?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Currently, an estimated 20% of the population in Sub-Saharan Africa is food insecure with the incidence of hunger and malnutrition still rising. This trend is amplified by the socio-economic consequences of the COVID-19 pandemic. In contrast, more than a third of the harvestable perishable produce is lost due to a lack of preservation or failure to utilize preservation as is the case for underutilized crops (UCs). Moreover, some of the preservation techniques utilized are poor, leading to the deterioration of food quality, especially the micronutrients. In this study, we thus exemplarily investigated the impact of different drying settings on the quality of two highly nutritious UCs, namely cocoyam and orange-flesh sweet potato (OFSP) (40, 60, and 80 °C for cocoyam and 40, 50, 60, and 70 °C for OFSP) to deduce the optimum quality retention and further develop a theoretical design of processing units and processing guidelines for decentralized food processing. Drying cocoyam at 80 °C and OFSP at 60 °C, respectively resulted in a relatively shorter drying time (135 and 210 min), a lower total color difference (2.29 and 11.49-13.92), greater retentions for total phenolics (0.43 mg GAE/100 gDM and 155.0-186.5 mg GAE/100 gDM), total flavonoid (128 mg catechin/100 gDM and 79.5-81.7 mg catechin/100 gDM) and total antioxidant activity (80.85% RSA and 322.58-334.67 mg AAE/100 gDM), respectively for cocoyam and OFSP. The β-carotene, ascorbic acid and vitamin A activity per 100 gDM of the OFSP flours ranged between 6.91- 9.53 mg, 25.90 − 35.72 mg, and 0.53 − 0.73 mg RAE, respectively.
1. Introduction
The incidence of hunger and malnutrition is rising in Africa, especially Sub-Saharan Africa (SSA) where 20% of the population is undernourished.[Citation1] An assessment of the global incidence of hidden hunger found that SSA has a “severe to an alarmingly high incidence of hidden hunger.”[Citation2] The severity of hidden hunger is becoming more entrenched in modern African societies that are facing an abandonment of agriculture and a high rate of rural to urban migration. For example, evidence from Tanzania suggests that migrants to urban areas neglect the consumption of traditional staple foods in favor of convenient and easy to access foods that are often unhealthy.[Citation3] The increasing climate change, population growth, and the worsening effect of the COVID-19 pandemic on the economies particularly in SSA will increase the adverse effects on food and nutrition security.[Citation4]
Despite the numerous edible plant species available worldwide, more than 60% of human energy needs are provided by maize, rice, wheat, and potato.[Citation5] However, with the world population projected to grow beyond 8.5 billion by the year 2030[Citation6] and the competing utilization of cereals in livestock farming, the demand for these crops will exceed their supply.[Citation7] Furthermore, the overdependence on these carbohydrate-based crops is one of the causes for the recent increase in malnutrition and chronic diet-related diseases namely hypertension, cardiovascular diseases, cancer, and diabetes in developing countries.[Citation5]
To combat this, several UCs have shown the potential to improve food and nutrition as they contain important health-promoting phytochemicals, vitamins, macro and micronutrients.[Citation8] Therefore, diversification of diets to include UCs could be a vital approach for improving food and nutrition in SSA.[Citation5]
Root and tuber crops are important sources of food and nutrition in developing countries, only second to cereals. Cocoyam is characterized by high yields per acre of land with a high quality of starch, various micro-nutrients, and medicinal value.[Citation9] Concurrently, OFSP contains bioactive compounds such as polyphenols, carotenoids, ascorbic acid, and anthocyanins with health-promoting benefits[Citation10,Citation11] as well as macronutrients including carbohydrates, vitamins, and dietary fiber.[Citation12,Citation13] These aspects make the two crops nutritionally superior as compared to cereals. As such, they can be used as raw materials to manufacture functional foods such as baby formula and food for people with chronic ailments.[Citation14] However, both crops are underutilized in the SSA. The uptake and commercialization of cocoyam and OFSP are hampered by their short storage time due to their high moisture content.[Citation9] Therefore, processing of the two crops into dried products such as slices, chips and flour could not only improve their storability but also improve their degree of utilization. For instance, the flour from dried and milled slices and chips could be utilized to fortify foodstuffs derived from readily available staple crops and also for partial substitution of wheat flour in bakery products in SSA.[Citation9]
Within the rural communities in SSA, traditional food processing techniques such as sun-drying are commonly utilized to preserve roots and tubers. Sun drying is not only labor and time-intensive but also adversely affects the overall quality of the product.[Citation15] The lack of appropriate technologies, combined with insufficient dissemination of proper processing methodologies across small-scale farmers and rural communities contribute significantly to quantitative and qualitative losses of the UCs. In recent years, several drying methodologies have been developed in the context of retaining essential nutrients in dried products. summarizes the most recent results from investigations on the processing and preservation of UCs. The results demonstrate the potential of UCs to increase food and nutritional security. However, further investigations on proper drying techniques, process set-ups, and their optimum settings are required to establish the optimal settings for maximum retention of product quality.
Table 1. Summary of selected UCs studied and drying systems used.
The current study aims to investigate the effect of different drying conditions on the overall quality attributes of two UCs namely cocoyam and OFSP. The overarching objective of this study is to assess the potential of the use of cocoyam and OFSP to produce innovative food products to increase food and nutritional security across small-scale holders and women groups in SSA. Overall, it is expected that the results from this study will help in the reduction of post-harvest losses and provide income-generating options for small-scale processors by giving an indication of the optimal process settings for the design of low-tech decentralized technologies.
2. Materials and methods
2.1. Materials utilized
Cocoyam tubers (Colocasia esculenta L. Schott) and OFSP roots (Ipomoea batatas L.cv.CRI-Apomuden) were harvested at maturity. The tubers were carefully harvested by hand and inspected to discard the ones with visible blemishes. The freshly harvested tubers were immediately refrigerated at 4 °C and utilized for experiments within 1–5 days.
2.2. Sample pre-processing
Cocoyam tubers were peeled with a sharp knife, washed in deionized water, and carefully dried with a towel. To ensure uniform material properties, the first and last 25 mm at both ends of the tubers were trimmed off. The midsection was then sliced into 4 mm slice thickness with a Graef Vivo V20EU bench slicer (GRAEF GmbH, Arnsberg, Germany). A core cutter was then utilized to cut the slices to 25 mm diameter slices.
