Abstract
Monocultures have been the preferred production route in the bio-industry, where contamination has been a major bottleneck. In nature, microorganisms usually exist as part of organized communities and consortia, gaining benefits from co-habitation, keeping invaders at bay. There is increasing interest in the use of co-cultures to tackle contamination issues, and simultaneously increase productivity and product diversity. The feasibility of extending the natural phenomenon of co-habitation to the biomanufacturing industry in the form of co-cultures requires careful and systematic consideration of several aspects. This article will critically examine and review current work on microbial co-cultures, with the intent of examining the concept and proposing a design pipeline that can be developed in a biomanufacturing context.
Introduction
Axenic monocultures are predominantly used in biomanufacturing, due to the ease of monitoring and to meet stringent safety regulations [Citation1]. However, such monocultures are at high risk of contamination that results in capital and product losses during manufacturing [Citation2,Citation3]. Controlled, symbiotic co-cultures possess features that provide solutions to surmount these bottlenecks. Though not universally applicable to all cell systems, co-cultures have shown improvements in yields of biomass, lipids [Citation4] and high-value products [Citation5].
Symbiotic microbial communities have existed from the beginning of time, within benthic mats and fossil remains [Citation6–8]. The first human civilizations used combinations of various microbes, for the production of fermented food and alcoholic beverages [Citation9,Citation10]. Nowadays, industry has harnessed microorganisms as a means of production, due to their innate abilities to synthesize complex compounds and the ease of scale-up. Cells derived from mammals, such as Chinese Hamster Ovary cells [Citation11,Citation12], HeLA cells and mouse cells are workhorses of the biopharmaceutical industry, alongside yeast [Citation13,Citation14] and bacteria [Citation9], which are used predominantly in the food industry, due to their quick turn-around times. The need for sustainable production routes has seen microorganisms deployed for bioremediation of water and soils and as carbon capture and storage options to minimize greenhouse gas emissions. Microbial communities are increasingly being investigated for the production of valuable accessory pigments [Citation15–17] and in microbial fuel cells for electricity generation [Citation18,Citation19].
Maintaining axenic cultures has proved to be expensive and labor intensive, given the recurrent problem of contamination by bacteria, viruses, protozoa, yeast, fungi and microplasma [Citation20]. Parasites or grazers can out-compete the working cell culture and influence cell health and production outputs. The Fifth Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production by Langer [Citation20] reported that a failure rate of 7%, would amount to US$1–2 billion in expenses. Across 434 biomanufacturing companies, contamination was the main reason for batch spoilage. Biomanufacturing with the help of defined artificial co-cultures and consortia may hold a key to increase production rates and tackle contamination [Citation21–23].
In recent years, researchers have started to question whether an axenic culture is strictly the best way forward, as in the natural environment, microorganisms thrive alongside other organisms. As thinking processes have evolved, research into harnessing consortia into biotechnological applications has increased [Citation21] and thanks to synthetic biology and “omics” analysis, the knowledge pool on microbial communication is expanding.
This review aims to examine critically the utility and characteristics of controlled co-cultures in biomanufacturing. An insight into natural consortia and the characteristics that are relevant and transferrable to the industrial world is presented followed by a case study scenario of the application of this principle in developing processes that employ microalgae.
Microbial consortia
Consortia in nature
Microbial consortia are encountered within various natural habitats, such as mammalian guts [Citation24], foods [Citation25], soils [Citation26–28], water bodies and wastes [Citation29]. A question that arises is, ‘Why do naturally occurring microorganisms prefer to live as part of a community? As with human communities, in which a group of individuals play a role in the advancement of society, so do microorganisms. Microbial associations may be symbiotic [Citation6,Citation30,Citation31], which include mutualism and commensalism [Citation32], parasitic or predator–prey type [Citation33–35].
Compared to a single taxon, microbial assemblages have been proven resilient when faced with adverse conditions [Citation36] and resist invasion from other species [Citation37]. A consortium can overcome challenges through communication [Citation38–41] and division of labor [Citation22,Citation23,Citation36,Citation37], evolving into a stable assemblage [Citation42,Citation43]. Biofilms are good examples of community assemblages [Citation44–46]. Studies conducted by Brenner et al. [Citation39] elucidate the bi-directional patterns present within complex systems, which shape and govern the mode in which the populations within the matrix grow, evolve and assert their roles [Citation47].
