. 2011. In vitro antifungal activities of bacteria associated with maize husks and cobs. Res. J. Microbiol. 6: 418–424. doi:10.3923/jm.2011.418.424
- Eom, A.-H., Hartnett, D. C., Wilson, G., and Figge, D. 1999. The effect of fire, mowing and fertilizer amendment on arbuscular mycorrhizas in tallgrass prairie. Am. Midland Nat. 142: 55–70. doi:10.1674/0003-0031(1999)142[0055:TEOFMA2.0.CO;2]
- Fauzi, M. T., and Paulitz, T. C. 1994. The effect of plant growth regulators and nitrogen on fusarium head blight of the spring wheat cultivar max. Plant Dis. 78: 289–292. doi:10.1094/PD-78-0289
- Fei, W., and Liu, Y. 2023. Biotrophic fungal pathogens: a critical overview. Appl. Biochem. Biotechnol. 195: 1–16. doi:10.1007/s12010-022-04087-0
- Fernandes, M. S., and Rossiello, R. O. P. 1995. Mineral nitrogen in plant physiology and plant nutrition. Crit. Rev. Plant Sci. 14: 111–148. doi:10.1080/07352689509701924
- Fernandez, M. R., DePauw, R. M., Clarke, J. M., and Lefkovitch, L. P. 1996. Red smudge in durum wheat reduces seedling vigour. Can. J. Plant Sci. 76: 321–324. doi:10.4141/cjps96-055
- Figueroa, M., Hammond-Kosack, K. E., and Solomon, P. S. 2018. A review of wheat diseases-a field perspective. Mol. Plant Pathol. 19: 1523–1536. doi:10.1111/mpp.12618
- Fitt, B. D. L., Goulds, A., and Polley, R. W. 1988. Eyespot (Pseudocercosporella herpotrichoides) epidemiology in relation to prediction of disease severity and yield loss in winter wheat? - A review. Plant Pathol. 37: 311–328. doi:10.1111/j.1365-3059.1988.tb02081.x
- Fleitas, M. C., Schierenbeck, M., Gerard, G. S., Dietz, J. I., Golik, S. I., Campos, P. E., and Simón, M. R. 2018a. How leaf rust disease and its control with fungicides affect dough properties, gluten quality and loaf volume under different N rates in wheat. J. Cereal Sci. 80: 119–127. doi:10.1016/j.jcs.2018.02.003
- Fleitas, M. C., Schierenbeck, M., Gerard, G. S., Dietz, J. I., Golik, S. I., and Simón, M. R. 2018b. Breadmaking quality and yield response to the green leaf area duration caused by fluxapyroxad under three nitrogen rates in wheat affected with tan spot. Crop Prot. 106: 201–209. doi:10.1016/j.cropro.2018.01.004
- Fones, H. N., Bebber, D. P., Chaloner, T. M., Kay, W. T., Steinberg, G., and Gurr, S. J. 2020. Threats to global food security from emerging fungal and oomycete crop pathogens. Nat. Food 1: 332–342. doi:10.1038/s43016-020-0075-0
- Fox, R. H., and Hoffman, L. D. 1981. The effect of N fertilizer source on grain yield, N uptake, soil pH, and lime requirement in no‐till corn. Agron. J. 73: 891–895. doi:10.2134/agronj1981.00021962007300050032x
- Francioli, D., Lentendu, G., Lewin, S., and Kolb, S. 2021. DNA metabarcoding for the characterization of terrestrial microbiota-pitfalls and solutions. Microorganisms 9: 361. doi:10.3390/microorganisms9020361
- Francioli, D., Schulz, E., Lentendu, G., Wubet, T., Buscot, F., and Reitz, T. 2016. Mineral vs. organic amendments: microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 7: 1446. doi:10.3389/fmicb.2016.01446
- Francioli, D., van Ruijven, J., Bakker, L., and Mommer, L. 2020. Drivers of total and pathogenic soil-borne fungal communities in grassland plant species. Fungal Ecol. 48: 100987. doi:10.1016/j.funeco.2020.100987
- Freeman, J., and Ward, E. 2004. Gaeumannomyces graminis, the take-all fungus and its relatives. Mol. Plant Pathol. 5: 235–252. doi:10.1111/j.1364-3703.2004.00226.x
- Gangopadhyay, S., and Chattopadhyay, S. B. 1975. Total silica and brown spot disease development of rice under varying levels of nitrogen. Curr. Sci. 44: 92–94.
- Gao, C., Montoya, L., Xu, L., Madera, M., Hollingsworth, J., Purdom, E., Singan, V., Vogel, J., Hutmacher, R. B., Dahlberg, J. A., Coleman-Derr, D., Lemaux, P. G., and Taylor, J. W. 2020. Fungal community assembly in drought-stressed sorghum shows stochasticity, selection, and universal ecological dynamics. Nat. Commun. 11: 34. doi:10.1038/s41467-019-13913-9
- Gebrel, E., Gad, M., and Farouk, M. 2019. Response of some wheat cultivars to different nitrogen fertilizer rates and their relation to rust diseases. Egypt. J. Agron. 42: 243–254. doi:10.21608/agro.2019.14921.1169
- Geisseler, D., and Scow, K. M. 2014. Long-term effects of mineral fertilizers on soil microorganisms – a review. Soil Biol. Biochem. 75: 54–63. doi:10.1016/j.soilbio.2014.03.023
- Gheorgies, C. 1974. Research concerning the influence of certain soil and crop factors upon the Septoria tritici leaf blotch of wheat. Lucràri stiintifice-Institutul Agronomic. Bucuresti Seria Agron. 15: 113–119.
- Gilbert, G. S., and Parker, I. M. 2016. The evolutionary ecology of plant disease: a phylogenetic perspective. Annu. Rev. Phytopathol. 54: 549–578. doi:10.1146/annurev-phyto-102313-045959
- Gilbert, G. S., and Webb, C. O. 2007. Phylogenetic signal in plant pathogen-host range. Proc. Natl. Acad. Sci. U S A. 104: 4979–4983. doi:10.1073/pnas.0607968104
- Gilland, B. 2002. World population and food supply. Food Policy 27: 47–63. doi:10.1016/S0306-9192(02)00002-7
- Giller, K. E., Delaune, T., Silva, J. V., Descheemaeker, K., van de Ven, G., Schut, A. G., van Wijk, M., Hammond, J., Hochman, Z., Taulya, G., Chikowo, R., Narayanan, S., Kishore, A., Bresciani, F., Teixeira, H. M., Andersson, J. A., and van Ittersum, M. K. 2021. The future of farming: who will produce our food? Food Sec. 13: 1073–1099. doi:10.1007/s12571-021-01184-6
- Goswami, R. S., and Kistler, H. C. 2004. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 5: 515–525. doi:10.1111/j.1364-3703.2004.00252.x
- Goutam, U., Kukreja, S., Yadav, R., Salaria, N., Thakur, K., and Goyal, A. K. 2015. Recent trends and perspectives of molecular markers against fungal diseases in wheat. Front. Microbiol. 6: 861. doi:10.3389/fmicb.2015.00861
- Grewal, S. K., and Kang, M. S. 1990. Influence of nitrogen fertilization on Fusarium sheath rot and yield of rice. Plant Dis. Res. 5: 47–52.
- Grybauskas, A. P., Their, A. L., and Sammons, D. J. 1988. Effect of chloride fertilizers on development of powdery mildew of winter wheat. Plant Dis. 72: 605–608. doi:10.1094/PD-72-0605
- Gunnell, P. S. 1984. Aggregate sheath spot of rice in California. Plant Dis. 68: 529. doi:10.1094/PD-68-529
- Guo, J., Shi, G., Kalil, A., Friskop, A., Elias, E., Xu, S. S., Faris, J. D., and Liu, Z. 2020. Pyrenophora tritici-repentis race 4 isolates cause disease on tetraploid wheat. Phytopathology 110: 1781–1790. doi:10.1094/PHYTO-05-20-0179-R
- Guo, Q., Kamio, A., Sharma, B. S., Sagara, Y., Arakawa, M., and Inagaki, K. 2006. Survival and subsequent dispersal of rice sclerotial disease fungi, Rhizoctonia oryzae and Rhizoctonia oryzae-sativae, in paddy fields. Plant Dis. 90: 615–622. doi:10.1094/PD-90-0615
- Guo, X., Li, Y., Fan, J., Li, L., Huang, F., and Wan, W. 2012. Progress in the study of false smut disease in rice. J. Agric. Sci. Technol. 2: 1211–1217.
