Abstract
Despite the emergence of various treatment strategies for rectal cancer based on neoadjuvant chemoradiotherapy, there is currently a lack of reliable biomarkers to determine which patients will respond well to neoadjuvant chemoradiotherapy. Through collecting hematological and biochemical parameters data of patients prior to receiving neoadjuvant chemoradiotherapy, we evaluated the predictive value of systemic inflammatory indices for pathological response and prognosis in rectal cancer patients. We found that baseline GRIm-Score was an independent predictor for MPR in rectal cancer patients. However, no association was observed between several commonly systemic inflammation indices and long-term outcome.
Introduction
The burden of colorectal cancer (CRC) is on the rise worldwide due to increasing prevalence (Citation1). Especially in China, where the all-age incidence is the highest with unique clinical characteristics—nearly half of all CRC tumors are located in the rectum (Citation2). Currently, the standard therapy for locally advanced rectal cancer (LARC) is fluorouracil-based neoadjuvant chemoradiotherapy (nCRT), followed by total mesorectal excision (TME) and adjuvant chemotherapy. Although the pathologic complete response (pCR) rate of LARC patients has improved to about 30%(Citation3), there are still no reliable biomarkers to identify patients who response well to nCRT.
Cancer-related inflammation is known to be inextricably associated with tumor development and progression (Citation4). In recent years, various blood parameters that can reflect systemic inflammatory status have been widely used to determine the severity of inflammation and predict the prognosis of cancer patients, such as Gustave Roussy Immune Score (GRIm-Score), systemic immune-inflammation index (SII) and neutrophil-to-lymphocyte ratio (NLR). GRIm-Score is composed of three independent biomarkers albumin (ALB), lactic dehydrogenase (LDH) and NLR. GRIm-Score has been reported to be a powerful prognostic biomarker in hepatocellular carcinoma, non-small cell lung cancer and gastric cancer (Citation5–7). In a recent study by Peng et al., a high GRIm-Score was found to be significantly associated with poor survival outcomes in CRC patients (Citation8), whereas the evidence for the predictive performance of GRIm-Score for rectal cancer remains inadequate. In addition, both NLR and SII are frequently-used markers that reflects the local immune response and systemic inflammation. A multicenter study in Italy showed that LARC patients with higher baseline SII and NLR values were associated with lower pCR rates and poorer survival outcomes (Citation9). However, some studies reported conflicting findings that neither SII nor NLR were associated with pCR in LARC patients (Citation10) and were not prognostic biomarkers for rectal cancer (Citation11). Therefore, the aim of this study was to determine the predictive and prognostic value of GRIm-Score, NLR, and SII, as well as other commonly used systemic inflammation indices, in rectal cancer patients undergoing nCRT.
Methods
Study design and patients
Data from patients with rectal cancer who received preoperative transcatheter rectal arterial chemoembolization (TRACE) and nCRT at Daping Hospital between July 2013 and May 2022, were retrospectively analyzed. These patients were enrolled in a prospective trial registered at ClinicalTrials.gov (NCT03601156). The study protocol was approved by the Ethics Committee of our hospital and all procedures performed in this study complied with the Declaration of Helsinki. The inclusion criteria were (1) patients aged 18 years or older; (2) pathological diagnosis of adenocarcinoma; (3) patients with cT2-4/cN-any/M0-1; (4) distance from the lower edge of the tumor to the anal verge (AV) ≤15 cm; and (5) Eastern Cooperative Oncology Group (ECOG) performance status 0-1. The exclusion criteria were patients with (1) severe comorbidities or mental diseases that may hinder treatment cooperation; (2) active systemic inflammatory or autoimmune diseases that could affect inflammatory parameters; (3) a history of chemoradiotherapy.
The procedure of preoperative intraarterial chemoembolization was performed as described previously (Citation12). All patients underwent TRACE, followed by long-course radiotherapy (cumulative dose of 45 Gy) and S-1 chemotherapy, and the total mesorectal excision (TME). Four to eight weeks after surgery, all patients received a postoperative mFOLFOX6 or CAPOX regimens for 4–6 months.
