?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study aimed to identify the influence of rice protein hydrolysates (RPH) (degree of hydrolysis DH of 2, 6, and 10%) and rice protein isolate (RPI) on the formation and stabilization of the volatile oil droplets and to verify how protein hydrolysate affects the distribution of components on the surface and physicochemical properties of the spray-dried microparticles. In general, RPHs could reduce the interfacial tension (3.78–4.04 mN/m) compared to non-hydrolyzed protein (4.74 mN/m). However, no significant difference was observed between RPH samples. A positive correlation was observed between the interfacial tension and the oil droplet size, in which RPH emulsions showed 8.29–8.66 µm while RPI was 9.06 µm. DH influenced the surface composition of the spray-dried RPH emulsions. RPH10 powder showed a greater presence of protein (3.3%) on the particle surface and consequently exhibited high oil retention (50.0%). In contrast, this sample presented high hygroscopicity attributed to the protein surface. This study provides insights into differences between RPH powders, which can potentially impact the powder functionality and protection of the active compound.
1. Introduction
Using plant protein as a stabilizing agent in oil-in-water emulsions in combination with polysaccharides is being recognized as a significant advancement in microencapsulation with environmental benefits. This trend has garnered the interest of both industry and academia, leading to research into novel, natural, and sustainable sources of emulsifiers. Among these, plant proteins show great promise as substitutes for synthetic emulsifiers and animal proteins.[Citation1] Because plant proteins generally exhibit lower emulsifying properties than animal proteins, various studies have successfully employed enzymatic modifications of vegetable proteins to enhance their emulsifying and functional characteristics for microencapsulating lipophilic compounds using diverse encapsulation techniques.[Citation2–4]
Rice protein has already been reported as a good nutritional source and antioxidant component.[Citation5] However, the results for some techno-functional properties are not very satisfactory.[Citation6] Gomes and Kurozawa[Citation7] showed that rice protein hydrolysates have better emulsifying capabilities than non-hydrolyzed proteins. Enzymatic hydrolysis provides a practical approach to enhance the functionality of proteins that have been degraded due to extraction and isolation conditions.[Citation8] This method implies a reaction between proteolytic enzymes and proteins, leading to structural alterations by cleaving the peptide bonds within the protein chain. Consequently, this process generates short-chain peptides characterized by reduced molecular weights, exposed hydrophobic groups, and improved solubility and emulsifying capabilities.
The strategy involving enzymatic modification of plant proteins imparts distinct attributes to spray-dried microparticles. Nonetheless, there exists limited data regarding the correlation between this technique and the distribution of elements on the microparticles’ surface, which represents a gap in this research domain. Investigating, analyzing, and applying insights into the surface composition of microparticles can contribute to optimizing the formulation of encapsulated products.
As far as we know, investigations into changes in functional properties, especially rehydration, have indicated that they are influenced by particle surface composition.[Citation9,Citation10] Our previous study reported the effects of different DH levels (2, 6, and 10%) on the physicochemical properties of spray-dried linseed oil powders, especially on lipid oxidation during storage.[Citation11] However, changes in chemical composition on the spray-dried surface of emulsion stabilized by rice protein hydrolysates, which are expected to play an essential role in the microencapsulation process of essential orange oil, have not been investigated.
Most active substances present in essential orange oils are extremely vulnerable to oxidative and chemical degradation reactions which may impart an unpleasant taste and loss of functionalities because of evaporation, oxidation, exposure to light, or chemical interactions. The primary compound of orange essential oil, d-limonene, is a terpene that corresponds to about 90 wt% of the mixture, and it is a valuable volatile compound that is applied in a variety of products in the industry, such as in cleaning products, cosmetics, and foodstuff. Therefore, is extremely important to use a method that can avoid this loses and reaction, in this case, microencapsulation is a valuable technique for this purpose.[Citation12]
In this study, we investigated the impact of rice protein hydrolysates on the formation of a protein film on the particle surfaces of spray-dried orange oil powder. The changes in surface chemical composition of the powders were determined by X-ray photoelectron spectroscopy (XPS), followed by an evaluation of the correlation between these results and the powder recovery (as an index of efficiency and economy in the spray-drying process), hygroscopicity (as an index of physical stability during preservation), particle size (as a determining factor in packaging conditions), and efficiency of microencapsulation.
2. Material and methods
2.1. Materials
Orange essential oil was provided by Citrosuco S/A Agroindústria (Matão, Brazil). The wall materials maltodextrin 10 DE Mor-Rex® 1910 and rice protein isolate (RPI) (with a protein concentration of 90% dry weight) were generously contributed by Ingredion (Mogi Guaçu, Brazil) and Gramkow (Joinville, Brazil), respectively. The commercial protease Flavourzyme was procured from Sigma-Aldrich (St. Louis, Mo., U.S.A). All other reagents utilized were of analytical grade.
2.1. Enzymatic hydrolysis of rice protein
The action of the protease Flavourzyme hydrolyzed rice protein isolate to obtain protein hydrolysates with the degree of hydrolysis (DH) of 2, 6, or 10% was conducted as described by Gomes and Kurozawa,[Citation3] using the pH-stat method.[Citation13] Hydrolysis was conducted under optimal enzyme conditions at 50 °C and pH 6.0. A solution containing RPI (5 g/100 mL) was prepared with deionized water and stirred for 1 h at room temperature to ensure complete rehydration and the hydrolysis was carried out using an enzyme/substrate ratio of 2 g/100 g protein. The solutions were stirred and the temperature was kept constant with an external system connected to a thermostatic bath. The control of pH was made by continuous titration of 0.1 mol/L NaOH and the volume of NaOH consumed throughout the hydrolysis reaction was used to calculate the degree of hydrolysis (DH) (EquationEq. 1(1)
(1) ). When the required DH was reached, the enzyme was inactivated by heating at 85 °C for 10 min, followed by cooling in an ice bath. The hydrolyzed mixture was centrifuged at 5000 rpm for 15 min and the supernatant was frozen until further analysis. In that study, rice protein hydrolysates (RPH) were denoted as RPHX, with X representing the degree of hydrolysis (DH).