- Gupta, K. J., Brotman, Y., Segu, S., Zeier, T., Zeier, J., Persijn, S. T., Cristescu, S. M., Harren, F. J. M., Bauwe, H., Fernie, A. R., Kaiser, W. M., and Mur, L. A. J. 2013. The form of nitrogen nutrition affects resistance against Pseudomonas syringae pv. phaseolicola in tobacco. J. Exp. Bot. 64: 553–568. doi:10.1093/jxb/ers348
- Han, Y., Feng, J., Han, M., and Zhu, B. 2020. Responses of arbuscular mycorrhizal fungi to nitrogen addition: a meta-analysis. Glob. Chang. Biol. 26: 7229–7241. doi:10.1111/gcb.15369
- Hane, J. K., Lowe, R. G. T., Solomon, P. S., Tan, K.-C., Schoch, C. L., Spatafora, J. W., Crous, P. W., Kodira, C., Birren, B. W., Galagan, J. E., Torriani, S. F. F., McDonald, B. A., and Oliver, R. P. 2007. Dothideomycete plant interactions illuminated by genome sequencing and EST analysis of the wheat pathogen Stagonospora nodorum. Plant Cell. 19: 3347–3368. doi:10.1105/tpc.107.052829
- Higgins, S., Fitt, B. D. L., and White, R. P. 1986. The development of eyespot (Pseudocercosporella herpotrichoides) leasions in winter wheat crops. J. Plant Dis. Prot. 93: 210–220.
- Hinzman, L., Bauer, M., and Daughtry, C. 1986. Effects of nitrogen fertilization on growth and reflectance characteristics of winter wheat. Remote Sens. Environ. 19: 47–61. doi:10.1016/0034-4257(86)90040-4
- Hodge, A., and Fitter, A. H. 2010. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. U S A. 107: 13754–13759. doi:10.1073/pnas.1005874107
- Hofer, K., Barmeier, G., Schmidhalter, U., Habler, K., Rychlik, M., Hückelhoven, R., and Hess, M. 2016. Effect of nitrogen fertilization on Fusarium head blight in spring barley. Crop Prot. 88: 18–27. doi:10.1016/j.cropro.2016.05.007
- Hollingsworth, C. R., Motteberg, C. D., Wiersma, J. V., and Atkinson, L. M. 2008. Agronomic and economic responses of spring wheat to management of fusarium head blight. Plant Dis. 92: 1339–1348. doi:10.1094/PDIS-92-9-1339
- Hornby, D. 1998. Take-all disease of cereals: a regional perspective. CAB International, Wallingford, UK.
- Hosford, R. M. 1982. Tan spot—developing knowledge 1902–1981, virulent races and wheat differentials, methodology, rating systems, other leaf diseases, literature. In Tan Spot of Wheat and Related Diseases Workshop, North Dakota Agricultural Experiment Station; Hosford, R. M., Ed. pp. 1–24.
- Howard, D. D., Chambers, A. Y., and Logan, J. 1994. Nitrogen and fungicide effects on yield components and disease severity in wheat. J. Prod. Agric. 7: 448–454. doi:10.2134/jpa1994.0448
- Huang, H., Nguyen Thi Thu, T., He, X., Gravot, A., Bernillon, S., Ballini, E., and Morel, J.-B. 2017. Increase of fungal pathogenicity and role of plant glutamine in nitrogen-induced susceptibility (NIS) to rice blast. Front. Plant Sci. 8: 265. doi:10.3389/fpls.2017.00265
- Huber, D. M. 1987. Amelioration of tan spot-infected wheat with nitrogen. Plant Dis. 71: 49–50. doi:10.1094/PD-71-0049
- Huber, D. M. 1990. Fertilizers and soil-borne diseases. Soil Use Manag. 6: 168–172. doi:10.1111/j.1475-2743.1990.tb00830.x
- Huber, D. M., and Haneklaus, S. 2007. Managing nutrition to control plant disease. Landbauforschung Volkenrode 57: 313–322.
- Huber, D. M., and Watson, R. D. 1974. Nitrogen form and plant disease. Annu. Rev. Phytopathol. 12: 139–165. doi:10.1146/annurev.py.12.090174.001035
- Hunter, T. 1989. Occurrence of Tapesia yallundae, teleomorph of Pseudocercosporella herpotrichoides, on unharvested wheat culms in England. Plant Pathol. 38: 598–603. doi:10.1111/j.1365-3059.1989.tb01457.x
- Jach-Smith, L. C., and Jackson, R. D. 2018. N addition undermines N supplied by arbuscular mycorrhizal fungi to native perennial grasses. Soil Biol. Biochem. 116: 148–157. doi:10.1016/j.soilbio.2017.10.009
- Jacquet, F., Jeuffroy, M.-H., Jouan, J., Le Cadre, E., Litrico, I., Malausa, T., Reboud, X., and Huyghe, C. 2022. Pesticide-free agriculture as a new paradigm for research. Agron. Sustain. Dev. 42: 24. doi:10.1007/s13593-021-00742-8
- Jefwa, J. M., Sinclair, R., and Maghembe, J. A. 2006. Diversity of glomale mycorrhizal fungi in maize/Sesbania intercrops and maize monocrop systems in Southern Malawi. Agroforest. Syst. 67: 107–114. doi:10.1007/s10457-004-2370-4
- Jensen, B., and Munk, L. 1997. Nitrogen‐induced changes in colony density and spore production of Erysiphe graminis f.sp. hordei on seedlings of six spring barley cultivars. Plant Pathol. 46: 191–202. doi:10.1046/j.1365-3059.1997.d01-224.x
- Jones, R., and Duncan, H. 1981. Effect of nitrogen fertilizer, planting date, and harvest date on aflatoxin production in corn inoculated with Aspergillus flavus. Plant Dis. 65: 741–744. doi:10.1094/PD-65-741
- Joshi, A. K., Ortiz-Ferrara, G., Crossa, J., Singh, G., Alvarado, G., Bhatta, M. R., Duveiller, E., Sharma, R. C., Pandit, D. B., Siddique, A. B., Das, S. Y., Sharma, R. N., and Chand, R. 2007. Associations of environments in South Asia based on spot blotch disease of wheat caused by Cochliobolus sativus. Crop Sci. 47: 1071–1081. doi:10.2135/cropsci2006.07.0477
- Junaid, M., Khan, H., Ali, A., Ahmad, M., and Raziq, F. 2009. Response of various maize cultivars to different levels of Nitrogen against Bipolaris maydis shoemaker under natural epiphytotic conditions. Sarhad J. Agric 25: 243–249.
- Jung, S. C., Martinez-Medina, A., Lopez-Raez, J. A., and Pozo, M. J. 2012. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 38: 651–664. doi:10.1007/s10886-012-0134-6
- Kahiluoto, H., Ketoja, E., Vestberg, M., and Saarela, I. 2001. Promotion of AM utilization through reduced P fertilization 2. Field studies. Plant Soil 231: 65–79. doi:10.1023/A:1010366400009
- Kavamura, V. N., Hayat, R., Clark, I. M., Rossmann, M., Mendes, R., Hirsch, P. R., and Mauchline, T. H. 2018. Inorganic nitrogen application affects both taxonomical and predicted functional structure of wheat rhizosphere bacterial communities. Front. Microbiol. 9: 1074. doi:10.3389/fmicb.2018.01074
- Kayin, G. B., Öztüfekci, S., Akin, H. F., Karaata, E. U., Katkat, A. V., and Turan, A. T. 2015. Effect of Bacillus subtilis Ch-13, nitrogen and phosphorus on yield, protein and gluten content of wheat (Triticum aestivum L.). Uludağ Üniversitesi Ziraat Fakültesi Dergisi 29: 19–29.