For OFSP, roots in two conditions (peeled and unpeeled) were processed for the drying experiment. The peeling of the roots was done manually with a stainless-steel knife after the roots were cleaned with clean water. The peeled or unpeeled OFSP tubers were cut into uniform slices of 3 mm thickness using a Ritter E16 bench slicer (Ritterwerk GmbH, Gröbenzell, Germany) then pretreated in a 0.5% Na2S2O5 solution for 5 minutes to minimize enzymatic browning.
2.3. Experimental design and drying experiments
2.3.1. Cocoyam
The experimental plan consisted of one level of drying air velocity (2 m·s−1) and three levels of drying temperature (40, 60, and 80 °C). Drying experiments were conducted using a HT Mini cabinet dryer (Innotech Ingenieursgesellschaft mbH, Altdorf, Germany). At the outset of drying, the dryer was operated with all trays in place at the target settings for 30 minutes to achieve uniform steady-state conditions. The cocoyam slices were then dehydrated until a final moisture content of 0.11 − 0.14 gW/gDM was reached. As the drying experiments proceeded, 15 slices were drawn from the dryer at once every 30 minutes for weight measurement (3 slices), color attributes (3 slices), and bioactive composition (9 slices). The slices drawn for weight measurement and color attributes were returned to the dryer while the slices for determination of bioactive composition were sealed within vacuum-grade bags using a LAVA V300 vacuum sealer (LANDIG Sondergerätebau, Bad Saulgau, Germany) and kept in a freezer at −28 °C until further analysis.
2.3.2. Orange-fleshed sweet potato
The peeled or unpeeled OFSP slices were dried at 4 different temperatures (40, 50, 60, and 70 °C) using the dryer that was preheated for 30 min as described in sub-section 2.3.1. The treated OFSP slices (300 g) were spread evenly in a thin –layer on a perforated tray and dehydrated to a moisture content of 0.10 − 0.13 gW/gDM. During the drying experiment, both the weight of the sample and CIELAB color parameters were determined at a regular interval of 30 min until the termination of the drying experiment. At the end of drying, samples were drawn from the drying cabinet for chemical analysis.
2.4. Moisture content determination
For moisture content determination, samples were weighed using a Sartorius Excellence E2000D digital weighing balance, ±0.001 g (Sartorius AG, Göttingen, Germany) for cocoyam and the weighing scale PCB 10000-1 (KERN & SOHN GmbH, Balingen, Germany) for OFSP. At the end of each drying run, the slices were dried in a Memmert Oven Drier (Memmert GmbH, Büchenbach, Germany) at 105 °C for 24 hours to establish the dry matter content (DM). The drying rate of the slices was calculated using EquationEqn. (1)(1)
(1) .
(1)
(1)
Where DR = drying rate (gW/gDM·min), Mt and Mt+Δt = moisture content at drying time t and t + Δt (drying timestep, min).
2.5. Color measurement
Instrumental color (L*, a*, b*) values of the cocoyam and OFSP were taken using Chromameter CR400 (Konica Minolta, Marunouchi, Japan). The total color difference was calculated using EquationEqn. (2)(2)
(2) .
(2)
(2)
Where ΔE = total color difference, L* = lightness, a* = greenness/redness and b* = blueness/yellowness, i = initial value and t = value at time t.
The browning index (BI) of the cocoyam was calculated using EquationEqns (3)(3)
(3) and Equation(4)
(4)
(4) .
(3)
(3)
(4)
(4)
2.6. OFSP flour preparation
The drying temperature of 60 °C was selected to further dry the peeled and unpeeled OFSP slices for flour production based on the relatively faster drying rate and better color retention observed at the temperature during experiments. The peeled and unpeeled OFSP slices were prepared as described in sub-section 2.2 and dried to a moisture content of 0.1 gW/gDM. The dried OFSP samples were milled immediately after drying and the flour was packaged into high-density polyethylene bags and stored at 4 °C for further analysis.
2.7. Analysis of bioactive compounds
2.7.1. Extraction of cocoyam samples
The slices preserved for analysis of bioactive compounds were dehydrated with a Christ Epsilon 2-40 freeze dryer (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany) at −85 °C for 96 h. The dehydrated slices were then milled and flour kept in airtight containers awaiting chemical processing. During chemical processing, 0.3 g of each sample was mixed with 10 ml of 80% v/v ethanol and homogenized using a vortex mixer. The blend was then separated for 10 min at 5000 RPM using a Megafuge 16 centrifuge (Thermo Fisher Scientific, Waltham, United States). About 10 ml supernatant was collected using disposable pipettes into airtight containers and kept at −20 °C awaiting further analysis.
2.7.2. Analysis of total phenolic, flavonoid, and antioxidant activity in cocoyam
The Folin-Ciocalteu method was applied to evaluate the total phenolic content (TPC). The concentration of TPC was measured using an Agilent 8453 UV-Vis spectrophotometer (Agilent Technologies, Waldbronn, Germany) at 735.8 nm and expressed as mg of gallic acid per 100 g of dry matter (mg GA/100 gDM). The aluminum chloride method was utilized to evaluate the total flavonoid content (TFC). The concentration of TFC was measured using a spectrophotometer at 425 nm and expressed as mg catechin equivalent per 100 g of dry matter (mg CE/100 gDM). The total antioxidant activity (TAA) was evaluated using the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay. TAA was measured using a spectrophotometer at 515 nm and expressed as a percentage of Radical Scavenging Activity (% RSA).
2.7.3. Extraction of OFSP flour for TPC, TFC, and TAA analysis
The OFSP flour samples were analyzed for TPC, TFC, beta-carotene, ascorbic acid, and TAA. Before the analyses of TPC, TFC, and TAA, the OFSP flour samples were extracted as follows; about 2 g of the flour was added to 16 ml of the extraction solvent (80% methanol and 1% HCl) and kept in a dark enclosure for 24 h at 25 ± 2 °C. After incubation, the blend was separated for 30 min at 4000 rpm (2701 × g) using a Rotofix 32 A centrifuge (Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany). The supernatant was carefully transferred into a cleaned empty container and the residue was extracted two additional times. The three collections of supernatants were pooled together and stored at −20 °C until all analyses were done.