Communication through metabolites [Citation6,Citation48–50] plays a key-role in defining relationships, protection, evolution, selection of partners and division of labor [Citation40], as shown in . Primary metabolites shape growth, development and reproduction, as seen in quorum sensing. During quorum sensing, bacterial populations release regulatory metabolites, such as N-acylhomoserine lactones [Citation51–53], as the population density grows [Citation54]. The same applies to interactions in the rhizosphere, where sugars, polysaccahrides, amino acids and sterols are chemical cues [Citation55]. Secondary metabolites facilitate external interactions [Citation10,Citation56]: toxins, pigments, antibiotics, alkaloids and carotenoids, are accumulated by cells as responses to abiotic and/or biotic factors [Citation49,Citation57,Citation58], and can be extracted and marketed. A balanced competition within the consortium does not allow other microorganisms to be able to “readily plunder” nutrients. Division of labor has applications in bioremediation [Citation59,Citation60], with microorganisms working together, for example, to counteract the effect of toxins [Citation61–63]. Thanks to these overarching characteristics, consortia are robust and readily adaptable [Citation64], and better at outcompeting microbial contaminants and predators.
Figure 1. Communication within microbial communities. Metabolite exchanges (arrows) facilitate various modes in which microorganisms (geometrical shapes) exhibit intra- or inter-species interactions. Communication is used for (A) quorum sensing and defining the abundance of each species and (B) type of symbiosis and roles played by partners, such as in (C) protection and (D) nutrient acquisition and division of labor. Further to this, as the community evolves, so does the communication, with the effect of causing changes to the microbial communities that are part of it, for example, by recruiting new partners (E) or by evolving existing members (F).
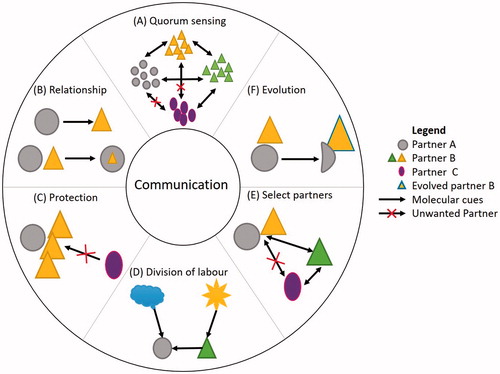
Microbial communities have successfully evolved in nature, from macro- to micro-sphere natural scenarios. This widespread natural occurrence gives reason to believe that synthetic consortia have the potential to drive production and improve industrial biotechnology.
Artificial co-cultures: learning from nature
The argument for moving towards co-cultures stems from the following: (a) current technology such as transcriptomics, metagenomics, metabolomics coupled to computer modeling allow for better understanding of microbial interactions [Citation65,Citation66], (b) contamination issues can be minimized or completely eliminated [Citation22,Citation23,Citation67]; (c) growth profiles of primary producers can be improved [Citation9,Citation68]; (d) the release of new molecules can be triggered [Citation69]; and (e) bioremediation and production can be coupled [Citation70]. From a biotechnological perspective, a good consortium would be scalable, robust, self-sustainable, reproducible, versatile in terms of feedstock and/or production [Citation38,Citation71–73] and profitable [Citation3,Citation74].
When constructing an artificial consortium, factors to consider include: priority effects, community backgrounds and competitiveness for resources. Overyielding or underyielding effects [Citation75] may arise, with overpowering microorganisms monopolizing the nutrients or with competition inhibiting growth of all members [Citation76,Citation77]. Nevertheless, artificial co-cultures have outperformed monocultures, when used for the production of antioxidants, pigments and aromatic compounds, as shown in .
Table 1. Microbial co-cultures in bio-production.
Co-culture design
A bottom-up pipeline is proposed in to design and set-up co-cultures. This involves starting with the end-product to then shortlisting a handful of suitable primary partners (A). The primary partner will then dictate the nature of the secondary partner (B), usually an aider, ideally with bioproduction capabilities. A two-way “trigger and response” system would be ideal, such as mutualism or a commensal symbiosis [Citation32]. It is important to realize that growth increments do not always translate into more products, as productivity can be additionally dependent on the activity of co-culture partners. This is true for microalgae, where co-culture of partner A with B may increase biomass of A, but appropriate stress inducers may be needed to increase specific product yields [Citation78,Citation79].