(1)
(1)
where B is the base consumption (mL); Nb is the normality of the base; Mp is the mass of protein (g); htot is the total number of peptide bonds in the protein substrate (7.40 meq/g rice protein, according to Zhao et al.[Citation14]); and α is the average degree of dissociation of the α -NH2 groups (EquationEq. 2
(2)
(2) ).
(2)
(2)
where pH is the value at which the enzyme hydrolysis was performed, and pKa is the average pKa for α-NH3+.
2.2. Investigation of oil droplet formation: interfacial tension
The effect of DH on the interfacial tension between essential orange oil and water was assessed at 25 °C using a Tracker-S tensiometer (Teclis, Longessaigne, France) through the rising (O/W) drop method. The experimental setup involved the aqueous phase (1.5% protein and 33.5% MD w/w) in the syringe and the lipid phase in the cuvette. Measurements were conducted in triplicate.
2.3. Preparation of emulsions
Based on our previous work[Citation7] on the performance of RPH on oil-in-water emulsions, the proportions of solids correspond to 45% of the total solids as follows: 10% of essential orange oil, 1.5% RPI or RPH, and 33.5% of maltodextrin. The emulsions were prepared using a rotor-stator homogenizer (Ultra-turrax IKA T18 Basic, Wilmington, USA) operating at 15,600 rpm for 2 min.
2.3.1. Droplet size distribution and mean droplet size of emulsions
The size distribution of oil droplets and their average size are characterized by the Sauter mean diameter (D3,2) and polydispersity index (PDI), respectively. These parameters were determined in triplicate using the laser diffraction technique with a Malvern Mastersizer 2000 instrument (Malvern Instruments Ltd., UK).
2.4. Production and characterization of orange oil spray-dried microparticles
All emulsions were subjected to spray drying using a laboratory-scale spray dryer (Lab Plant SD-06A, North Yorkshire, UK) comprising a dryer chamber measuring 500 × 215 mm and a double-fluid atomizer nozzle with a diameter of 0.5 mm, operated at 2.8 bar pressure. The emulsion was delivered to the spray dryer nozzle via a peristaltic pump at a feed rate of 485 mL/h at room temperature. Drying was conducted at inlet and outlet air temperatures of 180 ± 5 °C and 110 ± 5 °C, respectively.[Citation3]
2.4.1. Powder recovery
Powder recovery (%) was determined for each treatment and calculated as the ratio of the powder mass obtained at the spray dryer outlet to the mass of solids in the feed solution, expressed as a percentage.
2.4.2. Moisture content and water activity
The microparticle moisture content was assessed gravimetrically by drying samples in a vacuum oven at 70 °C until a consistent weight was achieved. The microparticle water activity (aw) was measured at 25 °C using a Novasina thermoconstanter (Novasina AG Zürich, Switzerland). Both analyses were conducted in triplicate.
2.4.3. Microparticle hygroscopicity
Two grams of the microparticle were placed in a desiccator containing a saturated NaCl solution (maintaining 75% relative humidity) and left at room temperature for 7 days. Powder hygroscopicity was quantified as the amount of moisture adsorbed, expressed in grams per 100 g of dry powder.[Citation15]
2.4.4. Microparticle size distribution
The microparticle size distribution was evaluated in triplicate using a laser light diffraction instrument (Mastersizer 2000, Malvern Instruments Ltd., UK). A small quantity of microparticles was dispersed in a stirred solution of 99.5% ethyl alcohol. The microparticle size distribution was continuously monitored during each subsequent measurement until the readings reached a stable state. The mean diameter was reported as the Brouckere diameter (D4,3).
2.4.5. Oil retention
The oil retention on the microparticles was determined by EquationEquation 2(2)
(2) .
(3)
(3)
The total oil content was determined through hydro-distillation using a Clevenger apparatus, following the method outlined by Jafari et al.[Citation16] with certain adjustments. Approximately 3 g of powders were dissolved in 150 mL of deionized water within a 500 mL round bottom flask. The flask was manually agitated for 2 min to facilitate dissolution of the microparticles. The Clevenger apparatus was then positioned atop the flask, with a condenser containing water circulating at 5 °C attached to the Clevenger. Distillation was carried out for a duration of 2 h, and the volume of distilled oil was directly observed in the Clevenger. This volume was multiplied by the density of orange essential oil (0.843 g cm−3) to determine the mass of the recovered oil. The theoretical oil content was calculated as the anticipated oil mass within the microparticles based on the emulsion formulation on a dry basis.
2.4.6. The surface composition by X-ray photoelectron spectroscopy (XPS)
The microparticle surface composition was analyzed using a K-Alpha spectrometer (X-ray Photoelectron Spectrometer, Thermo Scientific, England). The samples were placed in a high vacuum (2 × 10−7 Pa) and exposed to well-defined X-ray radiation (monochromatic AlK-α X-ray source, 1487 eV), resulting in the emission of photoelectrons from the outermost surface layers. Survey and high-resolution spectra were obtained using pass energies of 200 and 20 eV, respectively. Quantitative analysis was performed based on high-resolution spectra acquired from three different points located on various areas of the sample surface and averaged for accuracy.