Data collection
The following clinicopathological characteristics were collected: age, sex, ECOG, body mass index (BMI), clinical stage, distance from the lower edge of the tumor to the AV, tumor length, circumferential resection margin (CRM), extramural vascular invasion (EMVI), ypT category, ypN category, tumor regression grade (TRG), and vascular and perineural invasion. Blood test results of patients before any oncological treatment were retrospectively reviewed from the medical records, including hemoglobin, albumin (ALB), lactic dehydrogenase (LDH), carcinoembryonic antigen (CEA), cancer antigen 199 (CA199), white blood cell (WBC), neutrophil, lymphocyte, and platelet. Seven inflammatory indices were then evaluated based on their hematological and biochemical parameters, including systemic immune-inflammation index (SII), prognostic nutritional index (PNI), LDH to ALB ratio (LAR), neutrophil to lymphocyte ratio (NLR), derived neutrophil-to-lymphocytes ratio (dNLR), lung immune prognostic index (LIPI), and Gustave Roussy Immune Score (GRIm-Score). The SII was calculated as neutrophil × platelet/lymphocyte/1000. The PNI was calculated as 10 × ALB + 0.005 × lymphocyte, and the LAR was calculated as lymphocyte/albumin. The NLR was calculated as neutrophil/lymphocyte, while the dNLR was calculated as neutrophil/(WBC-neutrophil). Regarding the calculation of LIPI and GRIm-Score, some modifications were made on the basis of previous studies (Citation8,Citation13), as follows: the upper limit of LDH level was set at 250 U/mL for wet chemical system and 610 U/mL for dry chemical system. For the calculation of GRIm-Score, three high risk factors were considered, namely serum albumin less than 35 g/L, NLR greater than 75% percentile of our cohort and LDH exceeding the upper limit defined above. GRIm-Score for a given patient was defined as the number of these high-risk factors ranging from 0 to 3. The LIPI was defined as the number of two high-risk factors, dNLR greater than 3 and LDH exceeding the upper limit, ranging from 0 to 2.
Pathological assessment of tumor regression
All surgically resected specimens were subjected to histopathological examination and immunohistochemistry (IHC) analysis of DNA mismatch repair (MMR) protein patterns. The absence of any protein expression of MSH6, MSH2, MLH1, and PMS2 in the IHC results classified as mismatch repair-deficient (dMMR); otherwise, it was considered mismatch repair-proficient (pMMR). The pathological and tumor-regression grading (TRG) were evaluated according to the criteria of the American Joint Committee on Cancer (8th edition), as follows: TRG0 indicates no residual tumor cells in the surgical specimen, also known as pathologic complete response (pCR); TRG1 represents a near-complete response, with only viable single cells or small groups of cancer cells in the specimen; TRG2 indicates a partial response, which refers to residual cancer cells due to significant tumor regression; TRG3 indicates poor or no response, which refers to extensive residual cancer with no evident tumor regression. Furthermore, the major pathological response (MPR) was defined as the sum of TRG0 and TRG1.
Follow-up
Patients were followed up until March 1, 2023. Follow-up assessments were performed with evaluation every 6 months for the first 2 years and annually thereafter. Patients were followed up via telephone interview or outpatient examination. Overall survival (OS) was defined as the time from enrollment to the date of death or last follow-up.
Statistical analysis
Continuous variables are represented as median with interquartile range (IQR), and the differences between groups were examined using Wilcoxon test or Kruskal-Wallis test. Categorical data are presented as frequency and percentage, and the differences between group were examined using Fisher’s exact probability test. Spearman’s rank coefficient of correlation was used to evaluate association between systemic inflammation indices. Univariate logistic regression was used to evaluate the association of clinicopathological factors and systemic inflammatory indices with MPR or pCR. Univariate Cox regression was used to identify prognostic factors for OS.