Figure 2. Steps involved in constructing an artificial co-culture. A bottom-up approach is shown. The desired product is defined first (I), the microbial producers are short-listed next. This can be based on metabolite profiling or on natural associations (II). From selected candidates (III) co-cultures need to be investigated to elucidate the type of partnership (IV). The highest yielding co-culture is to be selected (V), optimized (VI) and upscaled (VII).
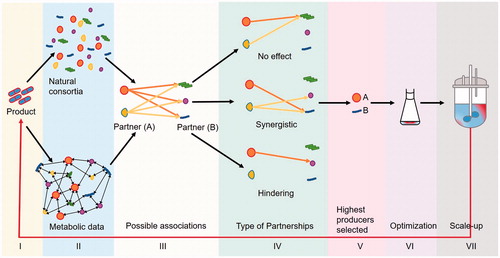
Shortlisting suitable candidates
The secondary partner (B) should possess some of the following characteristics: (a) be nontoxic, (b) be capable of co-habiting [Citation59], (c) match in growth rates, (d) provide nutrients and/or stimulators to enhance A [Citation80], (e) not cause underyielding effects [Citation75] (f) enhance the capability of A to utilize multiple feedstocks [Citation81], (g) remove inhibitory molecules (h) use A’s waste as a feed [Citation82], (i) maintain genetic integrity over prolonged periods of culture, and (j) function as a bioproducer.
Selecting co-culture partners
Co-culture partners are selected according to: (a) communication (metabolite/peptide/protein) profiling and/or (b) from existing natural associations. Screening based on communication profiling involves surveying the literature for secondary partners that release compounds to enhance the primary partner (A). Whilst, the second method consists of selecting partners from a natural symbiotic consortium. Angelis et al. [Citation69] tested combinations between eight Basidiomycetes and four strains of microalgae, to evaluate the best co-culture partners. The candidates were selected according to exopolysaccharide (EPS) production, on the basis that co-culturing fungi with algae would increase overall EPS production. An increased yield with a diverse composition of EPS was recovered, and the co-culture of Agaricus blazei (Basidiomycete) and Chlorella vulgaris (microalgae) was chosen for further studies [Citation69]. Similarly, Weissella confusa 11GU-1 (a yeast) and Propionibacterium freudenreichi JS15 (a bacterium) were deemed to be a working co-culture in bread-making, as the molecules released through their association served to be better antifungal, texture-building and anti-stalling agents [Citation83].
Co-culture media
A communal growth medium is required for co-culturing. Microorganisms isolated from symbiotic consortia will thrive in their original media. However, for artificial co-cultures, a new recipe has to be developed and tested. Conventionally, a growth medium of the primary partner, A [Citation4] or a mixed medium of A and B [Citation84] in which both partners can grow are used. In a mutualistic symbiosis, co-culturing in growth medium A, should be sufficient. In commensal symbiosis, a supplement to help partner B may be needed. For example, glucose, yeast extract [Citation4] and/or corn syrup [Citation85] were added to the algal media to assist the yeast strains.
Inoculation: ratio and timing
The inoculum density of each partner will affect the final co-culture outcome. This can be determined by analyzing the growth rate of the organisms in co-culture media. Buzzini [Citation85] demonstrated that when the inoculation ratio of Rhodotorula glutinis (yeast) and Debaryomyces castellii (starch accumulating bacteria) was 1:1, it resulted in a 150% increase in β-carotene production (by the yeast). This is not always the case, as seen in the C. vulgaris and R. glutinis (algae–yeast) co-culture where higher yields of lipids and biomass were achieved compared to monoculture, irrespective of the starting inoculum [Citation76]. The timing, order and growth phase at which the inocula are introduced into the culture vessel will influence the general structure of the co-culture and its performance. This phenomenon has been termed the priority effect [Citation86,Citation87], and can be an integral factor in bioreactor systems, as shown by Zhang et al. [Citation84]. The co-culturing of C. vulgaris and R. glutinis, achieved higher levels of biomass and lipids, reaching 17.3 and 70.9%, respectively, when each culture was inoculated in their respective log-phase, at a ratio of 1:1. Similarly, the co-culture of Dinoroseobacter shibae (a bacterium) and Thalassiosira pseudonana (a diatom), required T. pseudonana to be in exponential growth phase before the bacterial inoculation [Citation88].