2.5. Statistical analysis
The results were submitted to the analysis of variance (ANOVA) followed by Tukey’s (P < 0.05) post-hoc test using Statistical 7.0 software. Pearson’s correlation coefficient was also calculated to describe the relationship between interfacial tension and droplet size.
3. Results and discussion
3.1. Influence of interfacial tension on the droplet size
The interfacial tension reflects the surface activity of proteins at the oil/water interface, which is influenced by the type of protein adsorbed at the interface. As shown in , interfacial tensions were measured for different degrees of hydrolysis (DHs) to examine the spreading capacity of RPH around the oil droplets. The initial interfacial tension at the linseed oil/water interface was slightly lower (9.5–10.74 mN/m) for the RPH systems than for RPI (11.00 mN/m), where the lowest value was for the RPH10 system.
Figure 1. Interfacial tension at the interface between orange oil and rice protein isolate (RPI) and rice protein hydrolysates (RPH, with the degree of hydrolysis of 2, 6, and 10%) at 1.5% w/w.
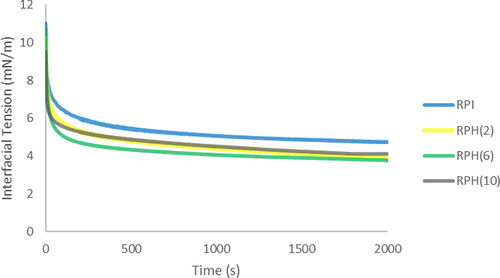
The reduction in interfacial tension can be attributed to the affinity of RPH for interfaces, as protein hydrolysis exposes more hydrophobic and hydrophilic sites, thereby increasing the number of molecules adsorbed at the oil/water interface. The same behavior was observed by lentil protein hydrolysate compared to non-hydrolyzed protein.[Citation17] Various interactions are likely to dominate in RPH samples, with hydrophobic and electrostatic interactions potentially influencing film formation and reducing interfacial tension.[Citation18] Assuming increased hydrophobicity after hydrolysis,[Citation19] the protein increases its contact points with the oil interface, causing the proteins to orient their polar groups toward the aqueous phase and their hydrophobic segments toward the oil phase more effectively. Moreover, hydrolysis led to a notable increase in surface adsorption kinetics and improved conformational flexibility, thereby resulting in a decrease in equilibrium interfacial tension.[Citation7] The equilibrium interfacial tensions were around 3.78-4.04 mN/m for all RPH, which was slightly lower than for RPI (4.74 mN/m). Regarding RPI, this would involve a gradually unfolding and molecular rearrangement, including an intermolecular association or aggregation of individual unfolded protein molecules at the interface. In general, systems containing RPH achieved equilibrium interfacial tension more rapidly than those with RPI.
However, the decreased interfacial tension does not depend only on the protein type. Based on our previous study,[Citation7] the size of the fatty acid chains and essential oil compounds, as well as the ratio of polar to non-polar groups of the oil interacting at the interface, may have played an important role in the stabilization mechanisms. In our previous study, using linseed oil as the lipid phase, the equilibrium interfacial tension was 9.88 ± 0.72 mN/m for RPH, which was much higher than this work. Linseed oil is primarily composed of long-chain unsaturated fatty acid that produces a bend in the molecule, decreasing the structural flexibility of the carbon chain.[Citation20] Thus, the hydrophobicity degree and energy required to break linseed oil into oil droplets are higher than the orange essential oil. Essential oils are primarily composed of small molecules (mono-terpenes) that tend to present more hydrophilic behavior than fatty acids.[Citation21] This results in a higher partition in the water phase that decreases the interfacial tension and facilitates the formation of oil droplets. The same behavior was observed by Barbon et al.[Citation22] who also verified the influence of the oil type on interfacial tension.
In addition, it is important to highlight that the interfacial tension values were obtained in this work on a laboratory scale, not directly translating into large-scale production due to several factors. For example, in lab-scale production, the surface area to volume ratio is typically higher than in large-scale production. This can lead to different behaviors in terms of surface tension effects. Besides that, it’s easier to control and measure surface tension in a lab setting due to the smaller volumes and more controlled environment. The mixing efficiency is another variable that may affect the interfacial tension. Usually, in lab-scale production, achieving a homogeneous mixture with consistent surface tension is generally easier.
For all samples, a high and positive correlation between interfacial tension and droplet size was observed by the +0.844 Pearson’s correlation coefficient. Since there was no difference between interfacial tension for all RPHs, no significant difference was observed between the RPH samples regarding the droplet size (P < 0.05) ().
Figure 2. Comparison between droplet size and interfacial tension of emulsions of orange oil stabilized by maltodextrin and rice protein isolate (RPI) or rice protein hydrolysates (RPH, with degree of hydrolysis of 2, 6, and 10%).