2.7.4. Analysis of TPC, TFC, and TAA in OFSP flour
TPC in the OFSP flour sample was measured following the Folin–Ciocalteu method. Extract (0.5 ml was mixed with 5 ml of Folin-Ciocalteu reagent (1 mol) after which 4 ml of sodium carbonate (7.5%, w/v) was added followed by a 2 h incubation at 25 ± 2 °C. The absorbance at 765 nm with a C-7000UV UV-Vis Spectrophotometer (Peak Instruments, Houston, TX, USA) was read. Gallic acid was utilized to develop a standard calibration curve and TPC was expressed as mg gallic acid equivalence per 100 gDM of the sample. The colorimetric method was applied for TFC analysis. About 5 mL of the extract was added to 0.15 ml of 5% sodium nitrite solution and 2 ml of distilled water and kept for 5 min after which 0.15 ml of 10% AlCl3 solution was added to the mix. The blend was incubated for 5 min under room temperature (25 ± 2 °C) after which 1 ml of 1 M NaOH was added, thoroughly mixed and the blend kept at ambient conditions for 15 min. The absorbance value was read at 415 nm using a spectrophotometer. Catechin was used for the standard calibration curve and TFC was expressed as mg catechin equivalence per 100 gDM. The phosphomolybdenum complex method was applied for the TAA measurement.
2.7.5. Analysis of beta-carotene and ascorbic acid in OFSP flour
The β-carotene content was analyzed spectrophotometrically where the absorbance was read at 450 nm using a C-7000UV UV-Vis Spectrophotometer (Peak Instruments, Houston, TX, USA). The conversion factor of 13 μg ß-carotene: 1 μg retinol activity equivalent (RAE) was used to calculate the vitamin A content. The ascorbic acid content in OFSP flour samples was measured using the 2,6-dichlorophenolindophenol titrimetric method.
2.9. Statistical analysis
Statistical analysis was conducted in the Python software (Python Software Foundation, Delaware, United States), using the statsmodels module. Analysis of Variance (ANOVA) was conducted and means comparisons were performed using Tukey's HSD test. Statistical tests were evaluated at the 0.05 level of significance.
3. Results and discussions
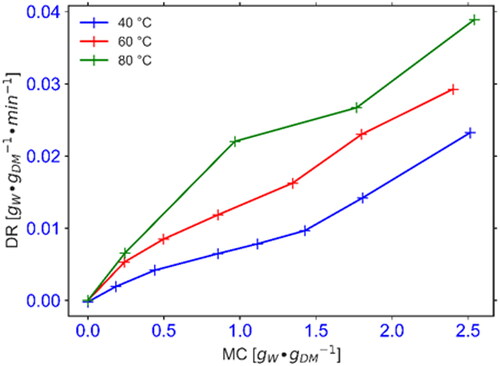
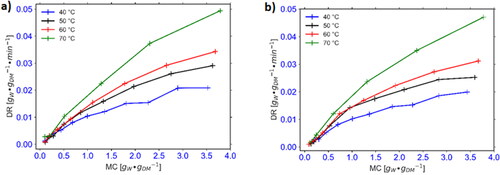
3.1. Drying rates for cocoyam and OFSP slices
and show the plots for drying rate against the moisture content for cocoyam and OFSP, respectively. The fresh cocoyam and OFSP samples utilized in this experiment had moisture contents of 2.13 ± 0.03 gW/gDM and 3.62 ± 0.14 gW/gDM, respectively. For the cocoyam, the average drying duration to reduce the moisture ratio to about 0.1 for experiments conducted at 40, 60, and 80 °C was found to be 330, 210, and 135 min, respectively. In the case of the OFSP, the effective drying time observed for 40, 50, 60, and 70 °C drying air temperatures were 300, 240, 210, and 150 min, respectively for the peeled OFSP and 360, 300, 270, and 180 min, respectively for the unpeeled OFSP. The drying rate curves for both crops reveal a two-stage falling rate behavior with a fast rate in the initial stage and a comparatively slower rate in the second stage. The effective drying time for the cocoyam decreased by 59.1% as the drying temperature increased from 40 to 80 °C whereas the drying duration for OFSP slices decreased by 50% with an increase in drying temperature from 40 °C to 70 °C. At all drying temperatures, the peeled OFSP had a slightly shorter drying time as compared to the unpeeled samples.
3.2. Color of dried samples
The color of processed food products is a significant attribute that is associated with the quality and the visual appearance of the products.[Citation24,Citation25]
3.2.1. Cocoyam
represents the CIELAB color indices (L*, a*, and b*), the total color difference (ΔE), and the browning index (BI) of the fresh and hot air-dried cocoyam. The drying process caused a slight decrease in L* value of the cocoyam slices from 86.28 ± 1.18 in the fresh slices to 80.26 ± 1.61, 82.46 ± 0.21, and 83.18 ± 0.75, respectively in the 40 °C, 60 °C, and 80 °C dried cocoyam. The decrease was statistically significant at 40 and 60 °C (p < 0.01 and p < 0.05, respectively) but not at 80 °C. This decline in L* could be attributed to oxidative reactions during drying as catalyzed by the drying temperature.[Citation26,Citation27] The extended duration of drying at 40 °C and 60 °C in comparison to 80 °C allowed adequate time for the oxidative reactions to occur with significance. Although the change in a* value was insignificant (p > 0.05), the drying process caused an increase in a* value from an initial value of 1.69 ± 0.39 to between 2.62-3.50 as the drying temperature decreased from 80 °C to 40 °C. The value of b* of 40 °C dried cocoyam samples (8.47 ± 0.12) increased significantly (p < 0.05) but decreased at 80 °C drying temperature (6.35 ± 0.33) as compared to the b* value of the fresh cocoyam (7.51 ± 0.70) (p > 0.05). The increase in yellowness, b* values at low drying temperature (40 °C) could be ascribed to the development of brown pigments during drying[Citation28] whereas the loss in b* values in cocoyam for 80 °C drying temperature can be associated with the degradation of heat-sensitive natural pigments in cocoyam.[Citation26,Citation27]
Table 2. CIELAB color values and browning index of fresh and dried cocoyam.