Reactor design and available technologies for co-culture
Bioreactors (photo, airlift, pulsed, stirred, packed, fixed-bed, fluidized, etc.) that can be run in continuous, semi-batch/fed-batch and batch modes have been devised for the culturing of axenic cultures, where monitoring and nutritional requirements are relatively simpler when compared to co-cultures. The challenges rest in finding suitable methods to maximize the growth of co-cultures.
One non-compartmentalized approaches, such as co-inoculation, pelletization [Citation89], biofilms, and encapsulation [Citation77], allow for close contact of the organisms facilitating metabolite exchange. However, this approach has problems with respect to monitoring population dynamics, third party contamination, and meeting nutritional requirements of the primary partner to ensure it is not outcompeted. In compartmentalized approaches the physical contact of the interacting organisms is limited [Citation70]. However, it offers the advantage of independent harvesting and easier monitoring of the bioreactor environment. Each culture is treated as a monoculture, whilst exploiting co-culture characteristics. Approaches here include: membrane segregation [Citation88] including dialysis/hydrogel system [Citation90], transwell systems [Citation70,Citation91] and adhesion matrix, bead entrapment [Citation77], agar plate growth [Citation92], growth in microfluidic channels, gaseous separation [Citation93], cell droplets [Citation94], and matrix immobilization [Citation95].
Critical considerations
Setting up a co-culture for a biotechnological application will involve compromising certain species characteristics. Trade-off between optimal conditions and the growth conditions, in the two or more species selected, need to be taken into account. Trade-off may involve a slower growth rate of the organisms, compared to optimal growth levels, but with higher product yields. This has an impact on processing times. However, the higher titers may outweigh the disadvantage. Viabilities of the co-culture can then be pre-determined with an overall system mass balance. Monitoring the population dynamics to prevent competition, over-/under-yielding effects [Citation96], contamination, toxicity, priority effects [Citation43,Citation86] and abiotic factors have to be addressed for system reproducibility and to prevent production failures or diminishing yields
Case study: microalgae co-cultures for biotechnological application
Microalgae can be prokaryotic (cyanobacteria) and eukaryotic photosynthetic microorganisms. They play a major role in the function of both aqueous and non-aqueous ecosystems due to their ability to grow photo-autotrophically, hence converting inorganic to organic matter that may serve as a source of nutrition for other microorganisms [Citation97]. The simplicity of microalgae, in terms of nutrient requirements and manipulation, makes them ideal candidates for biofuel production [Citation98–103], with some strains of Schizochytrium sp. reportedly accumulating oil up to 77% dry wt. [Citation104].
The multitude of high-value biomolecules, such as: astaxanthin, β-carotene, omega-3 fatty acids, phycocyanin, EPS, organic acids and allelopathic chemicals [Citation10,Citation105–108], that can be produced by these organisms, makes them organisms of commercial interest in the pharmaceutical and nutraceutical industries. However, their performance is affected by various factors, such as: contamination, pH, temperature, nutrient limitations, and light availability [Citation109–113]. Lipid accumulation [Citation114–118], and accumulation of other bio-active compounds is usually a response to stress caused by nutrient starvation, high light, temperature, pH and salinity [Citation119–123]. Usually, the biomolecules are chemically extracted, however, in the case of algae belonging to the genera Chlorella and Dunaliella, they are also secreted into the growth medium [Citation124].
Current established industrial productions include: β-carotene using Dunaliella salina [Citation125], astaxanthin using Haematococcus pluvialis [Citation126], proteins from Spirulina platensis [Citation127], fatty acids from Chlorella sp. [Citation128] and pigments using Nostoc sp. [Citation129]. Other products also include: lutein, xanthophylls, antimicrobials, anticoagulants in addition to carbohydrates (starch and other polysaccharides) [Citation71,Citation130–134]. lists examples of high-value products from species of microalgae, which have been commercially successful. The market value for lutein, for example, was estimated to be US $187 million in 2009 [Citation135] with astaxanthin products being worth about US $200 M per year [Citation136]. Though some of these compounds can be synthesized artificially, manufacturers are steering towards natural products, due to limitations in biological functions and implications in food safety [Citation137].