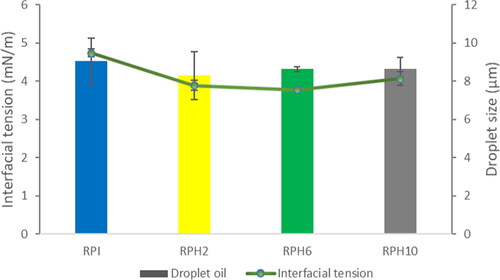
Generally, the formation of smaller droplet sizes depends on the ability of a biopolymer (or emulsifier) to be rapidly adsorbed on the oil-water interface and then undergo a conformational ordering to form a viscoelastic film surrounding the oil droplets. Therefore, a high interfacial tension means protein generated weak adsorption on the oil droplets. The droplet size of the RPH emulsions reached a minimum of 8.62 ± 0.32 µnm, while the particle size was 9.06 ± 1.21 µm for the RPI emulsion. As shown in our previous study, the hydrolysate samples with a specific molecular size and good solubility displayed their capability to form strong viscoelastic films at the oil droplet surface and decrease the droplet size.[Citation7]
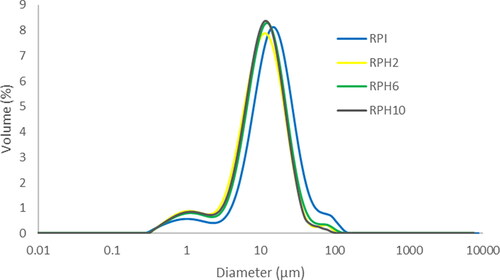
The droplet size distributions for all emulsions are presented in . Although the DH significantly reduced the droplet size compared to RPI, it could not avoid polydisperse distributions ranging from 0.5 to 100 um. The droplet size of all samples showed a predominant peak with a narrow distribution but showed two more peaks. The hydrolyzed proteins may not have quickly covered some of the oil droplets during emulsion preparation. Some droplets may be incompletely covered, indicating droplet-droplet aggregation (coalescence).
Figure 3. The size distribution of oil droplets in emulsions stabilized by maltodextrin and either rice protein isolate (RPI) or rice protein hydrolysate (RPH) with degrees of hydrolysis of 2%, 6%, and 10%.
Moreover, this can also be ascribed to certain insoluble proteins within the system. Nonetheless, the more robust film created by RPH6 aided in maintaining the integrity of the droplet interface during spray drying. In the fresh RPH6 emulsion, the span (1.97) of the spray-dried droplet size distribution was narrower compared to that of the RPI emulsion (2.11), leading to improved overall drying performance.
3.2. Impact of enzymatic hydrolysis of rice protein on particle surface composition
The concentrations of the chemical elements (carbon C, nitrogen N, and oxygen O) on the microparticle surface were quantified in three depth levels through X-ray photoelectron spectroscopy (XPS) () to understand their effect on the oil retention and some physicochemical characteristics of the microparticles.
Table 1. Effect of degree of hydrolysis (2, 6, and 10%) on the surface composition obtained from XPS for the spray-dried orange oil powders.
By analyzing the particle surface, we observed a slight increase in nitrogen on the particle surface as the degree of hydrolysis also increased. The lowest surface nitrogen content was detected for spray-dried microparticles containing RPI. Hydrolyzing the proteins increases their kinetic speed, and they become more capable of migrating to the surface of the atomized droplets during spray drying. This can be attributed to the smaller peptide size, leading to increased surface activity. Given the brief residence time of a droplet during the drying process, emulsifier surface adsorption is primarily diffusion-controlled.[Citation23] Therefore, a higher content of small peptides leads to greater nitrogen content on the powder surface.
It is worth emphasizing that the amount of nitrogen compared to the other chemical elements was lower due to the low quantity of protein/hydrolysates in the feed emulsions. Even so, we can state that even at low concentrations, the protein % on the particle surface displayed a strong and positive influence (Pearson’s correlation of 0.96) on the retention of orange essential oil (). It can be attributed to forming a less porous and more uniform matrix due to the smaller protein with specific characteristics, as demonstrated in our previous study.[Citation11]
Table 2. Physicochemical properties of orange oil microparticles formulated with maltodextrin (MD) and rice protein isolate (RPI) or rice protein hydrolysate (RPH, with degrees of hydrolysis of 2%, 6%, and 10%).
The integrity of the protein film on the surface powder also depends on how the protein behaves during the drying of atomized droplets. Water loss during drying causes a shrinkage of the protein film and its destabilization, together with oil leakage onto the powder surface, which leads to local changes in surface area and, thus, surface protein content.[Citation24] Therefore, based on the interfacial tension results and, consequently, the droplet size, we can assume that RPH could remain in the atomized droplet while drying.
Additionally, there exists a correlation between protein structures and their surface properties.[Citation25] Hydrolysis facilitates the liberation of amino acids and the formation of highly soluble small active peptides with lower molecular weights compared to the intact protein. This release reduces the size of the protein molecules,[Citation2,Citation3,Citation19] which may have facilitated their displacement to the microparticle surface.
3.3. Physicochemical characteristics of spray-dried emulsions
In this study, several properties of spray-dried emulsions were investigated, including powder recovery (as an efficiency and economic metric in the spray-drying process), hygroscopicity (as physical stability during preservation), and particle size (as a determining factor in packaging conditions). presents the properties of powders obtained from the spray drying of essential orange oil emulsions stabilized with different RPH, including non-hydrolyzed protein RPI.
Although the DHs have positively influenced powder recovery (P < 0.05), the recovery was only <30%. Other studies reported low recovery, which is also due to the matrix composition.[Citation25,Citation26] It is common for laboratory-scale spray dryers to have low solid recovery.[Citation27] This is partly due to the lower feed solids content in research-scale dryers compared to commercial dryers. Also, some of the problems related to low solid recovery are due to the wrong choice of wall material.[Citation25] Some wall material shows low glass transition temperature (Tg) and the relative degree of stickiness increases accordingly. Maltodextrins have high Tg, which depends on the DE value, often over the range of 140–180 °C.[Citation28] On the other hand, since Tg is directly related to the molecular weight of the compound, the enzymatic hydrolysis may depress the Tg of rice protein. In fact, in our previous work, microparticles of linseed oil encapsulated with MD and rice protein hydrolyzed by Flavourzyme (degree of hydrolysis 8%) had lower Tg (98.4 °C) than those encapsulated by MD and RPI (103 °C).[Citation2]
Even with the negative impact of protein hydrolysates on the glass transition temperature of spray-dried microparticles, the performance of the hydrolysates RPH6 and RPH10 in the emulsion achieved improved results in overcoming the coalescence of droplets, as well as the sticky interactions of droplets or particles on the wall, creating a surface film on the formed particles when compared to the RPI emulsion, thus increasing the recovery. Although the nature of the films of both RPI and RPH6 were similar, the hydrolysates could protect the oil droplet during drying.