The ΔE and BI values of the dried cocoyam slices indicated in revealed significant (p < 0.01) reductions in ΔE and BI from 8.64 to 2.29, and 13.99 to 9.68, respectively as the drying temperature increased from 40 °C to 80 °C. The longer duration of drying at the lower drying temperatures allowed enzymatic browning to occur under the action of the Polyphenol Oxidase enzyme (PPO).[Citation29] However, at higher drying temperatures, early and intermediate Maillard Reaction Products (MRP) which are colorless could have been formed in the short duration of drying. It is possible that these reactions did not proceed to the terminal stage where the colorless products could be converted to brown products.[Citation30]
3.2.2. OFSP
The L*, a*, b*, and ΔE values for the fresh and dried OFSP slices for the different drying air temperatures are presented in for the peeled and unpeeled OFSP slices respectively. The L*, a*, and b* values of the fresh OFSP slices measured between 73.1 ± 1.58, 20.90 ± 0.67, and 44.26 ± 1.83, for peeled OFSP and 70.68 ± 2.20, 19.73 ± 0.82, and 42.19 ± 1.45, for the unpeeled OFSP. The drying process caused darkening of the OFSP slices as affirmed by the decrease in L* values of between 58.68-71.69, and 50.19-69.38, respectively for the peeled and unpeeled dried OFSP slices as the drying temperatures increased from 40 to 70 °C. The decrease in L* which could be attributed to oxidative reactions during drying[Citation26,Citation27] was much higher among the OFSP slices dried at lower temperatures (40 and 50 °C) as compared with the 60 °C and 70 °C drying temperatures. Moreover, a significant (p < 0.05) reduction occurred in a* and b* values for both the peeled and unpeeled samples as compared to their fresh sample values (). The degree of change observed in a* value for both peeled and unpeeled OFSP dried slices was highest at 70 °C followed by 40 °C and 50 °C whereas the lowest change in a* value was observed in samples dried at 60 °C. A similar trend was observed for the b* where the reduction in b* value was higher for 40 and 70 °C drying temperatures as compared to 60 °C. The decrease in a* and b* values of the dried OFSP slices can be attributed to the degradation of carotenoids and the production of brown pigments during drying.[Citation24,Citation26,Citation27]
Table 3a. CIELAB color values of fresh and peeled OFSP dried slices.
Table 3b. CIELAB color values of fresh and unpeeled OFSP dried slices.
The ΔE has been used extensively to examine the color change in food products during processing. The ΔE in the final dried OFSP slices was significantly (p < 0.01) lower for 60 °C dried slices (11.49-13.92) and higher for the 40 °C (27.01-30.64) and 70 °C (21.77-22.93) dried samples. Generally, drying peeled and unpeeled OFSP slices at 60 °C air temperature resulted in higher retention of carotenoids and other plant pigments as indicated by the higher a* and b* and lower ΔE values in the final dried samples as compared with the 40, 50, and 70 °C drying temperatures.
3.3. Bioactive compounds
Bioactive compounds of plant origin contain antioxidant and anti-inflammatory attributes that are crucial in the prevention of various acute or chronic conditions.[Citation31]
3.3.1. Cocoyam
shows the changes in the bioactive composition of cocoyam slices as a result of the drying experiments. The TPC, TFC, and TAA content of the fresh cocoyam roots utilized for experiments were quantified to be 0.48 ± 0.02 mg GA/100 gDM, 143.31 ± 3.71 mg/100 gDM, and 80.98 ± 0.30% RSA, respectively.
Figure 3. Bioactive compounds in fresh and dried cocoyam. Identical letters on bars indicate no significant difference (p > 0.05).
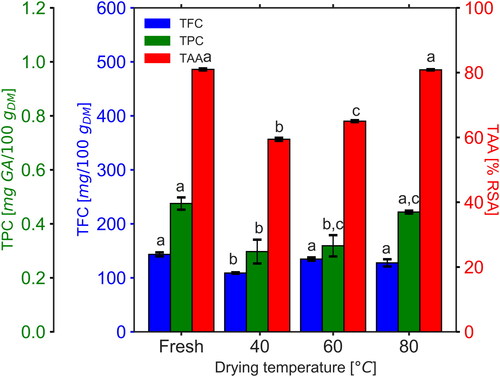
TPC was observed to significantly decrease after drying at 40 °C and 60 °C (p < 0.01). However, the change in TPC after drying at 80 °C was insignificant (p > 0.05). TFC was observed to be significantly decreased after drying at 40 °C (p < 0.01) as compared to the higher temperature settings. The decrease in phenolic compounds at 40 °C may be related to the degradation of phenolic compounds under the action of partial inactivation of oxidative enzymes possibly at the low drying temperatures.[Citation32] TAA was significantly reduced as a result of drying at 40 °C and 60 °C (p < 0.001) but no significant change was observed at 80 °C. At 80 °C, rapid inactivation of oxidative enzymes and the short duration of drying preserved the phenolic compounds which are known to exert antioxidant activity.[Citation33] Additionally, the early and intermediate MRP formed in the short duration of drying at 80 °C also possess radical scavenging activity and have an inhibitory effect on the Polyphenol Oxidase enzyme (PPO).[Citation30] These factors could have contributed to preventing the decrease in TAA at the higher temperature setting.
3.3.2. OFSP flour
shows the contents of bioactive compounds and total antioxidant activity in peeled and unpeeled OFSP flours. In this study, both the peeled and unpeeled OFSP flour contained significant levels of bioactive compounds. The values of β-carotene content and vitamin A activity of unpeeled OFSP flour (9.53 ± 0.31 mg/100 gDM and 0.73 ± 0.01 mg RAE/100 gDM, respectively) were about 27.49% higher than the values measured for the peeled OFSP flour (6.91 ± 0.42 mg/100gDM and 0.53 ± 0.01 mg RAE/100 gDM respectively). This suggests that the peeling caused a significant loss of β-carotene that consequently reduces the vitamin A activity in the flour. The ß-carotene values measured for OFSP flour in this study were higher than the 56.74 mg/100 gDM for peeled OFSP flour dried at 60 °C in a cabinet dryer reported by Ruttarattanamongkol et al.[Citation11] but lower than the 33.5-91.6 mg/100 gDM mentioned for flour of some OFSP cultivars by other authors.[Citation13]
Table 4. Average bioactive compounds values (expressed per 100 gDM) of peeled and unpeeled OFSP flour processed from 60 °C air-dried OFSP.