Table 2. A selection of high-value products derived from microalgae species as monocultures.
Microalgae co-cultures: current status
Microalgae are good candidates for co-culture, and research in this field is yet to harness its full potential. There is a considerable body of work on consortia and co-cultures in the wastewater treatment and anaerobic digestion, where microalgae are increasingly being investigated as co-culture partners. Here, we focus primarily on microalgae co-cultures that can be used in biomanufacturing. Work with bench scale and small pilot scale trials have been carried out on the interaction between microalgae and other microorganisms. Popularly, bacteria have been the focus of the investigation, as many bacterial species are endogenous in most non-axenic microalgal cultures. The tight-knit relationship that exists between bacteria and algae comes to the fact that many microalgae rely on exogenous sources of cobalamin (vitamin B12), thiamin (vitamin B1) and/or biotin (vitamin B7) to grow [Citation138–140]. These compounds are widely synthesized by a vast array of bacterial species [Citation68,Citation139,Citation141] and are available for consumption.
Investigations have shown that co-culture of the bacterium Mesorhizobium loti with the green alga Lobomonas rostrate [Citation138,Citation139] and the bacterium Sinorhizobium meliloti 1021 (Ensifer meliloti) with the green alga Chlamydomonas reinhardtii [Citation140] are based on vitamin associations. Furthermore, cobalamin producing bacteria, such as: Mesorhizobium sp., Mesorhizobium plurifurium, Roseomonas mucosa, S. meliloti Mn04-gfp, S. meliloti 1021, Alcaligenes faecalis, and Pseudomonas putida mt2, have also been shown to live in successful symbiotic associations with the microalgae C. reinhardtii, L. rostrate and C. nivalis [Citation138]. The studies concluded that the consortium established a defined algal morphology development, nutrient acquisition as well as bacterial growth [Citation140].
A further potentially important relationship is between microalgae and yeast, where the microalgae provide O2 for yeast to assimilate carbon substrates and the yeast release CO2 to aid algal photosynthesis. Work conducted in the co-culturing of yeast and algae has shown increases in overall biomass with an impact on lipid profiles. The coupling of microalgal species with a symbiotic organism led to an increase in biomass and desired products, and has gained popularity in bioremediation and biodiesel production, as shown in . When using microalgae assemblages for bioremediation, the waste streams are high in nutrients, which may cause bacterial strains to outgrow the algal strains. This would affect the lipid profile for biodiesel production, as bacterial strains are low lipid producers. Similarly, with no nutrient starvation, lipid synthesis may not occur within the algal strain. Thus, other forms of energy recovery, such as anaerobic digestion and hydrothermal liquefaction are more suitable.
Factors affecting microalgae co-cultures
As in monocultures, pH, nutrients, N/P ratio, availability of carbon source, light intensity and salinity will affect the growth kinetics of the co-culture. Likewise, the priority effects and history of the community, as discussed in the section “Co-culture media”, will influence the co-culture. A limiting step would be co-culturing an organism with a higher growth rate compared to algae (bacteria/yeast), which may result in the algae population being outcompeted, with light limitation due to shading, and competition, being factors affecting the final product yield [Citation37,Citation139,Citation142].
Studies carried out by Cai et al. [Citation143] investigated the growth and biochemical composition of alga Isochrysis galbana and the yeast Ambrosiozyma cicatricosa co-cultures for aquaculture food. A co-culture inoculum ratio of 1:1 was employed yielding a higher biomass of 1.32 g/L compared to the maximum obtained from I. galbana 8701 (1.17 g/L) and A. cicatricosa (0.31 g/L) monocultures, with enhancements in C14 and C18 fatty acid content, 18.85 and 9.03% of the total fatty acids. At the conclusion of the experimental period, the co-culture population was 96.64% algae cells. Zhang et al. [Citation84] demonstrated that inoculating C. vulgaris and R. glutinis co-culture during logarithmic growth improved biomass and lipid yields of 17.3 and 70.9%, with seeding ratios of 1:1 and 1:2 (yeast:algae).