As reported in our previous study,[Citation11] wherein rice protein hydrolysate was employed for microencapsulating flaxseed oil, protein hydrolysates offer a significant kinetic advantage by more readily migrating to the surface of the sprayed emulsion droplet during spray drying, thereby forming a surface film on the resultant particles. This observation was also corroborated by the XPS results, where more proteins were found on the surface of microparticles using RPH6 and RPH10 ().
Despite the numerous advantages presented by surface modification of droplets/particles to reduce stickiness, powder recovery also greatly depends on the scale of operation. Several authors have noted that recovery rates are higher in larger-scale setups because the fraction lost constitutes a smaller portion of the total production volume. Conversely, at the laboratory scale, recovery rates remain suboptimal, typically falling within the range of 20–70%.[Citation29] Generally, low recovery is due to product loss on the walls of the drying chamber. The amounts are relatively constant.
Spray drying investigations conducted at the production plant scale incur significant expenses. Therefore, employing a laboratory-scale spray dryer simplifies the system and establishes new drying models for scaling up to the production plant level while utilizing minimal material. Thus, we can affirm that hydrolysate protein can be used as a “smart drying aid” to minimize the stickiness during the spray-drying process. It presents new opportunities for insights into microencapsulation details by spray drying at different scales. Besides that, the drying process proved to be efficient in avoiding microbial growth on the powders since it showed low levels of moisture content (0.75–1.16%) and aw (0.07–0.149), as they are essential factors for storage.
Water adsorption plays a crucial role in the microencapsulation of oils as the presence of water can impact lipid oxidation, powder flowability, and caking.[Citation30] presents the hygroscopicity of all samples with varying DH. DH significantly affected (P < 0.05) the water adsorption capacity of the samples. The powders containing RPH exhibited higher hygroscopicity compared to those with RPI. This difference could be attributed to structural alterations in the enzyme-treated RPI, resulting in increased accessibility, exposure of hydrophilic regions, and enhanced solubility.[Citation2]
Another possible reason for this result could be the reduction in the surface oil content of the final powder, exposing a major number of active sites to water molecule adsorption, as also observed by Pereyra-Castro et al.[Citation31] In microparticles containing RPH6, the N/C ratio was higher than those with RPI based on XPS results (). It could indicate that the formulation with RPI has a higher concentration of unencapsulated oily material, as also observed by Porras-Saavedra et al.[Citation32] Besides that, this result can also be attributed to the higher surface affinity of the RPH6 compared to RPI, which migrate preferentially to the droplet’s surface during drying. Different results were found on lipid surface enrichment depending on the nature of proteins. Gaiani et al.[Citation33] reported that casein exhibits lower lipid surface enrichment compared to whey proteins, attributed to its lower surface tension at the air-water interface.
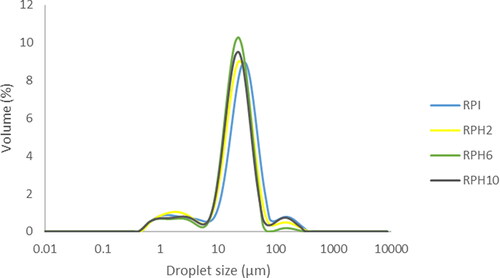
Additionally, particle size can influence powder hygroscopicity because moisture absorption predominantly occurs on the particle surface. Consequently, smaller particle sizes provide a relatively larger surface area for water absorption to take place, and vice versa. In the present study, powder hygroscopicity increased as particle size decreased (Pearson’s correlation of −0.85). Overall, all powders had relatively small particles (13.28–19.75 µm) and were affected by the presence of protein hydrolysates (P < 0.05). The variation in the particle size may also be attributed to the agglomeration of microparticles because of the presence of surface oil (). By analyzing the particle size distribution, a small displacement of the RPI curve can be observed, which results in a slight increase of the peak on the right that corresponds to agglomerates.
3.4. Oil retention
Oil retention represents the quantity of oil retained within powder particles, comprising both surface oil and internal oil entrapped within the wall matrix. DH significantly affected oil retention (P < 0.05) (). The lowest oil retention level (23.03%) was obtained for the RPI wall system, while the highest (49.97%) was for the sample with RPH10.
An increase in flavor retention can be related to the rapid formation of a semi-permeable membrane at the beginning of the drying process.[Citation34] As hydrolyzed proteins have higher kinetic velocity than intact proteins and the emulsifier surface adsorption is mainly adopted diffusion-controlled, the glassy hydrolysate protein film on the surface: (i) prevented flavor release; (ii) minimized the permeation of both oxygen in the particle and water from the surrounding environment; and (iii) also reduced the adhesive interactions of the particles on the drying wall. This membrane serves as a barrier to most flavor compounds while remaining permeable to water molecules, thus preventing the loss of volatile flavors.[Citation35] Although protein molecular size is a significant factor, several authors have pointed out that other factors, such as surface hydrophobicity, hydrodynamic radius, the number of ionizable groups, and the aggregation state of the protein, also play a crucial role in protection and encapsulation.[Citation24]
The use of RPI led to a reduction in oil retention, possibly due to its compact globular structure that confers lower molecular flexibility and poor emulsifying capability compared with protein hydrolysates.[Citation7] The RPH presented a good capacity for adsorption on the oil-water interface because of their amphiphilic molecular structure, which was improved by enzymatic hydrolysis. Such modification enables the adsorption of RPH at the oil-water interface more efficiently, thus reducing the interfacial tension of the system () and favoring the kinetic stabilization of the emulsion. Therefore, enough wall material is available to form a sufficiently strong structural matrix around the droplet, improving the protection of the essential orange oil during drying and, consequently, increasing the oil retention. These physicochemical characteristics of the emulsifying matrices also directly influenced the droplet size, affecting the oil retention. Many authors reported that a smaller droplet size reflects greater oil retention,[Citation36,Citation37] as observed in our results for the RPH10 sample. Thus, it can be reasonably hypothesized that for the RPH10 emulsions, both the droplet size and interfacial protein load contributed to the oil retention of the essential orange oil when compared with RPI. Carmona et al.[Citation12] studied the effect of the emulsion droplet size on the properties of essential orange oil powders obtained by spray drying. The authors observed that flavor retention markedly decreased as droplet mean diameter increased, concluding that is a significant factor to be considered for flavor retention.