Nevertheless, both flour types contained considerable amounts of ascorbic acid, the amount in the peeled OFSP flour (35.72 ± 0.66 mg/100 gDM) was about 1.38 times higher than the values determined in the unpeeled flour (25.90 ± 0.18 mg/100 gDM). The higher loss of ascorbic acid in unpeeled OFSP samples as compared to the peeled samples could be a result of extensive oxidation and thermal breakdown due to the longer duration of drying time.[Citation34] Total phenolics and flavonoids are also important bioactive compounds with antioxidant properties. The TPC, TFP, and TAA values of the unpeeled OFSP flours were higher by 16.87%, 2.69%, and 3.61%, respectively than the corresponding values for the peeled OFSP flour. The values of TPC observed in the current study (155.0-186.5 mg/100 gDM) for both peeled and unpeeled OFSP flours were higher than the 49.8-107.9 mg GAE/100 g reported for OFSP flours produced from 1 mm slice thickness, pretreated with 1% and 3% citric acid and dried at 55-65 °C reported by Kuyu et al..[Citation13] The variations in the results could be ascribed to differences in cultivar, pre-drying, and drying operations.[Citation35] TFC and TAA values were higher among the unpeeled OFSP flour than the peeled OFSP flour sample. This study has shown that both peeled and unpeeled OFSP flours are good sources of bioactive compounds for biofortification in other raw materials or food products low in these compounds.
3.4. General discussions
Diversification of diets to include UCs could play a significant role in improving food and nutrition security and household incomes, especially for women in rural communities in developing countries.[Citation5] While UCs contribute significantly to the macro-and micro-nutrients consumed by rural communities, their seasonality and perishability prevent their utilization to their full potential.[Citation5,Citation29] Decentralized processing of UCs using a combination of improved traditional methods to produce novel products with extended shelf life could improve their storability, marketing, and diversification of household nutritional sources and incomes.[Citation5] Sun and solar drying, production of blended flours and soups, and production of baked products are appropriate and fairly-utilized methods of decentralized processing in SSA.[Citation12,Citation16] However, insufficient knowledge on suitable process set-ups and settings as well as quality control could result in quantitative and qualitative losses of the product.[Citation15] Very often the definition of quality parameters differs between processors and consumers; with processors being more concerned with sugar content, firmness, and color while consumers tend to focus on the overall appearance.[Citation36] Food processors, therefore, aspire to minimize changes in the overall easily perceptible quality during food drying. However, with increasing consumer awareness of food nutrition, it is also essential to produce products that appeal to and meet all the demands.[Citation16] Furthermore, in disadvantaged population groups, particularly in SSA but also globally, natural food additives developed from UCs can offer as an alternative resource for micro-nutrients, vitamins, and minerals and thus play a crucial role in alleviating malnutrition and severe consequences of hidden hunger.[Citation37]
This study provides an exemplary approach to the establishment of optimal processing settings with the product quality in mind for cocoyam and OFSP which are common UCs in various countries in SSA. Based on this information, the central needs for the design of appropriate decentralized processing units were defined.
In this study, experiments were conducted to investigate the influence of different temperatures (40, 60, and 80 °C for cocoyam and 40, 50, 60, and 70 °C for OFSP) on quality attributes such as color and bioactive compounds of slices and flour. For cocoyam, extensive color changes were observed for the lowest temperature (40 °C, ΔE = 8.64) and further reduced by 59.87% for 60 °C and 73.46% for 80 °C. The longer duration of drying at the lower drying temperatures (i.e., 40, 60 °C) allowed enzymatic browning to occur under the action of the Polyphenol Oxidase enzyme (PPO).[Citation29] However, as a result of the short drying duration at the higher drying temperature (i.e., 80 °C), Maillard reactions may have produced colorless products which did not proceed to the terminal stage of conversion to brown products.[Citation30] For peeled and unpeeled OFSP, higher color changes were found in samples dried at 40 °C which could be attributed to enzymatic degradation of plant pigments worsened by the longer drying time,[Citation32] and at 70 °C as a result of the thermal degradation of natural pigments at the high drying temperature.[Citation34] Similar findings were also obtained by Saxena et al.[Citation38] for jackfruits and Md Saleh et al.[Citation39] for carrots.
Cocoyam and OFSP tubers contain various bioactive compounds including phenolic compounds such as flavonoids and in particular anthocyanins.[Citation29,Citation40] The combined effect of these compounds and the rich vitamin content in the tubers exhibit a strong antioxidant potential.[Citation40,Citation41] In comparison to control samples, hot-air drying at 40 °C and 60 °C resulted in a decrease in TPC, TFC, and TAA. This could be attributed to the enzymatic degradation of phenolic compounds at lower drying temperatures.[Citation32] However, the change in TPC, TFC, and TAA at 80 °C was insignificant. This could be because rapid inactivation of oxidative enzymes and the short duration of drying preserved the phenolic compounds which are known to exert antioxidant activity.[Citation33] Moreover, Maillard reaction products formed at 80 °C could have inhibited the Polyphenol Oxidase enzyme (PPO) in the short duration of drying.[Citation30]
This could be associated with the degradation of the phenolic compounds by oxidative enzymes at the low drying temperatures and aggravated by the prolonged dying time.[Citation32] For OFSP flour, the unpeeled samples showed high retention of TPC, TFC, and TAA as compared to the peeled flour samples. Moreover, β-carotene retention and vitamin A activity were higher in unpeeled OFSP flour by 27.49% as compared to the peeled flour. This observation can be attributed to the considerably higher contents of bioactive compounds in the peels of OFSP as compared to the flesh.[Citation42] Conversely, unpeeled OFSP flours exhibited a 37.92% decrease in ascorbic acid as compared to peeled OFSP flours possibly as a result of extensive oxidation and thermal breakdown due to the longer drying duration.[Citation34]
The process of identification and selection of optimal drying settings is a balancing act between preservation of product quality, optimization of process throughput, reduction of production costs, and ensuring resource efficiency.[Citation43] Therefore, a multi-objective approach rather than a singular objective (i.e., drying temperature or drying time) approach is required for holistic optimization of the process. Previous studies have shown that focusing on temperature alone can lead to significant degradation in quality attributes.[Citation44] At the same time, focusing on the reduction of drying time (i.e., using higher temperatures) can also lead to a myriad of reactions that can destroy heat-labile nutrients and structural attributes within the product.[Citation30,Citation43]
In SSA, as summarized in several drying techniques are implemented to dehydrate UCs. While traditional open air and direct sun drying are the easiest to adopt at a low cost and technological knowledge;[Citation45] the unpredictable weather conditions render the drying process uncontrollable. As a result, throughput is uncertain and the quality of the final product is non-uniform. Therefore, it is essential to adopt solar-assisted drying techniques for better processing of UCs. Solar dryers such as solar greenhouse dryers, solar mixed-mode dryers, solar tunnel dryers, and solar-hybrid dryers provide various advantages over traditional open-air drying such as increased drying rates while preserving product quality.[Citation46] During peak daily insolation, drying temperatures greater than 30 °C can be attained depending on the solar drying technology.[Citation45] In the context of rural communities and this study, utilization of low-tech and low-cost solar units for agro-processing is justified. However, such units should be developed with the process and quality criteria recommended in this study in mind.