To avoid non-linear fittings involved in continuous variables such as SII, PNI, and PAR, these variables were first categorized according to corresponding median value in the whole population. Stepwise logistic regression was used to develop a model to predict MPR using R package StepReg (Version 1.4.4). Candidate variables were selected based on likelihood ratio test for inclusion (P < 0.05) and exclusion (P < 0.15). Predictors with crude P < 0.1 on univariate logistic regression were considered for multivariate analysis. Stepwise Cox regression using the same variable selection criteria was used to identify independent prognostic factors for OS. The death risk score (DRS) was calculated by taking linear predictor returned by fitted values. The predictive performance of established MPR model was examined using area under the curve (AUC) in the receiver operating characteristic curve (ROC) analysis. The differences in AUC between models was examined using Delong’s test in R package pROC (version 1.18.0). The Kaplan-Meier curve was used to visualize OS, and the log-rank tests was used to compare the differences in OS between groups. With regard to DRS, patients were divided into low-risk and high-risk groups with a cutoff of 75%. The prognostic values of DRS, pre-cM stage, pre-cN stage, and hemoglobin for 2-year, 3-year and 5-year OS were determined by time-dependent ROC curve analysis, and the differences in AUC between group were examined by timeROC R package (version 0.4). All reported P values in this study were two-sided, with P < 0.05 considered statistically significant. All analyses were performed using R software version 4.2.3.
Results
Demographics and patient characteristics
The demographic and clinicopathological characteristics of patients are shown in . A total of 123 patients were enrolled in this study, with a median age of 59 years (52–68 years). The majority (65.85%) of patients were male. Most patients (91.87%) had pathological stage II-III rectal cancer, and 10 were diagnosed with metastases before participating in this study. In terms of tumor location, there were 59 (47.97%) cases in the lower rectum (0–5 cm from the anal verge), 56 (45.53%) cases in the middle rectum (5–10 cm from the anal verge), and 8 (6.5%) in the upper rectum (>10 cm from the anal verge). Notably, 61 (49.59%) patients had ypT0-2 rectal cancer, compared with 3 patients (2.44%) prior to treatment, indicating a significant downstaging. Regarding pathological response, the pCR rate was 18.70%, and the MPR rate was 45.53%. The proportion of patients with abnormally elevated serum CEA and CA199 was 40.65% and 16.26%, respectively.
Table 1. Clinicopathological characteristics of patients.
Relationship between systemic inflammation indices and baseline clinical characteristics
shows the boxplots of the distribution of log-transformed SII and NLR values in rectal cancer patients with cancer staging T2-3 and T4, respectively. Clinical staging T4 had significantly higher SII (P = 0.034) and NLR (P = 0.018) values compared to clinical staging T2-3. In addition, the stacked column diagram showed that GRIm-Score ≥ 1 was significantly distributed in rectal patients with tumor length > 4 cm (P = 0.008, ) or baseline serum CEA concentration > 5 ng/mL (P = 0.037, ). However, there were no significant differences between LIPI and any clinical characteristic variables. These results suggested that tumor burden had impact on systemic inflammation status. Further correlation analysis revealed no significant correlation between any systemic inflammation indices and baseline serum CEA or CA199 levels (Supplementary Figure 1). Supplementary Tables 1 and 2 summarize the relationship between inflammation indices and baseline clinical characteristics.
Figure 1. Distribution of SII, NLR, and GRIm-Score indices in rectal cancer patients with different clinical cancer staging, tumor lengths, and baseline CEA levels. (A) Boxplot showing the distribution of log-transformed SII values in rectal cancer patients with cancer staging T2-3 and T4. (B) Boxplot showing the distribution of log-transformed NLR values in rectal cancer patients with cancer staging T2-3 and T4. (C) A stacked column diagram showing the proportion of GRIm-Score ≥ 1 in rectal cancer patients with tumor length > 4 cm. (D) A stacked column diagram showing the proportion of GRIm-Score ≥ 1 in rectal cancer patients with serum CEA concentration > 5 ng/mL.