On the other hand, it can be observed that despite the similar droplet size and interfacial tension observed in the RPH2 and RPH10 emulsions, the oil retention of the RPH10 spray-dried powder was significantly higher (). This observation indicates that there are parameters other than droplet size or interfacial tension that affect oil retention. Several studies reported an accumulation of protein on the particle surface during spray-drying, resulting in the formation of a surface protective film.[Citation23,Citation38,Citation39] In this case, the RPH2 could not be maintained at the surface during spray drying and forming a surface film on the formed particles, providing a protective barrier. Andersen et al.[Citation40] demonstrated that the thickness of the encapsulation layer is one of the key factors governing the rate of diffusional loss of aroma compounds. This hypothesis was corroborated by XPS results regarding particle surfaces, whereas a thicker protein layer was observed in RPH6 samples ().
When comparing samples RPH10 and RPH6, we observed similar behavior in oil retention, powder recovery, and interfacial tension. From an economic perspective and considering the hydrolysis time of the rice protein, RPH6 would be the obvious choice. However, the choice of hydrolysate depends on the specific process or product application. For example, in our published paper by Gomes and Kurozawa,[Citation3] we demonstrated that hydrolyzing rice protein enhances its antioxidant capacity. Since hydrolysates with a higher degree of hydrolysis exhibited greater antioxidant capacity, the particles formulated with RPH10 may confer greater oxidative stability.
4. Conclusion
This study is the first to characterize the impact of rice protein hydrolysates on the particle surfaces of spray-dried orange oil powder and correlate with powder properties. The use of rice protein hydrolysates in combination with maltodextrin as wall materials proved to be efficient as a natural green label and sustainable ingredient for the microencapsulation of essential oil via spray drying. The hydrolyzed proteins decreased the interfacial tension and consequently displayed smaller droplet sizes than the non-hydrolyzed protein. Thus, emulsions stabilized by hydrolyzed protein combined with maltodextrin showed a higher stability behavior compared to intact protein emulsions. These effects directly influenced the protein’s interaction with oil and maltodextrin and the formation of microparticles.
The results showed that microparticles containing RPH had greater oil retention than RPI samples. The protein hydrolysate with DH 10% doubled the oil retention (50.0%) compared to RPI (23.0%), due to the formation of a protein film on the particle surface, as demonstrated by XPS analysis. This surface film also improved powder recovery, with RPH6 and RPH10 hydrolysates performing better in the emulsion by effectively preventing sticky interactions of droplets or particles on the wall dryer and, as a consequence, improving solid recovery. On the other hand, the hygroscopicity of the powders was higher for the RPH sample. Additionally, the RPH powders had relatively small particles that influenced their hygroscopicity when compared to RPI.
Determining correlations between emulsion properties, surface powder composition, and final product properties is important. These correlations could allow us to determine optimal microparticle formulation and improve bioactive compounds’ protection and controlled release.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Drusch, S.; Klost, M.; Kieserling, H. Current Knowledge on the Interfacial Behaviour Limits Our Understanding of Plant Protein Functionality in Emulsions. Curr. Opin. Colloid Interface Sci. 2021, 56, 101503. DOI: 10.1016/j.cocis.2021.101503.
- Gomes, M. H. G.; Kurozawa, L. E. Influence of Rice Protein Hydrolysate on Lipid Oxidation Stability and Physico-Chemical Properties of Linseed Oil Microparticles Obtained through Spray-Drying. LWT 2021, 139, 110510. DOI: 10.1016/j.lwt.2020.110510.
- Gomes, M. H. G.; Kurozawa, L. E. Improvement of the Functional and Antioxidant Properties of Rice Protein by Enzymatic Hydrolysis for the Microencapsulation of Linseed Oil. J. Food Eng. 2020, 267, 109761. DOI: 10.1016/j.jfoodeng.2019.109761.
- Tamm, F.; Herbst, S.; Brodkorb, A.; Drusch, S. Functional Properties of Pea Protein Hydrolysates in Emulsions and Spray-Dried Microcapsules. Food Hydrocoll. 2016, 58, 204–214. DOI: 10.1016/j.foodhyd.2016.02.032.
- Han, S. W.; Chee, K. M.; Cho, S. J. Nutritional Quality of Rice Bran Protein in Comparison to Animal and Vegetable Protein. Food Chem. 2014, 172, 766–769. DOI: 10.1016/j.foodchem.2014.09.127.
- Amagliani, L.; O'Regan, J.; Kelly, A. L.; O'Mahony, J. A. The Composition, Extraction, Functionality and Applications of Rice Proteins: A Review. Trends Food Sci. Technol. 2017, 64, 1–12. DOI: 10.1016/j.tifs.2017.01.008.