Through the investigations conducted within the current study, it can be concluded that a drying temperature of 80 °C and 60 °C are the optimal processing temperatures of cocoyam and OFSP respectively for the majority of quality attributes considered. Drying of cocoyam at 80 °C and OFSP at 60 °C not only reduces drying time but also preserves quality attributes including color, phenolic content; β-carotene, vitamin A, and antioxidant activity. However, the retention of yellow pigments (b*) in dried cocoyam was better at the drying temperature of 60 °C while retention of TFC was similar at both 60 °C and 80 °C. Additionally, the best retention of TPC was at the drying temperature of 80 °C.
Since higher temperatures of up to 80 °C are more technologically challenging to obtain for solar-assisted dryers while it is economically sound to utilize a single drying unit for multiple products, such a unit should be designed for temperatures up to 60 °C. This would enable rural farmers to process both cocoyam and OFSP as well as other products using a single unit with a reasonable compromise on product quality. The UPGRADE Plus project developed a modular solar-assisted low-tech drying unit which can be built from locally available materials and that enabled operation in rural areas of West Africa. provides a schematic of the dryer with an inside view of the drying chamber.
Figure 4. Schematic of the dryer developed in the UPGRADE Plus project, including a front view of the drying chamber’s inside.
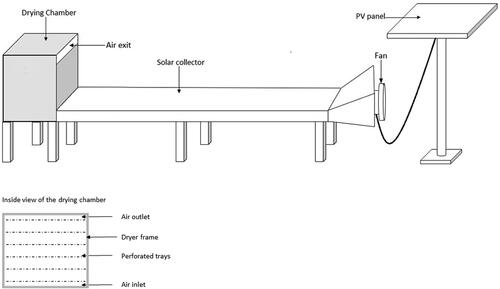
The modular dryer allows decentralized processing of multiple products. Due to ease of transportation and assembly it can also be used in a flexible manner. Replication of such solar assisted dryer systems for local processing of UCs such as cocoyam and OFSP could open up new market channels for small-scale farmers and reduce rural poverty. Furthermore, the income generated from value-added UCs products has the potential to promote self-sufficiency and empowerment of disadvantaged groups including women groups within SSA. The combination of optimal processing techniques and decentralized modular solar-driven units will thus contribute to improving the overall food and nutrition security in SSA.
4. Conclusion
The results obtained from the investigation conducted reveal that both cocoyam and OFSP products are rich sources of bioactive compounds and natural antioxidants. The overall retention of quality attributes in the final dried products strongly depends on the combined effect of drying time and temperature as well as pre-drying processes. TPC, TFC, and TAA of cocoyam declined significantly (p < 0.05) after drying at 40 °C as compared to 80 °C drying temperature. In the case of OFSP, the unpeeled had higher retention for TPC, TFC, β-carotene, vitamin A activity, and TAA but low ascorbic acid content than the peeled OFSP dried slices. In the range of drying settings investigated, hot air drying of cocoyam at 80 °C and OFSP at 60 °C resulted in a relatively shorter time, less color change, higher retention of bioactive compounds, and antioxidant activity in the dried products. Due to energetic and feasibility considerations for the realization of solar assisted drying systems the best compromise in terms of drying temperature was found to be 60 °C.
These results provide drying settings for decentralized processing of cocoyam and OFSP into innovative dried products with improved nutritional quality. Further investigations should focus on the optimization and further development of low-tech, off-grid, and renewable energy-driven processing technology including but not limited to the drying technology.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- FAO, IFAD, UNICEF, WFP, & WHO. The State of Food Security and Nutrition in the World 2019. In Safeguarding against Economic Slowdowns and Downturns. FAO: Rome, 2019. DOI: 10.1109/JSTARS.2014.2300145.
- Muthayya, S.; Rah, J. H.; Sugimoto, J. D.; Roos, F. F.; Kraemer, K.; Black, R. E. The Global Hidden Hunger Indices and Maps: An Advocacy Tool for Action. PLoS One 2013, 8, e67860. DOI: 10.1371/journal.pone.0067860.
- Cockx, L.; Colen, L.; De Weerdt, J.; P. S, G. Urbanization as a Driver of Changing Food Demand in Africa: Evidence from Rural-Urban Migration in Tanzania. Publications Office of the European Union: Luxembourg, 2019, JRC Technical Reports; Vol. JRC107918. DOI: 10.2760/515064.
- Piacentini, R. D.; Novara, I.; Mujumdar, A. S. Climate Change and Pandemics: New Challenges for Science and Technology. Drying Technol. 2020, 38, 1391–1392. DOI: 10.1080/07373937.2020.1786981.
- Baldermann, S.; Blagojević, L.; Frede, K.; Klopsch, R.; Neugart, S.; Neumann, A.; Ngwene, B.; Norkeweit, J.; Schröter, D.; Schröter, A.; et al. Critical Reviews in Plant Sciences Are Neglected Plants the Food for the Future? Crit. Rev. Plant Sci. 2016, 35, 106–119. DOI: 10.1080/07352689.2016.1201399.
- United Nations. World Population Prospects 2019: Highlights. https://population.un.org/wpp. (accessed Oct 24, 2019).