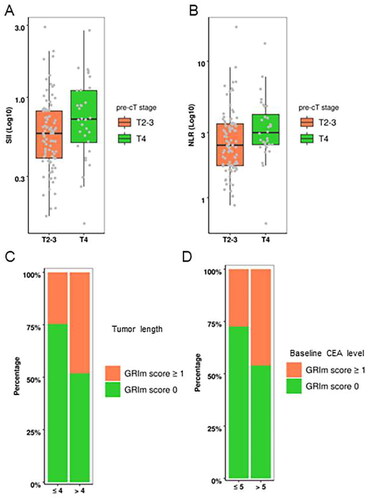
Table 2. The final logistic model to predict MRP.
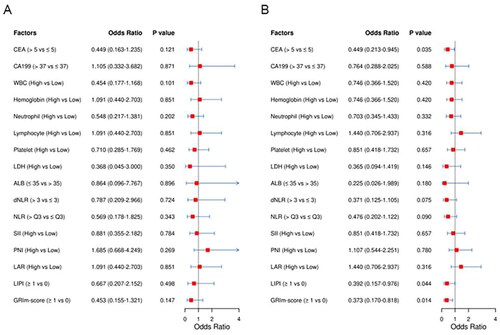
Univariate and multivariate analyses of risk factors for pCR and MPR
There were no significant differences in any hematological indices between rectal patients in the non-MPR group and MPR group (Supplementary Table 1). Nonetheless, the MPR rate in patients with GRIm-Score ≥ 1 had an approximately 23% lower MPR rate than those with a GRIm-Score of 0 (30.2% vs 53.8%, P = 0.014, Supplementary Table 3). In addition, a similar trend was observed for the LIPI index. These results suggested that indices combining several nutritional and inflammatory biomarkers, such as the GRIm-Score are superior to the use of a single hematologic biomarker in predicting the MPR rate. Furthermore, there were no statistically significant differences in any hematological indices between rectal patients with and without pCR (Supplementary Table 4, ). Univariate logistic regression analysis showed that LIPI and GRIm-Score were significantly associated with MPR (). Moreover, multivariate logistic regression analysis further revealed that GRIm-Score (OR = 0.394, 95% CI: 0.177–0.876, P = 0.012) and pre-cT stage (OR = 0.391, 95% CI: 0.160–0.954, P = 0.034) were independent predictors for MPR ().
Prognostic factors for OS
The overall median follow-up time was 34 months, ranging from 9 months to 106 months. The 1-year, 3-year, 5-year OS rates were 98.27%, 78.57% and 66.91%, respectively. Univariate Cox regression showed that ypN stage (HR = 3.0806, 95% CI: 1.4880 − 6.4002, P = 0.003) and pre-cM stage (HR = 11.1652 95% CI: 4.2754 − 29.1580, P < 0.0001) were significant risk factor for OS in rectal patients. Regarding baseline hematological and biochemical parameters, CA199 (HR = 3.3698, 95% CI: 1.5186 − 8.9632, P = 0.0039), hemoglobin (HR = 0.4252, 95% CI: 0.1971 − 0.9175, P = 0.0293), and LDH (HR = 3.3995, 95% CI: 1.3673 − 8.4523, P = 0.0085) were the only three risk factors significantly associated with OS (Supplementary Table 5). Multivariate Cox regression analysis showed that pre-cN stage, pre-cM stage and hemoglobin were independent prognostic factors for OS (). shows the Kaplan-Meier survival curves for OS stratified by pre-cN stage, pre-cM stage, hemoglobin levels and DRS levels. Time-dependent ROC analysis showed that the prognostic performance of DRS was superior to pre-cN stage, pre-cM stage and hemoglobin in predicting 3-year and 5-year OS (Supplementary Table 6 and Supplementary Figure 2). These results clearly demonstrated that nutritional status can provide better prognostic performance for OS than commonly adopted clinical stages.