- Gomes, M. H. G.; Kurozawa, L. E. Performance of Rice Protein Hydrolysates as a Stabilizing Agent on Oil-in-Water Emulsions. Food Res. Int. 2023, 172, 113099. DOI: 10.1016/j.foodres.2023.113099.
- Eckert, E.; Han, J.; Swallow, K.; Tian, Z.; Jarpa-Parra, M.; Chen, L. Effects of Enzymatic Hydrolysis and Ultrafiltration on Physicochemical and Functional Properties of Faba Bean Protein. Cereal Chem. 2019, 96, 725–741. DOI: 10.1002/cche.10169.
- Ho, T. M.; Ton, T. T.; Gaiani, C.; Bhandari, B. R.; Bansal, N. Changes in Surface Chemical Composition Relating to Rehydration Properties of Spray-Dried Camel Milk Powder during Accelerated Storage. Food Chem. 2021, 361, 130136. DOI: 10.1016/j.foodchem.2021.130136.
- Murrieta-Pazos, I.; Gaiani, C.; Galet, L.; Cuq, B.; Desobry, S.; Scher, J. Comparative Study of Particle Structure Evolution During Water Sorption: Skim and Whole Milk Powders. Colloids Surf. B Biointerfaces 2011, 87, 1–10. DOI: 10.1016/j.colsurfb.2011.05.001.
- Gomes, M. H. G.; Kurozawa, L. E. Compositional Aspect and Mechanism of Surface Formation of Spray-Dried Microparticles with Surface-Active Rice Protein Hydrolysates. J. Food Eng. 2024, 371, 112007. DOI: 10.1016/j.jfoodeng.2024.112007.
- Carmona, P. A. O.; Tonon, R. V.; da Cunha, R. L.; Hubinger, M. D. Influence of Emulsion Properties on the Microencapsulation of Orange Essential Oil by Spray Drying. J Coll Sci Biotechnol 2013, 2, 130–139. DOI: 10.1166/jcsb.2013.1042.
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier: London, 1986, p. 427.
- Zhao, Q.; Xiong, H.; Selomulya, C.; Chen, X. D.; Zhong, H.; Wang, S.; Sun, W.; Zhou, Q. Enzymatic Hydrolysis of Rice Dreg Protein: Effects of Enzyme Type on the Functional Properties and Antioxidant Activities of Recovered Proteins. Food Chem. 2012, 134, 1360–1367. DOI: 10.1016/j.foodchem.2012.03.033.
- Cai, Y. Z.; Corke, H. Production and Properties of Spray-Dried Amaranthus Betacyanin Pigments. J. Food Sci. 2000, 65, 1248–1252. DOI: 10.1111/j.1365-2621.2000.tb10273.x.
- Jafari, S. M.; He, Y.; Bhandari, B. Encapsulation of Nanoparticles of D-Limonene by Spray Drying: Role of Emulsifiers and Emulsifying Techniques. Dry. Technol. 2007, 25, 1069–1079. DOI: 10.1080/07373930701396758.
- Avramenko, N. A.; Low, N. H.; Nickerson, M. T. The Effects of Limited Enzymatic Hydrolysis on the Physicochemical and Emulsifying Properties of a Lentil Protein Isolate. Food Res. Int. 2013, 51, 162–169. DOI: 10.1016/j.foodres.2012.11.020.
- Klost, M.; Drusch, S. Functionalisation of Pea Protein by Tryptic Hydrolysis: Characterisation of Interfacial and Functional Properties. Food Hydrocoll. 2019, 86, 134–140. DOI: 10.1016/j.foodhyd.2018.03.013.
- Xu, X.; Liu, W.; Liu, C.; Luo, L.; Chen, J.; Luo, S.; McClements, D. J.; Wu, L. Effect of Limited Enzymatic Hydrolysis on Structure and Emulsifying Properties of Rice Glutelin. Food Hydrocoll. 2016, 61, 251–260. DOI: 10.1016/j.foodhyd.2016.05.023.
- Singh, K. K.; Mridula, D.; Rehal, J.; Barnwal, P. Flaxseed: A Potential Source of Food, Feed and Fiber. Crit. Rev. Food Sci. Nutr. 2011, 51, 210–222. DOI: 10.1080/10408390903537241.
- Zhang, J.; Bing, L.; Reineccius, G. A. Formation, Optical Property and Stability of Orange Oil Nanoemulsions Stabilized by Quallija Saponins. LWT-Food Sci. Technol. 2015, 64, 1063–1070. DOI: 10.1016/j.lwt.2015.07.034.
- Paulo, B. B.; Alvim, I. D.; Reineccius, G.; Prata, A. S. Performance of Oil-in-Water Emulsions Stabilized by Different Types of Surface-Active Components. Colloids Surf. B Biointerfaces 2020, 190, 110939. DOI: 10.1016/j.colsurfb.2020.110939.
- Landström, K.; Alsins, J.; Bergenståhl, B. Competitive Protein Adsorption between Bovine Serum Albumin and β-Lactoglobulin during Spray-Drying. Food Hydrocoll. 2000, 14, 75–82. DOI: 10.1016/S0268-005X(99)00047-8.
- Drusch, S.; Hamann, S.; Berger, A.; Serfert, Y.; Schwarz, K. Surface Accumulation of Milk Proteins and Milk Protein Hydrolysates at the Air-Water Interface on a Time-Scale Relevant for Spray-Drying. Food Res. Int. 2012, 47, 140–145. DOI: 10.1016/j.foodres.2011.04.037.
- Fang, Z.; Wang, R.; Bhandari, B. Effects of Type and Concentration of Proteins on the Recovery of Spray-Dried Sucrose Powder. Dry. Technol. 2013, 31, 1643–1652. DOI: 10.1080/07373937.2013.770011.