- Kazan, K., Gardiner, D. M., and Manners, J. M. 2012. On the trail of a cereal killer: recent advances in Fusarium graminearum pathogenomics and host resistance. Mol. Plant Pathol. 13: 399–413. doi:10.1111/j.1364-3703.2011.00762.x
- Keim, R., and Webster, R. K. 1973. Nitrogen fertilization and severity of stem rot of rice. Phytopathology 64: 178–183.
- Kema, G. H., Yu, D., Rijkenberg, F. H., Shaw, M. W., and Baayen, R. P. 1996. Histology of the pathogenesis of Mycosphaerella graminicola in wheat. Phytopathology 86: 777–786. doi:10.1094/Phyto-86-777
- Kerdraon, L., Barret, M., Laval, V., and Suffert, F. 2019. Differential dynamics of microbial community networks help identify microorganisms interacting with residue-borne pathogens: the case of Zymoseptoria tritici in wheat. Microbiome 7: 17. doi:10.1186/s40168-019-0736-0
- Khan, M. H., Bukhari, A., Dar, Z. A., and Rizvi, S. M. 2013. Status and strategies in breeding for rust resistance in wheat. Agric. Sci. 04: 292–301. doi:10.4236/as.2013.46042
- Khush, G. S., and Virk, P. S. 2002. Rice improvement: past, present, and future. In Crop Improvement: Challenges in the Twenty-First Century; Kang, M. S. Ed. Food Products Press, New York, pp 17–42.
- Kim, J. Y., Wu, J., Kwon, S. J., Oh, H., Lee, S. E., Kim, S. G., Wang, Y., Agrawal, G. K., Rakwal, R., Kang, K. Y., Ahn, I.-P., Kim, B.-G., and Kim, S. T. 2014. Proteomics of rice and Cochliobolus miyabeanus fungal interaction: insight into proteins at intracellular and extracellular spaces. Proteomics (Eng). 14: 2307–2318. doi:10.1002/pmic.201400066
- Knott, D. R. 1989. The Wheat Rusts - Breeding for Resistance. Springer, Berlin Heidelberg, Berlin, Heidelberg.
- Koeck, M., Hardham, A. R., and Dodds, P. N. 2011. The role of effectors of biotrophic and hemibiotrophic fungi in infection. Cell. Microbiol. 13: 1849–1857. doi:10.1111/j.1462-5822.2011.01665.x
- Koiso, Y., Li, Y., Iwasaki, S., Hanaoka, K., Kobayashi, T., Sonoda, R., Fujita, Y., Yaegashi, H., and Sato, Z. 1994. Ustiloxins, antimitotic cyclic peptides from false smut balls on rice panicles caused by Ustilaginoidea virens. J. Antibiot. (Tokyo) 47: 765–773. doi:10.7164/antibiotics.47.765
- Kostandi, S. F., and Soliman, M. F. 1991. Effect of nitrogen rates at different growth stages on corn yield and common smut disease [Ustilago maydis (D.C.) Corda]. J. Agron. Crop Sci. 167: 53–60. doi:10.1111/j.1439-037X.1991.tb00933.x
- Kostandi, S. F., and Soliman, M. F. 1997. Smut disease incidence and mineral composition of corn as affected by N fertilizer sources and K application rates. J. Agron. Crop Sci. 178: 197–204. doi:10.1111/j.1439-037X.1997.tb00491.x
- Kostandi, S. F., Soliman, M. F., and Ghaly, A. A. 1997. Smut disease and yield performance in corn (Zea mays L.) as influenced by nitrapyrin, urea and zinc applications in coarse-textured soils. J. Agron. Crop Sci. 179: 219–226. doi:10.1111/j.1439-037X.1997.tb00520.x
- Krupinsky, J. M., Halvorson, A. D., Tanaka, D. L., and Merrill, S. D. 2007. Nitrogen and tillage effects on wheat leaf spot diseases in the Northern Great Plains. Agron. J. 99: 562–569. doi:10.2134/agronj2006.0263
- Krupinsky, J. M., and Tanaka, D. L. 2001. Leaf spot diseases on winter wheat influenced by nitrogen, tillage, and haying after a grass-alfalfa mixture in the conservation reserve program. Plant Dis. 85: 785–789. doi:10.1094/PDIS.2001.85.7.785
- Kudsk, P., and Mathiassen, S. K. 2020. Pesticide regulation in the European Union and the glyphosate controversy. Weed Sci. 68: 214–222. doi:10.1017/wsc.2019.59
- Kwak, Y.-S., and Weller, D. M. 2013. Take-all of wheat and natural disease suppression: a review. Plant Pathol. J. 29: 125–135. doi:10.5423/PPJ.SI.07.2012.0112
- Lamari, L., and Bernier, C. C. 1991. Genetics of tan necrosis and extensive chlorosis in tan spot of wheat caused by Pyrenophora tritici-repentis. Phytopathology 81: 1092–1095. doi:10.1094/Phyto-81-1092
- Last, F. T. 1953. Some effects of temperature and nitrogen supply on wheat powdery mildew. Ann. Appl. Biol. 40: 312–322. doi:10.1111/j.1744-7348.1953.tb01085.x
- Luo, L., Zhang, Y., and Xu, G. 2020. How does nitrogen shape plant architecture? J. Exp. Bot. 71: 4415–4427. doi:10.1093/jxb/eraa187
- Lefevere, H., Bauters, L., and Gheysen, G. 2020. Salicylic acid biosynthesis in plants. Front. Plant Sci. 11: 338. doi:10.3389/fpls.2020.00338
- Leitch, M. H., and Jenkins, P. D. 1995. Influence of nitrogen on the development of Septoria epidemics in winter wheat. J. Agric. Sci. 124: 361–368. doi:10.1017/S0021859600073329
- Leite, M., Freitas, A., Silva, A. S., Barbosa, J., and Ramos, F. 2021. Maize food chain and mycotoxins: a review on occurrence studies. Trends Food Sci. Technol. 115: 307–331. doi:10.1016/j.tifs.2021.06.045
- Lemmens, M., Haim, K., Lew, H., and Ruckenbauer, P. 2004. The effect of nitrogen fertilization on fusarium head blight development and deoxynivalenol contamination in wheat. J. Phytopathol. 152: 1–8. doi:10.1046/j.1439-0434.2003.00791.x
- Leonard, K. J. 1977. Virulence, temperature optima, and competitive abilities of isolines of races T and 0 of Bipolaris maydis. Phytopathology 67: 1273–1279. doi:10.1094/Phyto-67-1273
- Li, D., Tang, Q., Zhang, Y., Qin, J., Li, H., Chen, L., Yang, S., Zou, Y., and Peng, S. 2012. Effect of nitrogen regimes on grain yield, nitrogen utilization, radiation use efficiency, and sheath blight disease intensity in super hybrid rice. J. Integr. Agric. 11: 134–143. doi:10.1016/S1671-2927(12)60791-3
- Linquist, B. A., Byous, E., Jones, G., Williams, J. F., Six, J., Horwath, W., and van Kessel, C. 2008. Nitrogen and potassium fertility impacts on aggregate sheath spot disease and yields of rice. Plant Prod. Sci. 11: 260–267. doi:10.1626/pps.11.260
- Liu, H., and Brettell, L. E. 2019. Plant defense by VOC-induced microbial priming. Trends Plant Sci. 24: 187–189. doi:10.1016/j.tplants.2019.01.008
- Lofgren, L. A., LeBlanc, N. R., Certano, A. K., Nachtigall, J., LaBine, K. M., Riddle, J., Broz, K., Dong, Y., Bethan, B., Kafer, C. W., and Kistler, H. C. 2018. Fusarium graminearum: pathogen or endophyte of North American grasses? New Phytol. 217: 1203–1212. doi:10.1111/nph.14894
- Long, D. H., Lee, F. N., and TeBeest, D. O. 2000. Effect of nitrogen fertilization on disease progress of rice blast on susceptible and resistant cultivars. Plant Dis. 84: 403–409. doi:10.1094/PDIS.2000.84.4.403
- Lorrain, C., Gonçalves Dos Santos, K. C., Germain, H., Hecker, A., and Duplessis, S. 2019. Advances in understanding obligate biotrophy in rust fungi. New Phytol. 222: 1190–1206. doi:10.1111/nph.15641
- Lucas, J. A., Dyer, P. S., and Murray, T. D. 2000. Pathogenicity, host-specificity, and population biology of Tapesia spp., causal agents of eyespot disease of cereals. In Advances in Botanical Research; Callow, J. A. Eds. Academic Press: San Diego, CA, pp 225–258.