Figure 3. Kaplan-Meier survival curve for OS stratified by pre-cN stage, pre-cM stage, hemoglobin levels and DRS levels. (A) pre-cN stage. (B) pre-cM stage. (C) Hemoglobin. (D) DRS.
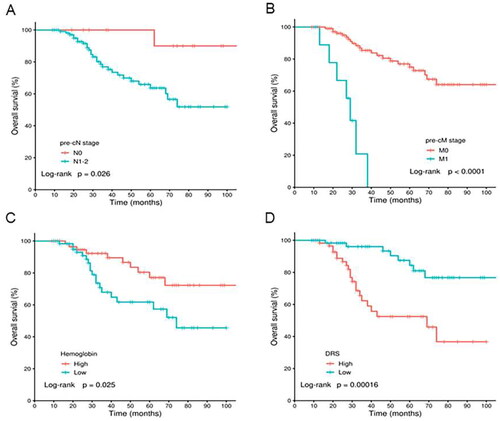
Table 3. The final prognostic model for OS.
Discussion
Many studies demonstrated that ypT/N-category and pCR are independent prognostic factors for LARC (Citation14,Citation15). However, there is still a lack of economical and noninvasive methods to predict tumor response and clinical outcome. Given the extensive impact of inflammation on tumor development and progression (Citation4), the predictive and prognostic value of systemic inflammation indicators has received increased attention in recent years. In this study, therefore, we analyzed 123 cases of stage II-IV rectal cancer treated with nCRT to explore the predictive performance of several inflammation-related biomarkers on pathological responses and prognosis. Results of our univariate analysis found that low levels of baseline LIPI (P = 0.044) and GRIm-Score (P = 0.014) were significantly associated with MPR, whereas only GRIm-Score (P = 0.023) was identified as an independent indicator for MPR. Surprisingly, many inflammation-related biomarkers were found not to be independent predictors of OS in rectal cancer patients. Hemoglobin was the only inflammation-related biomarker identified in this study as an independent prognostic factor for OS in patients with rectal cancer. This finding is supported by previous studies showing that rectal cancer patients with anemia were less likely to achieve pCR and had worse survival outcomes (Citation16,Citation17).
Tumor immune microenvironment has been found to affect chemoradiation resistance and clinical outcomes in many solid tumors (Citation18–20). And there is a complex interplay between local immune response and the systemic inflammatory status. A variety of cytokines, inflammatory proteins, and immune cells in the local tumor microenvironment can be detected in peripheral blood, suggesting that systemic inflammation indices based on peripheral blood cell counts and biochemical parameters can reflect local tumor immunity to a certain extent (Citation21). Therefore, many studies have investigated the association between systemic inflammation indices and responsiveness to nCRT in rectal cancer, but the results remain controversial. Zhang et al. conducted a retrospective analysis and found that LARC patients with baseline NLR < 2 exhibited better response to nCRT and tend to survival longer (Citation22), while other researches do not support this conclusion. In a study involving 1237 LARC patients who received standard neoadjuvant therapy, inflammatory indices such as NLR and PLR were neither predictive of pCR nor prognostic for long-term outcomes (Citation23). Similarly, another retrospective study did not find the predictive value of baseline NLR and PLR in pathological response of rectal cancer patients (Citation24). More importantly, the negative results from a prospective study indicate that both NLR and PLR are not suitable biomarkers for predicting response and prognosis in patients undergoing nCRT for LARC (Citation25), which is also consistent with our results. However, published literature suggests that SII may be as candidates to help identify subgroup population who would benefit from neoadjuvant therapy (Citation26), and the survival in rectal cancer patients with high PNI level (≥ 45) were significantly better than those with low PNI level (< 45)(Citation27). In the present study, we found neither a significant correlation between tumor response and low SII level in pretreatment circulating blood nor an association between baseline PNI and clinical outcomes. In the aforementioned studies, LARC patients received standard neoadjuvant chemoradiotherapy such as long-term radiotherapy (45–50.4 Gy in 25 fractions) in combination with fluorouracil-based chemotherapy or short-course radiotherapy (25 Gy in 5 fractions), while in our study, patients with rectal cancer underwent a TRACE before neoadjuvant therapy, which may cause fluctuations in various hematological and biochemical indicators. Additionally, the lack of clearly defined cut-off values for systemic inflammatory indices also leads to non-comparability between study results.