- Lebot, V. Tropical Root and Tuber Crops: Cassava, Sweet Potato, Yams and Aroids. Crop Production Science in Horticulture; CABI: Oxfordshire, 2009; Vol. 17.
- Raice, R. T.; Chiau, E.; Sjoholm, I.; Bergenstahl, B. The Loss of Aroma Components of the Fruit of Vangueria Infausta L. (African Medlar) after Convective Drying. Drying Technol. 2015, 33, 887–895. DOI: 10.1080/07373937.2014.995804.
- Zhang, Z.; Wei, Q.; Liu, C.; Li, D.; Liu, C.; Jiang, N. Comparison of Four Pretreatments on the Drying Behavior and Quality of Taro (Colocasia esculenta L. Schott) Slices during Intermittent Microwave Vacuum-Assisted Drying. Drying Technol. 2017, 35, 1347–1357. DOI: 10.1080/07373937.2017.1323761.
- Lagnika, C.; Jiang, N.; Song, J.; Li, D.; Liu, C.; Huang, J.; Wei, Q.; Zhang, M. Effects of Pretreatments on Properties of Microwave-Vacuum Drying of Sweet Potato Slices. Drying Technol. 2019, 37, 1901–1914. DOI: 10.1080/07373937.2018.1543702.
- Ruttarattanamongkol, K.; Chittrakorn, S.; Weerawatanakorn, M.; Dangpium, N. Effect of Drying Conditions on Properties, Pigments and Antioxidant Activity Retentions of Pretreated Orange and Purple-Fleshed Sweet Potato Flours. J. Food Sci. Technol. 2016, 53, 1811–1822. DOI: 10.1007/s13197-015-2086-7.
- Chikpah, S. K.; Korese, J. K.; Hensel, O.; Sturm, B. Effect of Sieve Particle Size and Blend Proportion on the Quality Properties of Peeled and Unpeeled Orange-Fleshed Sweet Potato Composite Flours. Foods 2020, 9, 722–740. DOI: 10.3390/foods9060740.
- Kuyu, C. G.; Tola, Y. B.; Mohammed, A.; Ramaswamy, H. S. Determination of Citric Acid Pretreatment Effect on Nutrient Content, Bioactive Components, and Total Antioxidant Capacity of Dried Sweet Potato Flour. Food Sci. Nutr. 2018, 6, 1724–1733. DOI: 10.1002/fsn3.747.
- Pereira, P. R.; Silva, J. T.; Verícimo, M. A.; Paschoalin, V. M. F.; Teixeira, G. A. P. B. Crude Extract from Taro (Colocasia esculenta) as a Natural Source of Bioactive Proteins Able to Stimulate Haematopoietic Cells in Two Murine Models. J. Funct. Foods 2015, 18, 333–343. DOI: 10.1016/j.jff.2015.07.014.
- Sturm, B.; Hensel, O. Pigments and Nutrients during Vegetable Drying Process, Dried Products Storage and Their Associated Colour Changes Products. In Handbook of Drying of Vegetables and Vegetable Products; Zhang, M., Bhandari, B., Fang, Z., Eds.; CRC Press, 2016; pp 257–277.
- Adeboyejo, F. O.; Aderibigbe, O. R.; Obarayi, M. T.; Sturm, B. Comparative Evaluation of Instant “Poundo” Cocoyam (Colocasia esculenta) and Yam (Dioscorea Rotundata) Flours Produced by Flash and Cabinet Drying. Int. J. Food Sci. Technol. 2021, 56, 1482–1490. DOI: 10.1111/ijfs.14703.
- Mutuli, G. P.; Mbuge, D. O. Effect of Drying on the Nutritional and Organoleptic Characteristics of African Leafy Vegetables, Jute Mallow (Corchorus Olitorius L.) and Cowpea (Vigna unguiculata). J. Biosyst. Eng. 2018, 43, 211–218. DOI: 10.5307/JBE.2018.43.3.211.
- Aderibigbe, O. R.; Ezekiel, O. O.; Owolade, S. O.; Korese, J. K.; Sturm, B.; Hensel, O. Exploring the Potentials of Underutilized Grain Amaranth (Amaranthus Spp.) along the Value Chain for Food and Nutrition Security: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 656–669. DOI: 10.1080/10408398.2020.1825323.
- Ronoh, E.; Kanali, C.; Mailutha, J.; Shitanda, D. Thin Layer Drying Kinetics of Amaranth (Amaranthus Cruentus) Grains in a Natural Convection Solar Tent Dryer. Afr. J. Food Agric. Nutr. Dev. 2010, 10, 2218–2233. DOI: 10.4314/ajfand.v10i3.54080.
- Xing, S.; Keding, G. B.; Pawelzik, E. Effects of Processing on Nutrient Composition in Guava- and Jackfruit-Based Snacks. Afr. J. Food Sci. 2021, 15, 236–253. DOI: 10.5897/AJFS2021.2104.
- Ngadze, R. T.; Linnemann, A. R.; Nyanga, L. K.; Fogliano, V.; Verkerk, R. Local Processing and Nutritional Composition of Indigenous Fruits: The Case of Monkey Orange (Strychnos Spp.) from Southern Africa. Food Rev. Int. 2017, 33, 123–142. DOI: 10.1080/87559129.2016.1149862.
- Akintayo, O. A.; Adegbaju, K. E.; Akeem, S. A.; Balogun, M. A.; Adediran, O. J.; Aruna, T. E.; Onwudinjo, H. O.; Akintayo, F. M.; Adesina, B. O.; Ojo, P. K.; Kolawole, F. L. Effect of Parboiling and Drying Pretreatment on the Cooking Time and Quality Attributes of Bambara Groundnut. J. Food Process. Preserv. 2021, 45, e15351. DOI: 10.1111/jfpp.15351.
- Hussein, J.; Ilesanmi, J.; Filli, K.; Sanusi, M. Effects of Drying Methods on the Chemical Properties of Okra (Abelmoschus Esculentus L. Moench) Slices. Curr. J. Appl. Sci. Technol. 2018, 26, 1–10. DOI: 10.9734/CJAST/2018/38796.
- Doymaz, İ. Drying Kinetics, Rehydration and Colour Characteristics of Convective Hot-Air Drying of Carrot Slices. Heat Mass Transfer 2017, 53, 25–35. DOI: 10.1007/s00231-016-1791-8.