- Luo, C., Ma, L., Zhu, J., Guo, Z., Dong, K., and Dong, Y. 2021. Effects of nitrogen and intercropping on the occurrence of wheat powdery mildew and stripe rust and the relationship with crop yield. Front. Plant Sci. 12: 637393. doi:10.3389/fpls.2021.637393
- Ma, M., Jiang, X., Wang, Q., Ongena, M., Wei, D., Ding, J., Guan, D., Cao, F., Zhao, B., and Li, J. 2018a. Responses of fungal community composition to long-term chemical and organic fertilization strategies in Chinese Mollisols. MicrobiologyOpen 7: 1–12. doi:10.1002/mbo3.597
- Ma, M., Ongena, M., Wang, Q., Guan, D., Cao, F., Jiang, X., and Li, J. 2018b. Chronic fertilization of 37 years alters the phylogenetic structure of soil arbuscular mycorrhizal fungi in Chinese Mollisols. AMB Express 8: 10. doi:10.1186/s13568-018-0587-2
- Maity, S. S., and Sanyal, R. P. 2002. Effect of inorganic nutrients on leaf blight severity in wheat caused by Helminthosporium sativum. Ann. Plant Prot. Sci. 10: 106–110.
- Manoharan, L., Kushwaha, S. K., Ahrén, D., and Hedlund, K. 2017. Agricultural land use determines functional genetic diversity of soil microbial communities. Soil Biol. Biochem. 115: 423–432. doi:10.1016/j.soilbio.2017.09.011
- Marschner, P., Gerendás, J., and Sattelmacher, B. 1999. Effect of N concentration and N source on root colonization by Pseudomonas fluorescens 2-79RLI. Plant Soil 215: 135–141. doi:10.1023/A:1004373007606
- Martínez, S. 2021. Stem rot management by nitrogen and potassium fertilization and effect on grain yield and quality of rice in Uruguay. Can. J. Plant Pathol. 43: 783–793. doi:10.1080/07060661.2021.1922939
- Maywald, N. J., Mang, M., Pahls, N., Neumann, G., Ludewig, U., and Francioli, D. 2022. Ammonium fertilization increases the susceptibility to fungal leaf and root pathogens in winter wheat. Front. Plant Sci. 13: 946584. doi:10.3389/fpls.2022.946584
- Meier, M. A., Lopez-Guerrero, M. G., Guo, M., Schmer, M. R., Herr, J. R., Schnable, J. C., Alfano, J. R., and Yang, J. 2021. Rhizosphere microbiomes in a historical maize-soybean rotation system respond to host species and nitrogen fertilization at the genus and subgenus levels. Appl. Environ. Microbiol. 87: 1–13. doi:10.1128/AEM.03132-20
- Mendes, R., Garbeva, P., and Raaijmakers, J. M. 2013. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 37: 634–663. doi:10.1111/1574-6976.12028
- Menzies, J. D., et al. 1970. Factors affecting plant pathogen populations in soil. In Root Diseases and Soil-Borne Pathogens; Toussoun, T. A., Eds. University of Califonia Press: Berkeley, pp 16–21.
- Meyer, S. L. F., Everts, K. L., Gardener, B. M., Masler, E. P., Abdelnabby, H. M. E., and Skantar, A. M. 2016. Assessment of DAPG-producing Pseudomonas fluorescens for management of meloidogyne incognita and Fusarium oxysporum on Watermelon. J. Nematol. 48: 43–53.
- Milazzo, C., Zulak, K. G., Muria-Gonzalez, M. J., Jones, D., Power, M., Bransgrove, K., Bunce, M., and Lopez-Ruiz, F. J. 2021. High-throughput metabarcoding characterizes fungal endophyte diversity in the phyllosphere of a barley crop. Phytobiomes J. 5: 316–325. doi:10.1094/PBIOMES-09-20-0066-R
- Miller, J. D. 2008. Mycotoxins in small grains and maize: old problems, new challenges. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 25: 219–230. doi:10.1080/02652030701744520
- Miller, T. C., and Webster, R. K. 2001. Soil sampling techniques for determining the effect of cultural practices on Rhizoctonia oryzae-sativae inoculum in rice field soils. Plant Dis. 85: 967–972. doi:10.1094/PDIS.2001.85.9.967
- Motallebi, P., Niknam, V., Ebrahimzadeh, H., Hashemi, M., and Enferadi, S. T. 2017. Exogenous methyl jasmonate treatment induces defense response against Fusarium culmorum in wheat seedlings. J. Plant Growth Regul. 36: 71–82. doi:10.1007/s00344-016-9620-3
- Mueller, R. C., and Bohannan, B. J. M. 2015. Shifts in the phylogenetic structure of arbuscular mycorrhizal fungi in response to experimental nitrogen and carbon dioxide additions. Oecologia 179: 175–185. doi:10.1007/s00442-015-3337-z
- Munkvold, G. P., and Desjardins, A. E. 1997. Fumonisins in maize: can we reduce their occurrence? Plant Dis. 81: 556–565. doi:10.1094/PDIS.1997.81.6.556
- Mur, L. A. J., Santosa, I. E., Laarhoven, L. J. J., Holton, N. J., Harren, F. J. M., and Smith, A. R. 2005. Laser photoacoustic detection allows in planta detection of nitric oxide in tobacco following challenge with avirulent and virulent Pseudomonas syringae Pathovars. Plant Physiol. 138: 1247–1258. doi:10.1104/pp.104.055772
- Mur, L. A. J., Simpson, C., Kumari, A., Gupta, A. K., and Gupta, K. J. 2017. Moving nitrogen to the centre of plant defence against pathogens. Ann. Bot. 119: 703–709. doi:10.1093/aob/mcw179
- Murrell, E. G., Hanson, C. R., and Cullen, E. M. 2015. European corn borer oviposition response to soil fertilization practices and arbuscular mycorrhizal colonization of corn. Ecosphere 6: 1–12. doi:10.1890/ES14-00501.1
- Mutiga, S. K., Morales, L., Angwenyi, S., Wainaina, J., Harvey, J., Das, B., and Nelson, R. J. 2017. Association between agronomic traits and aflatoxin accumulation in diverse maize lines grown under two soil nitrogen levels in Eastern Kenya. Field Crops Res. 205: 124–134. doi:10.1016/j.fcr.2017.02.007
- Nakata, K., and Kawamura, E. 1939. Studies on rice sclerotial diseases. Mat. Agric. Improv. Agric. For. Min. 139: 1–176.