LIPI is an inflammation-related index composed of dNLR and LDH, which is proposed to predict prognosis in lung cancer. Arıkan et al. first investigated the predictive performance of baseline LIPI in LARC patients treated with nCRT, but reported a conflicting result. High LIPI was associated with worse disease-free survival compared with low LIPI, while patients with high LIPI had better response rates (Citation28). In the current study, rectal cancer patients with low levels of baseline LIPI were more likely to have good tumor response, but only GRIm-Score, not LIPI, was found to be an independent predictive factor of MPR. GRIm-Score is a novel prognostic scoring system that combines three independent biomarkers to provide a more comprehensive understanding of immune properties of TME. Cancer patients with high GRIm-Score are more likely to have hypoalbuminemia and high levels of LDH and NLR, all of which are markers of poor prognosis (Citation29–31). Evidence reveals that a high GRIm-Score is closely associated with poor survival in many malignancies (Citation32–34). Our study is the first to investigate the predictive significance of GRIm-Score in rectal cancer patients. GRIm-Score can provide an economic and practical method for predicting pathological response in rectal cancer patients treated with nCRT and help to personalize management decisions in this patient population. Notably, a favorable tumor response to neoadjuvant therapy is generally related to longer survival for patients with rectal cancer. However, the association between GRIm-Score and survival in this study was not observed, which may be due to the small sample size and relatively short duration of follow-up. The limitations of this study also include the retrospective design and single-institution patient cohort. Therefore, the results still need to be confirmed in a future validation cohort. Therefore, caution should be exercised when attempting to extrapolate our results to other countries.
Conclusions
Through collecting hematological and biochemical parameters data of patients prior to receiving nCRT, we evaluated the predictive value of systemic inflammatory indices for pathological response and prognosis in rectal cancer patients. We found that baseline GRIm-Score was an independent predictor for MPR in rectal cancer patients. However, no association was observed between several commonly systemic inflammation indices and long-term outcome. Clinical N and M staging as well as hemoglobin were identified to be independent prognostic factors for OS. Future multicenter studies with larger sample sizes are required to future validate the findings of this study.
Authors’ contributions
Conception and design: WNY, XML, HX and JML. Provision of study materials or patients: CC, YF, ND, MXL, CXL and CYQ. Collection and assembly of data: WNY, XML, CFL and YXY. Data analysis and interpretation: all authors. Writing–original draft: WNY and XML. Writing–review and editing: all authors. Supervision: HX and JML.
Supplemental Material
Download PDF (569.7 KB)Supplemental Material
Download PDF (369 KB)Disclosure statement
The authors have no conflicts of interest to declare.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660.
- Qu R, Ma Y, Zhang Z, Fu W. Increasing burden of colorectal cancer in China. Lancet Gastroenterol Hepatol. 2022;7(8):700.
- Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99(7):918–928. doi:10.1002/bjs.8702.
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205.
- Li Y, Pan Y, Lin X, Hou J, Hu Z, Xu L, et al. Development and validation of a prognostic score for hepatocellular carcinoma patients in immune checkpoint inhibitors therapies: the hepatocellular carcinoma modified Gustave Roussy Immune score. Front Pharmacol. 2021;12:819985. doi:10.3389/fphar.2021.819985.
- Lenci E, Cantini L, Pecci F, Cognigni V, Agostinelli V, Mentrasti G, et al. The Gustave Roussy immune (GRIm)-score variation is an early-on-treatment biomarker of outcome in advanced non-small cell lung cancer (NSCLC) patients treated with first-line pembrolizumab. J Clin Med. 2021;10(5):1005.