- Moon, J. H.; Pan, C.; Yoon, W. B. Drying Characteristics and Thermal Degradation Kinetics of Hardness, Anthocyanin Content and Colour in Purple- and Red-Fleshed Potato (Solanum tuberosum L.) during Hot Air Drying. Int. J. Food Sci. Technol. 2015, 50, 1255–1267. DOI: 10.1111/ijfs.12740.
- Demiray, E.; Tulek, Y. Color Degradation Kinetics of Carrot (Daucus Carota L.) Slices during Hot Air Drying. J. Food Process. Preserv. 2015, 39, 800–805. DOI: 10.1111/jfpp.12290.
- Koca, N.; Burdurlu, H. S.; Karadeniz, F. Kinetics of Colour Changes in Dehydrated Carrots. J. Food Eng. 2007, 78, 449–455. DOI: 10.1016/j.jfoodeng.2005.10.014.
- Argyropoulos, D.; Khan, M. T.; Müller, J. Effect of Air Temperature and Pre-Treatment on Color Changes and Texture of Dried Boletus Edulis Mushroom. Drying Technol. 2011, 29, 1890–1900. DOI: 10.1080/07373937.2011.594194.
- Ndisya, J.; Mbuge, D.; Kulig, B.; Gitau, A.; Hensel, O.; Sturm, B. Hot Air Drying of Purple-Speckled Cocoyam (Colocasia esculenta L. Schott) Slices: Optimisation of Drying Conditions for Improved Product Quality and Energy Savings. Therm. Sci. Eng. Prog. 2020, 18, 100557. DOI: 10.1016/j.tsep.2020.100557.
- Atrooz, O. M. The Effects of Maillard Reaction Products on Apple and Potato Polyphenoloxidase and Their Antioxidant Activity. Int. J. Food Sci. Tech. 2008, 43, 490–494. DOI: 10.1111/j.1365-2621.2006.01478.x.
- Teodoro, A. J. Bioactive Compounds of Food: Their Role in the Prevention and Treatment of Diseases. Oxid. Med. Cell. Longevity 2019, 2019, 3765986–3765984. DOI: 10.1155/2019/3765986.
- Rinaldo, D. Carbohydrate and Bioactive Compounds Composition of Starchy Tropical Fruits and Tubers, in Relation to Pre and Postharvest Conditions: A Review. J. Food Sci. 2020, 85, 249–259. DOI: 10.1111/1750-3841.15002.
- Karakaya, S.; El, S. N.; Taş, A. A. Antioxidant Activity of Some Foods Containing Phenolic Compounds. Int. J. Food Sci. Nutr. 2001, 52, 501–508. DOI: 10.1080/09637480020027000-6-6.
- Vega-Gálvez, A.; Lemus-Mondaca, R.; Bilbao-Sáinz, C.; Fito, P.; Andrés, A. Effect of Air Drying Temperature on the Quality of Rehydrated Dried Red Bell Pepper (Var. Lamuyo). J. Food Eng. 2008, 85, 42–50. DOI: 10.1016/j.jfoodeng.2007.06.032. .
- Van Hal, M. Quality of Sweet Potato Flour during Processing and Storage. Food Rev. Int. 2000, 16, 1–37. DOI: 10.1081/FRI-100100280.
- Shewfelt, R. L. What is Quality? Postharvest Biol. Technol. 1999, 15, 197–200. DOI: 10.1016/S0925-5214(98)00084-2.
- Padulosi, S.; Thompson, J.; Rudebjer, P. Fighting Poverty, Hunger and Malnutrition with Neglected and Underutilized Species (NUS): Needs, Challenges and the Way Forward. Biodiversity International: Rome, 2013.
- Saxena, A.; Maity, T.; Raju, P. S.; Bawa, A. S. Degradation Kinetics of Colour and Total Carotenoids in Jackfruit (Artocarpus Heterophyllus) Bulb Slices during Hot Air Drying. Food Bioprocess Technol. 2012, 5, 672–679. DOI: 10.1007/s11947-010-0409-2.
- Md Saleh, R.; Kulig, B.; Hensel, O.; Sturm, B. Investigation of Dynamic Quality Changes and Optimization of Drying Parameters of Carrots (Daucus Carota Var. laguna). J Food Process Eng. 2020, 43, 1–17. DOI: 10.1111/jfpe.13314.
- Eleazu, C. O. Characterization of the Natural Products in Cocoyam (Colocasia esculenta) Using GC–MS. Pharm. Biol. 2016, 54, 2880–2885. DOI: 10.1080/13880209.2016.1190383..
- Ndisya, J.; Gitau, A.; Mbuge, D.; Arefi, A.; Bădulescu, L.; Pawelzik, E.; Hensel, O.; Sturm, B. Vis-NIR Hyperspectral Imaging for Online Quality Evaluation during Food Processing: A Case Study of Hot Air Drying of Purple-Speckled Cocoyam (Colocasia esculenta L. Schott). Processes 2021, 9, 1804. DOI: 10.3390/pr9101804.
- Brar, A.; Bhatia, A. K.; Pandey, V.; Kumari, P. Biochemical and Phytochemical Properties of Potato: A Review. Chem. Sci. Rev. Lett. 2017, 6, 117–129.
- Khawas, P.; Dash, K. K.; Das, A. J.; Deka, S. C. Drying Characteristics and Assessment of Physicochemical and Microstructural Properties of Dried Culinary Banana Slices. Int. J. Food Eng. 2015, 11, 667–678. DOI: 10.1515/ijfe-2015-0094. .
- Bhatta, S.; Stevanovic Janezic, T.; Ratti, C. Freeze-Drying of Plant-Based Foods. Foods 2020, 9, 87. DOI: 10.3390/foods9010087.
- Chua, K. J.; Chou, S. K. Low-Cost Drying Methods for Developing Countries. Trends Food Sci. Technol. 2003, 14, 519–528. DOI: 10.1016/j.tifs.2003.07.003.
- Ayua, E.; Mugalavai, V.; Simon, J.; Weller, S.; Obura, P.; Nyabinda, N. Comparison of a Mixed Modes Solar Dryer to a Direct Mode Solar Dryer for African Indigenous Vegetable and Chili Processing. J. Food Process. Preserv. 2017, 41, e13216. DOI: 10.1111/jfpp.13216.