- Neumann, S., Paveley, N. D., Beed, F. D., and Sylvester-Bradley, R. 2004. Nitrogen per unit leaf area affects the upper asymptote of Puccinia striiformis f.sp. tritici epidemics in winter wheat. Plant Pathol. 53: 725–732. doi:10.1111/j.1365-3059.2004.01107.x
- Nguyen, N. H., Song, Z., Bates, S. T., Branco, S., Tedersoo, L., Menke, J., Schilling, J. S., and Kennedy, P. G. 2016. FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 20: 241–248. doi:10.1016/j.funeco.2015.06.006
- Nie, S., Lei, X., Zhao, L., Brookes, P. C., Wang, F., Chen, C., Yang, W., and Xing, S. 2018. Fungal communities and functions response to long-term fertilization in paddy soils. Appl. Soil Ecol. 130: 251–258. doi:10.1016/j.apsoil.2018.06.008
- Nishad, R., Ahmed, T., Rahman, V. J., and Kareem, A. 2020. Modulation of plant defense system in response to microbial interactions. Front. Microbiol. 11: 1298. doi:10.3389/fmicb.2020.01298
- Noritake, T., Kawakita, K., and Doke, N. 1996. Nitric oxide induces phytoalexin accumulation in potato tuber tissues. Plant Cell Physiol. 37: 113–116. doi:10.1093/oxfordjournals.pcp.a028908
- O'Donnell, K., Ward, T. J., Geiser, D. M., Corby Kistler, H., and Aoki, T. 2004. Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genet. Biol. 41: 600–623. doi:10.1016/j.fgb.2004.03.003
- Oerke, E.-C., and Dehne, H.-W. 2004. Safeguarding production—losses in major crops and the role of crop protection. Crop Prot. 23: 275–285. doi:10.1016/j.cropro.2003.10.001
- Oliver, R. P., and Ipcho, S. V. S. 2004. Arabidopsis pathology breathes new life into the necrotrophs-vs.-biotrophs classification of fungal pathogens. Mol. Plant Pathol. 5: 347–352. doi:10.1111/j.1364-3703.2004.00228.x
- Orton, E. S., Deller, S., and Brown, J. K. M. 2011. Mycosphaerella graminicola: from genomics to disease control. Mol. Plant Pathol. 12: 413–424. doi:10.1111/j.1364-3703.2010.00688.x
- Ownley, B. H., Duffy, B. K., and Weller, D. M. 2003. Identification and manipulation of soil properties to improve the biological control performance of phenazine-producing Pseudomonas fluorescens. Appl. Environ. Microbiol. 69: 3333–3343. doi:10.1128/AEM.69.6.3333-3343.2003
- Parry, M., Rosenzweig, C., Iglesias, A., Livermore, M., and Fischer, G. 2004. Effects of climate change on global food production under SRES emissions and socio-economic scenarios. Global Environ. Change 14: 53–67. doi:10.1016/j.gloenvcha.2003.10.008
- Pérez-de-Luque, A., Tille, S., Johnson, I., Pascual-Pardo, D., Ton, J., and Cameron, D. D. 2017. The interactive effects of arbuscular mycorrhiza and plant growth-promoting rhizobacteria synergistically enhance host plant defences against pathogens. Sci. Rep. 7: 10. doi:10.1038/s41598-017-16697-4
- Perfect, S. E., and Green, J. R. 2001. Infection structures of biotrophic and hemibiotrophic fungal plant pathogens. Mol. Plant Pathol. 2: 101–108. doi:10.1046/j.1364-3703.2001.00055.x
- Petti, C., Reiber, K., Ali, S. S., Berney, M., and Doohan, F. M. 2012. Auxin as a player in the biocontrol of Fusarium head blight disease of barley and its potential as a disease control agent. BMC Plant Biol. 12: 9. doi:10.1186/1471-2229-12-224
- Philippot, L., Raaijmakers, J. M., Lemanceau, P., and van der Putten, W. H. 2013. Going back to the roots: the microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 11: 789–799. doi:10.1038/nrmicro3109
- Pholthaweechai, U., and Pengnoo, A. 2022. Effect of nitrogen and Bacillus subtilis SM1 strain on controlling Rigidoporus microporus NK6 strain the cause of white root rot disease in vitro testing. Songklanakarin J. Plant Sci. 8: 44–49.
- Piening, L. J. 1972. Effects of leaf rust on nitrate in rye. Can. J. Plant Sci. 52: 842–843. doi:10.4141/cjps72-139
- Pimentel, D., Acquay, H., Biltonen, M., Rice, P., Silva, M., Nelson, J., Lipner, V., Giordano, S., Horowitz, A., and D'Amore, M. 1992. Environmental and economic costs of pesticide use. BioScience 42: 750–760. doi:10.2307/1311994
- Pogăcean, M. O., and Gavrilescu, M. 2009. Plant protection products and their sustainable and environmentally friendly use. Environ. Eng. Manag. 8: 608–627.
- Porras-Alfaro, A., Herrera, J., Natvig, D. O., and Sinsabaugh, R. L. 2007. Effect of long-term nitrogen fertilization on mycorrhizal fungi associated with a dominant grass in a semiarid grassland. Plant Soil 296: 65–75. doi:10.1007/s11104-007-9290-9
- Pozo, M. J., Verhage, A., García-Andrade, J., García, J. M., Azcón-Aguilar, C. 2009. Priming plant defence against pathogens by arbuscular mycorrhizal fungi. In Mycorrhizas - Functional Processes and Ecological Impact; edited by Azcón-Aguilar, C., Eds. Springer Berlin Heidelberg: Berlin, Heidelberg, pp 123–135.
- Prew, R. D., Church, B. M., Dewar, A. M., Lacey, J., Penny, A., Plumb, R. T., Thorne, G. N., Todd, A. D., and Williams, T. D. 1983. Effects of eight factors on the growth and nutrient uptake of winter wheat and on the incidence of pests and diseases. J. Agric. Sci. 100: 363–382. doi:10.1017/S0021859600033529
- Raaijmakers, J. M., Bonsall, R. F., and Weller, D. M. 1999. Effect of population density of Pseudomonas fluorescens on production of 2,4-diacetylphloroglucinol in the rhizosphere of wheat. Phytopathology 89: 470–475. doi:10.1094/PHYTO.1999.89.6.470
- Raaijmakers, J. M., and Mazzola, M. 2012. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu. Rev. Phytopathol. 50: 403–424. doi:10.1146/annurev-phyto-081211-172908
- Raaijmakers, J. M., and Weller, D. M. 1998. Natural plant protection by 2,4-diacetylphloroglucinol-producing Pseudomonas spp. in take-all decline soils. MPMI 11: 144–152. doi:10.1094/MPMI.1998.11.2.144
- Raemaekers, R. H., and Tinline, R. D. 1981. Epidemic of diseases caused by Cochliobolus sativus on rainfed wheat in Zambia. Can. J. Plant Pathol. 3: 211–214. doi:10.1080/07060668109501350
- Ramirez, M. L., Reynoso, M. M., Farnochi, M. C., and Chulze, S. 2006. Vegetative compatibility and mycotoxin chemotypes among Fusarium graminearum (Gibberella zeae) isolates from wheat in Argentina. Eur. J. Plant Pathol. 115: 139–148. doi:10.1007/s10658-006-0009-1
- Rani, R., Sharma, V. K., Pannu, P. P. S., and Lore, J. S. 2015. Influence of nitrogen fertilizer dose on false smut of rice (Oryza sativa) caused by Ustilaginoidea virens. Indian J. Agric. Sci. 85: 19–22.
- Rees, R. G., Platz, G. J., and Mayer, R. J. 1982. Yield losses in wheat from yellow spot: comparison of estimates derived from single tillers and plots. Aust. J. Agric. Res. 33: 899–908. doi:10.1071/AR9820899
- Reid, L. M., Zhu, X., and Ma, B. L. 2001. Crop rotation and nitrogen effects on maize susceptibility to gibberella (Fusarium graminearum) ear rot. Plant Soil 237: 1–14. doi:10.1023/A:1013311703454
- Roelfs, A. P. 1989. Epidemiology of the cereal rusts in North America. Can. J. Plant Pathol. 11: 86–90. doi:10.1080/07060668909501153
- Rojas, E. C., Sapkota, R., Jensen, B., Jørgensen, H. J. L., Henriksson, T., Jørgensen, L. N., Nicolaisen, M., and Collinge, D. B. 2020. Fusarium head blight modifies fungal endophytic communities during infection of wheat spikes. Microb. Ecol. 79: 397–408. doi:10.1007/s00248-019-01426-3
- Różewicz, M., Wyzińska, M., and Grabiński, J. 2021. The most important fungal diseases of cereals—problems and possible solutions. Agronomy 11: 714. doi:10.3390/agronomy11040714
- Ruijven, J., Ampt, E., Francioli, D., and Mommer, L. 2020. Do soil‐borne fungal pathogens mediate plant diversity–productivity relationships? Evidence and future opportunities. J. Ecol. 108: 1810–1821. doi:10.1111/1365-2745.13388
- Sánchez-Vallet, A., McDonald, M. C., Solomon, P. S., and McDonald, B. A. 2015. Is Zymoseptoria tritici a hemibiotroph? Fungal Genet. Biol. 79: 29–32. doi:10.1016/j.fgb.2015.04.001
- Santos, B. H. C. d., Ribeiro, R. C. F., Xavier, A. A., Neto, J. A. S., and Mizobutsi, E. H. 2018. Nitrogen fertilization and rhizobacteria in the control of Meloidogyne javanica in common bean plants. JAS 11: 430–437. doi:10.5539/jas.v11n1p430
- Santos, J. C., Finlay, R. D., and Tehler, A. 2006. Molecular analysis of arbuscular mycorrhizal fungi colonising a semi-natural grassland along a fertilisation gradient. New Phytol. 172: 159–168. doi:10.1111/j.1469-8137.2006.01799.x
- Sarabia, M., Cornejo, P., Azcón, R., Carreón-Abud, Y., and Larsen, J. 2017. Mineral phosphorus fertilization modulates interactions between maize, rhizosphere yeasts and arbuscular mycorrhizal fungi. Rhizosphere 4: 89–93. doi:10.1016/j.rhisph.2017.09.001
- Saravanakumar, D., Lavanya, N., Muthumeena, K., Raguchander, T., and Samiyappan, R. 2009. Fluorescent pseudomonad mixtures mediate disease resistance in rice plants against sheath rot (Sarocladium oryzae) disease. BioControl 54: 273–286. doi:10.1007/s10526-008-9166-9
- Savary, S. 1995. Direct and indirect effects of nitrogen supply and disease source structure on rice sheath blight spread. Phytopathology 85: 959–965. doi:10.1094/Phyto-85-959
- Savary, S., Castilla, N. P., Elazegui, F. A., McLaren, C. G., Ynalvez, M. A., and Teng, P. S. 1995. Direct and indirect effects of nitrogen supply and disease source structure on rice sheath blight spread. Ecol. Epidemiol. 85: 959–965. doi:10.1094/Phyto-85-959
- Savary, S., Willocquet, L., Elazegui, F. A., Castilla, N. P., and Teng, P. S. 2000. Rice pest constraints in tropical Asia: quantification of yield losses due to rice pests in a range of production situations. Plant Dis. 84: 357–369. doi:10.1094/PDIS.2000.84.3.357
- Savary, S., Willocquet, L., Pethybridge, S. J., Esker, P., McRoberts, N., and Nelson, A. 2019. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 3: 430–439. doi:10.1038/s41559-018-0793-y
- Savolainen, T., and Kytöviita, M.-M. 2022. Mycorrhizal symbiosis changes host nitrogen source use. Plant Soil 471: 643–654. doi:10.1007/s11104-021-05257-5
- Scheffer, R. P. 1997. The Nature of Disease in Plants. Cambridge University Press, Cambridge.