- Nakazawa N, Sohda M, Ubukata Y, Kuriyama K, Kimura A, Kogure N, et al. Changes in the Gustave Roussy immune score as a powerful prognostic marker of the therapeutic sensitivity of nivolumab in advanced gastric cancer: a multicenter, retrospective study. Ann Surg Oncol. 2022;29(12):7400–7406. doi:10.1245/s10434-022-12226-4.
- Tian S, Cao Y, Duan Y, Liu Q, Peng P. Gustave Roussy immune score as a novel prognostic scoring system for colorectal cancer patients: a propensity score matching analysis. Front Oncol. 2021;11:737283. doi:10.3389/fonc.2021.737283.
- Chiloiro G, Romano A, Mariani S, Macchia G, Giannarelli D, Caravatta L, et al. Predictive and prognostic value of inflammatory markers in locally advanced rectal cancer (PILLAR) – a multicentric analysis by the Italian Association of Radiotherapy and Clinical Oncology (AIRO) gastrointestinal study group. Clin Transl Radiat Oncol. 2023;39:100579.
- Guan B, Huang X, Xia H, Guan G, Xu B. Prognostic value of mesorectal package area in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy: a retrospective cohort study. Front Oncol. 2022;12:941786. doi:10.3389/fonc.2022.941786.
- Portale G, Cavallin F, Valdegamberi A, Frigo F, Fiscon V. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio are not prognostic biomarkers in rectal cancer patients with curative resection. J Gastrointest Surg. 2018;22(9):1611–1618. doi:10.1007/s11605-018-3781-2.
- Yang B, Shan J, Feng Y, Dai N, Li M, Chen C, et al. Transcatheter rectal arterial chemoembolization with oxaliplatin plus S-1 concurrent chemoradiotherapy can improve the pathological remission rate in locally advanced rectal cancer: a comparative study. Radiat Oncol. 2020;15(1):94. doi:10.1186/s13014-020-01540-4.
- Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4(3):351–357. doi:10.1001/jamaoncol.2017.4771.
- Ryu HS, Lee JL, Kim CW, Yoon YS, Park IJ, Lim SB, et al. Correlative significance of tumor regression grade and ypT category in patients undergoing preoperative chemoradiotherapy for locally advanced rectal cancer. Clin Colorectal Cancer. 2022;21(3):212–219. doi:10.1016/j.clcc.2022.02.001.
- Jäger T, Neureiter D, Urbas R, Klieser E, Hitzl W, Emmanuel K, et al. Applicability of American Joint Committee on cancer and College of American Pathologists regression grading system in rectal cancer. Dis Colon Rectum. 2017;60(8):815–826. doi:10.1097/DCR.0000000000000806.
- Bong JW, Lim SB, Ryu H, Lee JL, Kim CW, Yoon YS, et al. Effect of anaemia on the response to preoperative chemoradiotherapy for rectal cancer. ANZ J Surg. 2021;91(5):E286–E291. doi:10.1111/ans.16547.
- Rodrigues D, Simões J, Teixeira L, Aires F, Fernandes C, Rey C, et al. Baseline anaemia increases locally advanced rectal cancer mortality in older patients undergoing preoperative chemoradiation. Support Care Cancer. 2021;29(3):1403–1411. doi:10.1007/s00520-020-05618-3.
- Cosper PF, McNair C, González I, Wong N, Knudsen KE, Chen JJ, et al. Decreased local immune response and retained HPV gene expression during chemoradiotherapy are associated with treatment resistance and death from cervical cancer. Int J Cancer. 2020;146(7):2047–2058. doi:10.1002/ijc.32793.
- Nicolas AM, Pesic M, Engel E, Ziegler PK, Diefenhardt M, Kennel KB, et al. Inflammatory fibroblasts mediate resistance to neoadjuvant therapy in rectal cancer. Cancer Cell. 2022;40(2):168–184 e13. doi:10.1016/j.ccell.2022.01.004.