- Adhikari, B.; Howes, T.; Wood, B. J.; Bhandari, B. R. The Effect of Low Molecular Weight Surfactants and Proteins on Surface Stickiness of Sucrose during Powder Formation through Spray Drying. J. Food Eng. 2009, 94, 135–143. DOI: 10.1016/j.jfoodeng.2009.01.022.
- Fyfe, K.; Kravchuk, O.; Nguyen, A. V.; Deeth, H.; Bhandari, B. Influence of Dryer Type on Surface Characteristics of Milk Powders. Dry. Technol. 2011, 29, 758–769. DOI: 10.1080/07373937.2010.538481.
- Roos, Y.; Karel, M. Phase Transitions of Mixtures of Amorphous Polysaccharides and Sugars. Biotechnol. Prog. 1991, 7, 49–53. DOI: 10.1021/bp00007a008.
- Sosnik, A.; Seremeta, K. P. Advantages and Challenges of the Spray-Drying Technology for the Production of Pure Drug Particles and Drug-Loaded Polymeric Carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. DOI: 10.1016/j.cis.2015.05.003.
- Juarez-Enriquez, E.; Olivas, G. I.; Zamudio-Flores, P. B.; Ortega-Rivas, E.; Perez-Vega, S.; Sepulveda, D. R. Effect of Water Content on the Flowability of Hygroscopic Powders. J. Food Eng. 2017, 205, 12–17. DOI: 10.1016/j.jfoodeng.2017.02.024.
- Pereyra-Castro, S. C.; Alamilla-Beltrán, L.; Villalobos-Castillejos, F.; Porras-Saavedra, J.; Pérez-Pérez, V.; Gutiérrez-López, G. F.; Jiménez-Aparicio, A. R. Microfluidization and Atomization Pressure During Microencapsulation Process: Microstructure, Hygroscopicity, Dissolution and Flow Properties. LWT 2018, 96, 378–385. DOI: 10.1016/j.lwt.2018.05.042.
- Porras-Saavedra, J.; Alamilla-Beltrán, L.; Lartundo-Rojas, L.; de Jesús Perea-Flores, M.; Yáñez-Fernández, J.; Palacios-González, E.; Gutiérrez-López, G. F. Chemical Components Distribution and Morphology of Microcapsules of Paprika Oleoresin by Microscopy and Spectroscopy. Food Hydrocoll. 2018, 81, 6–14. DOI: 10.1016/j.foodhyd.2018.02.005.
- Gaiani, C.; Morand, M.; Sanchez, C.; Tehrany, E. A.; Jacquot, M.; Schuck, P.; Jeantet, R.; Scher, J. How Surface Composition of High Milk Proteins Powders Is Influenced by Spray-Drying Temperature. Colloids Surf. B Biointerfaces 2010, 75, 377–384. DOI: 10.1016/j.colsurfb.2009.09.016.
- Fernandes, R. V.; de, B.; Borges, S. V.; Botrel, D. A.; Silva, E. K.; Costa, J. M. G.; Da; Queiroz, F. Microencapsulation of Rosemary Essential Oil: Characterization of Particles. Dry. Technol. 2013, 31, 1245–1254. DOI: 10.1080/07373937.2013.785432.
- Huynh, T. V.; Caffin, N.; Dykes, G. A.; Bhandari, B.; Huynh, T. V.; Caffin, N.; Dykes, G. A.; Bhandari, B. Optimization of the Microencapsulation of Lemon Myrtle Oil Using Response Surface Methodology. Dry. Technol. 2008, 26, 357. DOI: 10.1080/07373930801898182.
- Peng, Q.; Meng, Z.; Luo, Z.; Duan, H.; Ramaswamy, H. S.; Wang, C. Effect of Emulsion Particle Size on the Encapsulation Behavior and Oxidative Stability of Spray Microencapsulated Sweet Orange Oil (Citrus Aurantium Var. Dulcis). Foods 2022, 12, 116. DOI: 10.3390/foods12010116.
- Soottitantawat, A.; Yoshii, H.; Furuta, T.; Ohkawara, M.; Linko, M. Microencapsulation by Spray Drying: Influence of Emulsion Size on the Retention of Volatile Compounds. Food Eng. Phys. Prop. 2003, 68, 2256–2262. DOI: 10.1111/j.1365-2621.2003.tb05756.x.
- Elversson, J.; Millqvist-Fureby, A. Aqueous Two-Phase Systems as a Formulation Concept for Spray-Dried Protein. Int J. Pharm. 2005, 294, 73–87. DOI: 10.1016/j.ijpharm.2005.01.015.
- Munoz-Ibanez, M.; Nuzzo, M.; Turchiuli, C.; Bergenståhl, B.; Dumoulin, E.; Millqvist-Fureby, A. The Microstructure and Component Distribution in Spray-Dried Emulsion Particles. Food Struct. 2016, 8, 16–24. DOI: 10.1016/j.foostr.2016.05.001.
- Andersen, A. B.; Risbo, J.; Andersen, M. L.; Skibsted, L. H. Oxygen Permeation through an Oil-Encapsulating Glassy Food Matrix Studied by ESR Line Broadening Using a Nitroxyl Spin Probe. Food Chem. 2000, 70, 499–508. DOI: 10.1016/S0308-8146(00)00102-3.
- Sosulski, F. W.; Imafidon, G. I. Amino Acid Composition and Nitrogen-to-Protein Conversion Factors for Animal and Plant Foods. J. Agric. Food Chem. 1990, 38, 1351–1356. DOI: 10.1021/jf00096a011.