- Scherm, B., Balmas, V., Spanu, F., Pani, G., Delogu, G., Pasquali, M., and Migheli, Q. 2013. Fusarium culmorum: causal agent of foot and root rot and head blight on wheat. Mol. Plant Pathol. 14: 323–341. doi:10.1111/mpp.12011
- Schmidt, J. E., Kent, A. D., Brisson, V. L., and Gaudin, A. C. M. 2019. Agricultural management and plant selection interactively affect rhizosphere microbial community structure and nitrogen cycling. Microbiome 7: 18. doi:10.1186/s40168-019-0756-9
- Semenov, M. V., Krasnov, G. S., Semenov, V. M., and van Bruggen, A. 2022. Mineral and organic fertilizers distinctly affect fungal communities in the crop rhizosphere. JoF 8: 251. doi:10.3390/jof8030251
- Semenov, M. V., Krasnov, G. S., Semenov, V. M., and van Bruggen, A. H. 2020. Long-term fertilization rather than plant species shapes rhizosphere and bulk soil prokaryotic communities in agroecosystems. Appl. Soil Ecol. 154: 103641. doi:10.1016/j.apsoil.2020.103641
- Sétamou, M., Cardwell, K. F., Schulthess, F., and Hell, K. 1997. Aspergillus flavus infection and aflatoxin contamination of preharvest maize in Benin. Plant Dis. 81: 1323–1327. doi:10.1094/PDIS.1997.81.11.1323
- Shao, D., Smith, D. L., Kabbage, M., and Roth, M. G. 2021. Effectors of plant necrotrophic fungi. Front. Plant Sci. 12: 687713. doi:10.3389/fpls.2021.687713
- Sharma, A., Kumar, V., Shahzad, B., Tanveer, M., Sidhu, G. P. S., Handa, N., Kohli, S. K., Yadav, P., Bali, A. S., Parihar, R. D., Dar, O. I., Singh, K., Jasrotia, S., Bakshi, P., Ramakrishnan, M., Kumar, S., Bhardwaj, R., and Thukral, A. K. 2019. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 1: 1446. doi:10.1007/s42452-019-1485-1
- Sharma, P., Duveiller, E., and Sharma, R. C. 2006. Effect of mineral nutrients on spot blotch severity in wheat, and associated increases in grain yield. Field Crops Res. 95: 426–430. doi:10.1016/j.fcr.2005.04.015
- Shipton, P. J. 1972. Influence of stubble treatment and autumn application of nitrogen to stubbles on the subsequent incidence of take-all and eyespot. Plant Pathol. 21: 147–155. doi:10.1111/j.1365-3059.1972.tb01749.x
- Simón, M. R., Cordo, C. A., Perelló, A. E., and Struik, P. C. 2003. Influence of nitrogen supply on the susceptibility of wheat to Septoria tritici. J. Phytopathol. 151: 283–289. doi:10.1046/j.1439-0434.2003.00720.x
- Simón, M. R., Fleitas, M. C., Castro, A. C., and Schierenbeck, M. 2020. How foliar fungal diseases affect nitrogen dynamics, milling, and end-use quality of wheat. Front. Plant Sci. 11: 569401. doi:10.3389/fpls.2020.569401
- Simón, M. R., Perelló, A. E., Cordo, C. A., and Struik, P. C. 2002. Influence of Septoria tritici on yield, yield components, and test weight of wheat under two nitrogen fertilization conditions. Crop Sci. 42: 1974–1981. doi:10.2135/cropsci2002.1974
- Singh, A. 2006. Cell membrane injury in flag leaf of wheat by brown rust (Puccinia recondita Rob. ex. Desm. f. sp. tritici) at different nitrogen levels. J. Phytological Research 19: 111–113.
- Singh, B. B., and Kaur, H. 2005. Effect of nitrogen fertilizer on Fusarium sheath rot in rice. J. Res. 42: 44–47.
- Singh, V., and Singh, R. N. 2006. Effect of mineral nutrition and environmental variables on the intensity of wheat spot blotch under rice-wheat system. Indian Phytopath. 59: 417–426.
- Sisterna, M. N., and Sarandón, S. J. 1996. Black point of wheat (Bipolaris sorokiniana (Sacc) Shoem. influenced by N fertilization under no till and conventional tillage. Cereal Res. Commun. 24: 217–221.
- Skamnioti, P., and Gurr, S. J. 2009. Against the grain: safeguarding rice from rice blast disease. Trends Biotechnol. 27: 141–150. doi:10.1016/j.tibtech.2008.12.002
- Slaton, N. A., Cartwright, R. D., Meng, J., Gbur, E. E., and Norman, R. J. 2003. Sheath blight severity and rice yield as affected by nitrogen fertilizer rate, application method, and fungicide. Agron. J. 95: 1489–1496. doi:10.2134/agronj2003.1489
- Smiley, R. W. 1978. Antagonists of Gaeumannomyces graminis from the rhizoplane of wheat in soils fertilized with ammonium- or nitrate-nitrogen. Soil Biol. Biochem. 10: 169–174. doi:10.1016/0038-0717(78)90092-5
- Smiley, R. W., and Cook, R. J. 1973. Relationship between take-all of wheat and rhizosphere pH in soils fertilized with ammonium vs. nitrate-nitrogen. Phytopathology 63: 882–890. doi:10.1094/Phyto-63-882
- Sommermann, L., Babin, D., Behr, J. H., Chowdhury, S. P., Sandmann, M., Windisch, S., Neumann, G., Nesme, J., Sørensen, S. J., Schellenberg, I., Rothballer, M., Geistlinger, J., Smalla, K., and Grosch, R. 2022. Long-term fertilization strategy impacts Rhizoctonia solani-microbe interactions in soil and rhizosphere and defense responses in lettuce. Microorganisms 10: 1717. doi:10.3390/microorganisms10091717
- Song, Y., Chen, D., Lu, K., Sun, Z., and Zeng, R. 2015. Enhanced tomato disease resistance primed by arbuscular mycorrhizal fungus. Front. Plant Sci. 6: 786. doi:10.3389/fpls.2015.00786
- Soonvald, L., Loit, K., Runno-Paurson, E., Astover, A., and Tedersoo, L. 2020. Characterising the effect of crop species and fertilisation treatment on root fungal communities. Sci. Rep. 10: 18741. doi:10.1038/s41598-020-74952-7
- Srinivasachary, Laetitia, W., and Serge, S. 2011. Resistance to rice sheath blight (Rhizoctonia solani Kühn) [(teleomorph: Thanatephorus cucumeris (A.B. Frank) Donk.] disease: current status and perspectives. Euphytica 178: 1–22. doi:10.1007/s10681-010-0296-7
- Stakman, E. C. 1924. The effect of fertilizers on the development of stem rust of wheat. J. Agric. Res. 27: 341–385.