- Liu H, Tang L, Li Y, Xie W, Zhang L, Tang H, et al. Nasopharyngeal carcinoma: current views on the tumor microenvironment’s impact on drug resistance and clinical outcomes. Mol Cancer. 2024;23(1):20. doi:10.1186/s12943-023-01928-2.
- Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493-503–e503. doi:10.1016/S1470-2045(14)70263-3.
- Zhang X, Li J, Peng Q, Huang Y, Tang L, Zhuang Q, et al. Association of markers of systemic and local inflammation with prognosis of patients with rectal cancer who received neoadjuvant radiotherapy. Cancer Manag Res. 2019;11:191–199. doi:10.2147/CMAR.S187559.
- Dudani S, Marginean H, Tang PA, Monzon JG, Raissouni S, Asmis TR, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictive and prognostic markers in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiation. BMC Cancer. 2019;19(1):664. doi:10.1186/s12885-019-5892-x.
- Lai S, Huang L, Luo S, Liu Z, Dong J, Wang L, et al. Systemic inflammatory indices predict tumor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Oncol Lett. 2020;20(3):2763–2770. doi:10.3892/ol.2020.11812.
- Gawiński C, Mróz A, Roszkowska-Purska K, Sosnowska I, Derezińska-Wołek E, Michalski W, et al. A prospective study on the roles of the lymphocyte-to-monocyte ratio (LMR), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) in patients with locally advanced rectal cancer. Biomedicines. 2023;11(11):3048. doi:10.3390/biomedicines11113048.
- Eraslan E, Adas YG, Yildiz F, Gulesen AI, Karacin C, Arslan UY. Systemic immune-inflammation index (SII) predicts pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. J Coll Physicians Surg Pak. 2021;31 (4):399–404. doi:10.29271/jcpsp.2021.04.399.
- Wang Y, Chen L, Zhang B, Song W, Zhou G, Xie L, et al. Pretreatment inflammatory-nutritional biomarkers predict responses to neoadjuvant chemoradiotherapy and survival in locally advanced rectal cancer. Front Oncol. 2021;11:639909. doi:10.3389/fonc.2021.639909.
- Arıkan R, Alkış H, Işık S, Yaşar A, Çelebi A, Majidova N, et al. Evaluation of predictive and prognostic importance of lung immune prognostic index in locally advanced rectal cancer patients treated with neoadjuvant chemoradiotherapy. Cureus. 2023;15(6):e40548. doi:10.7759/cureus.40548.
- Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019;43(2):181–193. doi:10.1002/jpen.1451.
- Claps G, Faouzi S, Quidville V, Chehade F, Shen S, Vagner S, et al. The multiple roles of LDH in cancer. Nat Rev Clin Oncol. 2022;19(12):749–762. doi:10.1038/s41571-022-00686-2.
- Hwang M, Canzoniero JV, Rosner S, Zhang G, White JR, Belcaid Z, et al. Peripheral blood immune cell dynamics reflect antitumor immune responses and predict clinical response to immunotherapy. J Immunother Cancer. 2022;10(6):e004688. doi:10.1136/jitc-2022-004688.
- Feng JF, Wang L, Yang X, Chen S. Gustave Roussy Immune Score (GRIm-Score) is a prognostic marker in patients with resectable esophageal squamous cell carcinoma. J Cancer. 2020;11(6):1334–1340.
- Li SJ, Zhao L, Wang HY, Zhou HN, Ju J, Du H, et al. Gustave Roussy Immune Score based on a three-category risk assessment scale serves as a novel and effective prognostic indicator for surgically resectable early-stage non-small-cell lung cancer: a propensity score matching retrospective cohort study. Int J Surg. 2020;84:25–40.
- Minichsdorfer C, Gleiss A, Aretin MB, Schmidinger M, Fuereder T. Serum parameters as prognostic biomarkers in a real world cancer patient population treated with anti PD-1/PD-L1 therapy. Ann Med. 2022;54(1):1339–1349.