- Sun, S.-K., and Snyder, W. C. 1981. The bakanae disease of the rice plant. In Fusarium: Diseases, Biology and Taxonomy; Nelson, P. E. Eds. The Pennsylvania University Press, University Park, pp 104–113.
- Sun, Y., Wang, M., Mur, L. A. J., Shen, Q., and Guo, S. 2020. Unravelling the roles of nitrogen nutrition in plant disease defences. IJMS 21: 572. doi:10.3390/ijms21020572
- Syed Ab Rahman, S. F., Singh, E., Pieterse, C. M. J., and Schenk, P. M. 2018. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 267: 102–111. doi:10.1016/j.plantsci.2017.11.012
- Szulc, P., Rybus-Zając, M., and Jagła, M. 2014. Influence of maize hybrid type (Zea Mays L.) and N dose on nitrogen eutrophication of the environment. Electron. J. Pol. Agric. Univ. 17: 1–10.
- Taberlet, P., Coissac, E., Pompanon, F., Brochmann, C., and Willerslev, E. 2012. Towards next-generation biodiversity assessment using DNA metabarcoding. Mol. Ecol. 21: 2045–2050. doi:10.1111/j.1365-294X.2012.05470.x
- Taheri, P., and Tarighi, S. 2011. Cytomolecular aspects of rice sheath blight caused by Rhizoctonia solani. Eur. J. Plant Pathol. 129: 511–528. doi:10.1007/s10658-010-9725-7
- Tanaka, E., Ashizawa, T., Sonoda, R., and Tanaka, C. 2008. Villosiclava virens gen. nov., comb. nov., teleomorph of Ustilaginoidea virens, the causal agent of rice false smut. Mycotaxon 106: 491–501.
- Tang, Q., Peng, S., Buresh, R. J., Zou, Y., Castilla, N. P., Mew, T. W., and Zhong, X. 2007. Rice varietal difference in sheath blight development and its association with yield loss at different levels of N fertilization. Field Crops Research 102: 219–227. doi:10.1016/j.fcr.2007.04.005
- Tedersoo, L., Bahram, M., Põlme, S., Kõljalg, U., Yorou, N. S., Wijesundera, R., Villarreal Ruiz, L., Vasco-Palacios, A. M., Thu, P. Q., Suija, A., Smith, M. E., Sharp, C., Saluveer, E., Saitta, A., Rosas, M., Riit, T., Ratkowsky, D., Pritsch, K., Põldmaa, K., Piepenbring, M., Phosri, C., Peterson, M., Parts, K., Pärtel, K., Otsing, E., Nouhra, E., Njouonkou, A. L., Nilsson, R. H., Morgado, L. N., Mayor, J., May, T. W., Majuakim, L., Lodge, D. J., Lee, S. S., Larsson, K.-H., Kohout, P., Hosaka, K., Hiiesalu, I., Henkel, T. W., Harend, H., Guo, L., Greslebin, A., Grelet, G., Geml, J., Gates, G., Dunstan, W., Dunk, C., Drenkhan, R., Dearnaley, J., Kesel, A. d., Dang, T., Chen, X., Buegger, F., Brearley, F. Q., Bonito, G., Anslan, S., Abell, S., and Abarenkov, K. 2014. Fungal biogeography. Global diversity and geography of soil fungi. Science 346: 1256688. doi:10.1126/science.1256688
- Thapa, S., Prasanna, R., Ramakrishnan, B., Sheoran, N., Kumar, A., Velmourougane, K., and Kumar, A. 2018. Interactive effects of Magnaporthe inoculation and nitrogen doses on the plant enzyme machinery and phyllosphere microbiome of resistant and susceptible rice cultivars. Arch. Microbiol. 200: 1287–1305. doi:10.1007/s00203-018-1540-0
- Thirkell, T., Cameron, D., and Hodge, A. 2019. Contrasting nitrogen fertilisation rates alter mycorrhizal contribution to barley nutrition in a field trial. Front. Plant Sci. 10: 1312. doi:10.3389/fpls.2019.01312
- Thompson, M. E. H., and Raizada, M. N. 2018. Fungal pathogens of maize gaining free passage along the silk road. Pathogens 7: 81. doi:10.3390/pathogens7040081
- Tiedemann, A. V. 1996. Single and combined effects of nitrogen fertilization and ozone on fungal leaf diseases on wheat. J. Plant Dis. Prot. 103: 409–419.
- Torsvik, V., Øvreås, L., and Thingstad, T. F. 2002. Prokaryotic diversity–magnitude, dynamics, and controlling factors. Science 296: 1064–1066. doi:10.1126/science.1071698
- Tosi, M., Deen, W., Drijber, R., McPherson, M., Stengel, A., and Dunfield, K. 2021. Long-term N inputs shape microbial communities more strongly than current-year inputs in soils under 10-year continuous corn cropping. Soil Biol. Biochem. 160: 108361. doi:10.1016/j.soilbio.2021.108361
- Trivedi, P., Delgado-Baquerizo, M., Anderson, I. C., and Singh, B. K. 2016. Response of soil properties and microbial communities to agriculture: implications for primary productivity and soil health indicators. Front. Plant Sci. 7: 990. doi:10.3389/fpls.2016.00990
- Tyc, O., Song, C., Dickschat, J. S., Vos, M., and Garbeva, P. 2017. The ecological role of volatile and soluble secondary metabolites produced by soil bacteria. Trends Microbiol. 25: 280–292. doi:10.1016/j.tim.2016.12.002
- van Bockhaven, J., Spíchal, L., Novák, O., Strnad, M., Asano, T., Kikuchi, S., Höfte, M., and de Vleesschauwer, D. 2015. Silicon induces resistance to the brown spot fungus Cochliobolus miyabeanus by preventing the pathogen from hijacking the rice ethylene pathway. New Phytol. 206: 761–773. doi:10.1111/nph.13270
- van Diepen, L. T. A., Lilleskov, E. A., and Pregitzer, K. S. 2011. Simulated nitrogen deposition affects community structure of arbuscular mycorrhizal fungi in northern hardwood forests. Mol. Ecol. 20: 799–811. doi:10.1111/j.1365-294X.2010.04969.x
- van Elsas, J. D., Chiurazzi, M., Mallon, C. A., Elhottova, D., Kristufek, V., and Salles, J. F. 2012. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. U S A. 109: 1159–1164. doi:10.1073/pnas.1109326109
- Verbruggen, E., van der Heijden, M. G. A., Rillig, M. C., and Kiers, E. T. 2013. Mycorrhizal fungal establishment in agricultural soils: factors determining inoculation success. New Phytol. 197: 1104–1109. doi:10.1111/j.1469-8137.2012.04348.x
- Veresoglou, S. D., Barto, E. K., Menexes, G., and Rillig, M. C. 2013. Fertilization affects severity of disease caused by fungal plant pathogens. Plant Pathol 62: 961–969. doi:10.1111/ppa.12014
- Veresoglou, S. D., and Rillig, M. C. 2012. Suppression of fungal and nematode plant pathogens through arbuscular mycorrhizal fungi. Biol. Lett. 8: 214–217. doi:10.1098/rsbl.2011.0874
- Waller, J. M. 1987. Rice diseases. Ex. Agric 23: 357–357. doi:10.1017/S0014479700017245
- Walters, D. R. 2003. Polyamines and plant disease. Phytochemistry 64: 97–107. doi:10.1016/s0031-9422(03)00329-7
- Walters, D. R., and Bingham, I. J. 2007. Influence of nutrition on disease development caused by fungal pathogens: implications for plant disease control. Ann Applied Biology 151: 307–324. doi:10.1111/j.1744-7348.2007.00176.x
- Wang, J., Rhodes, G., Huang, Q., and Shen, Q. 2018a. Plant growth stages and fertilization regimes drive soil fungal community compositions in a wheat-rice rotation system. Biol Fertil Soils 54: 731–742. doi:10.1007/s00374-018-1295-4
- Wang, Q., Ma, M., Jiang, X., Zhou, B., Guan, D., Cao, F., Chen, S., and Li, J. 2019. Long-term N fertilization altered 13C-labeled fungal community composition but not diversity in wheat rhizosphere of Chinese black soil. Soil Biol. Biochem. 135: 117–126. doi:10.1016/j.soilbio.2019.04.009
- Wang, Y., Ji, H., Hu, Y., Wang, R., Rui, J., and Guo, S. 2018b. Different selectivity in fungal communities between manure and mineral fertilizers: a study in an alkaline soil after 30 years fertilization. Front. Microbiol. 9: 2613. doi:10.3389/fmicb.2018.02613
- Wattenburger, C. J., Halverson, L. J., and Hofmockel, K. S. 2019. Agricultural management affects root-associated microbiome recruitment over maize development. Phytobiomes Journal 3: 260–272. doi:10.1094/PBIOMES-03-19-0016-R
- Webster, R. K., and Gunnell, P. S. 1992. Compendium of Rice Diseases. APS Press American Phytopathological Soc, St. Paul, Minn.
- Webster, R. K., Wick, C. M., Brandon, D. M., Hall, D. H., and Bolstad, J. 1981. Epidemiology of stem rot disease of rice: effects of burning vs. soil incorporation of rice residue. Hilg 49: 1–12. doi:10.3733/hilg.v49n03p012
- Weller, D. M., Raaijmakers, J. M., Gardener, B. B. M., and Thomashow, L. S. 2002. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 40: 309–348. doi:10.1146/annurev.phyto.40.030402.110010
- Williams, A., Manoharan, L., Rosenstock, N. P., Olsson, P. A., and Hedlund, K. 2017. Long-term agricultural fertilization alters arbuscular mycorrhizal fungal community composition and barley (Hordeum vulgare) mycorrhizal carbon and phosphorus exchange. New Phytol. 213: 874–885. doi:10.1111/nph.14196
- Willocquet, L., Savary, S. and Srinivasachary, 2011. Resistance to rice sheath blight (Rhizoctonia solani Kühn) [(teleomorph: Thanatephorus cucumeris (A.B. Frank) Donk.] disease: current status and perspectives. Euphytica 178: 1–22. doi:10.1007/s10681-010-0296-7
- Wilson, R. A., and Talbot, N. J. 2009. Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 7: 185–195. doi:10.1038/nrmicro2032
- Windisch, S., Sommermann, L., Babin, D., Chowdhury, S. P., Grosch, R., Moradtalab, N., Walker, F., Höglinger, B., El-Hasan, A., Armbruster, W., Nesme, J., Sørensen, S. J., Schellenberg, I., Geistlinger, J., Smalla, K., Rothballer, M., Ludewig, U., and Neumann, G. 2020. Impact of long-term organic and mineral fertilization on rhizosphere metabolites, root-microbial interactions and plant health of lettuce. Front. Microbiol. 11: 597745. doi:10.3389/fmicb.2020.597745
- Wu, F. 2007. Measuring the economic impacts of Fusarium toxins in animal feeds. An. Feed Sci. Technol. 137: 363–374. doi:10.1016/j.anifeedsci.2007.06.010
- Wu, W., Huang, J., Cui, K., Nie, L., Wang, Q., Yang, F., Shah, F., Yao, F., and Peng, S. 2012. Sheath blight reduces stem breaking resistance and increases lodging susceptibility of rice plants. Field Crops Res. 128: 101–108. doi:10.1016/j.fcr.2012.01.002
- Wu, W., Shah, F., Shah, F., and Huang, J. 2015. Rice sheath blight evaluation as affected by fertilization rate and planting density. Australas. Plant Pathol. 44: 183–189. doi:10.1007/s13313-014-0338-z
- Yaeno, T., and Iba, K. 2008. BAH1/NLA, a RING-type ubiquitin E3 ligase, regulates the accumulation of salicylic acid and immune responses to Pseudomonas syringae DC3000. Plant Physiol. 148: 1032–1041. doi:10.1104/pp.108.124529
- Yamasaki, H., and Cohen, M. F. 2006. NO signal at the crossroads: polyamine-induced nitric oxide synthesis in plants? Trends Plant Sci. 11: 522–524. doi:10.1016/j.tplants.2006.09.009
- Yi, Y., Luan, P., Liu, S., Shan, Y., Hou, Z., Zhao, S., Jia, S., and Li, R. 2022. Efficacy of Bacillus subtilis XZ18-3 as a biocontrol agent against Rhizoctonia cerealis on wheat. Agriculture 12: 258. doi:10.3390/agriculture12020258
- Zalila-Kolsi, I., Ben Mahmoud, A., Ali, H., Sellami, S., Nasfi, Z., Tounsi, S., and Jamoussi, K. 2016. Antagonist effects of Bacillus spp. strains against Fusarium graminearum for protection of durum wheat (Triticum turgidum L. subsp. durum). Microbiol. Res. 192: 148–158. doi:10.1016/j.micres.2016.06.012
- Zhai, F., Li, T., Qin, X., Zhao, X., Jiang, L., and Xie, Y. 2022. Effect of fertilisation on fungal community in topsoil of winter wheat field. Plant Soil Environ 68: 317–327. doi:10.17221/117/2022-PSE
- Zhang, W. 2018. Global pesticide use: Profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 8: 1–27.
- Zhang, W., Wu, L., Ding, Y., Yao, X., Wu, X., Weng, F., Li, G., Liu, Z., Tang, S., Ding, C., and Wang, S. 2017. Nitrogen fertilizer application affects lodging resistance by altering secondary cell wall synthesis in japonica rice (Oryza sativa). J. Plant Res. 130: 859–871. doi:10.1007/s10265-017-0943-3
- Zhao, Y., Selvaraj, J. N., Xing, F., Zhou, L., Wang, Y., Song, H., Tan, X., Sun, L., Sangare, L., Folly, Y. M. E., and Liu, Y. 2014. Antagonistic action of Bacillus subtilis strain SG6 on Fusarium graminearum. PloS One 9: e92486. doi:10.1371/journal.pone.0092486
- Zheng, Y., Kim, Y.-C., Tian, X.-F., Chen, L., Yang, W., Gao, C., Song, M.-H., Xu, X.-L., and Guo, L. 2014. Differential responses of arbuscular mycorrhizal fungi to nitrogen addition in a near pristine Tibetan alpine meadow. FEMS Microbiol. Ecol. 89: 594–605. doi:10.1111/1574-6941.12361
- Zimmermann, B., Claß-Mahler, I., von Cossel, M., Lewandowski, I., Weik, J., Spiller, A., Nitzko, S., Lippert, C., Krimly, T., Pergner, I., Zörb, C., Wimmer, M. A., Dier, M., Schurr, F. M., Pagel, J., Riemenschneider, A., Kehlenbeck, H., Feike, T., Klocke, B., Lieb, R., Kühne, S., Krengel-Horney, S., Gitzel, J., El-Hasan, A., Thomas, S., Rieker, M., Schmid, K., Streck, T., Ingwersen, J., Ludewig, U., Neumann, G., Maywald, N., Müller, T., Bradáčová, K., Göbel, M., Kandeler, E., Marhan, S., Schuster, R., Griepentrog, H.-W., Reiser, D., Stana, A., Graeff-Hönninger, S., Munz, S., Otto, D., Gerhards, R., Saile, M., Hermann, W., Schwarz, J., Frank, M., Kruse, M., Piepho, H.-P., Rosenkranz, P., Wallner, K., Zikeli, S., Petschenka, G., Schönleber, N., Vögele, R. T., and Bahrs, E. 2021. Mineral-ecological cropping systems—a new approach to improve ecosystem services by farming without chemical synthetic plant protection. Agronomy 11: 1710. doi:10.3390/agronomy11091710