Shu et al. [Citation144] investigated Chlorella sp. and Saccharomyces cerevisiae, at the following seeding ratios, 1:2, 1:1, 2:1, the best ratio was 2:1. (algae:yeast), with higher lipid and biomass produced. In the case of Scenedesmus obliquus with Candida tropicalis and S. cerevisiae, a ratio of 3:1 (algae:yeast) increased the algal biomass yield by 30% [Citation145].
Microalgae co-culture: future potential
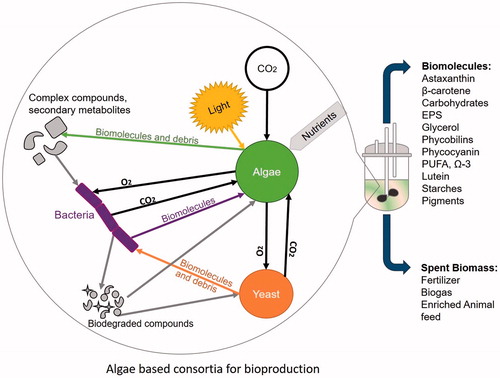
In the case of eukaryotic microalgae, the partnership with other organisms, such as bacteria, yeast or cyanobacteria may be beneficial in production outputs. Selecting symbiotic/synergistic/mutualistic organisms for artificial co-cultures, that themselves produce marketable products, allows for a biorefinery mode of production [Citation71,Citation72]. Extrapolating this concept to symbiotic poly-cultures, thus mimicking natural consortia in the laboratory, would fully exploit the system. A possible future multi-production scheme, for an algae photobioreactor is represented in .
Figure 3. Representation of a microalgae-based consortium for biotechnological applications. A photo-illuminated bioreactor for culturing an artificially created synergistic consortium between algae, yeast and bacteria within a small-scale reactor is represented. The microalgae take up carbon dioxide and produce oxygen (through photosynthesis) that is, consumed by the aerobic bacteria and yeast, which in turn supply carbon dioxide (through respiration) to be consumed by the algae. Cell secretions and degradation will release biomolecules (vitamins, proteins, carbohydrates, nucleic acids and secondary metabolites) into the growth media. The bacteria will break these materials into simpler compounds to be consumed by all members of the consortium.
Co-cultures and consortia: challenges and future possibilities
The literature presented in this review describes the benefits of a co-culture, with the design of co-cultures on trigger-response mechanisms to increase outputs [Citation49,Citation58]. However, slight variations in the culturing system could modify the behavior of the consortium and destabilize the synergistic balance, leading to loss of product. Potential reactor design based on the actual metabolic fluxes, as proposed by Stenuit and Agathos [Citation64], is a tool to be used to monitor and predict culture behavior, and from which to build upon for further optimization.
Understanding the underlying communication and population dynamics is necessary to engineer a successful industrial consortium. Identifying the extracellular chemical cues (metabolites/peptides/proteins) released by species within a co-culture/consortium would provide a canvas from which to develop the consortium production [Citation34,Citation57]. Various methods have been used to track molecular exchanges between microorganisms, outlined by Narihiro and Sekiguchi [Citation146] and Beale et al. [Citation147]. These include extraction using organic solvents, cation exchange [Citation148] combined with chromatography techniques and Mass Spectrometry [Citation149] in combination with intracellular metabolic profiling [Citation150,Citation151]. Challenges exist with respect to trapping and concentrating the molecules of interest [Citation91,Citation147], sample processing, and separation of intra- and extra-cellular metabolites. In addition, the interference from matrix components, such as salts found in growth media of marine algae need to be considered [Citation151,Citation152].
Co-culture database
Natural consortia have evolved over long periods and the associations constructed by the microorganisms themselves have progressed through selection phenomena to produce the extant scenarios. In the biotechnological environment, it would be unworkable to screen all positive associations. A valuable tool would be to have an open access database, detailing successful and failed, co-culture trials, with proper documentation of extracellular compound yields and relevant metadata. This would be beneficial for academic research and facilitate the transition from bench-scale to industrial applications.
Databases have found their role in engineering and more recently in synthetic biology. The compilation of databases, such as the Synthetic Biology Open Language database allows the user to search and find the right combinations to meet the research requirements. The standardization of key aspects that govern biological phenomena has propelled research in synthetic biology. In a similar fashion, databases have been created for the metabolites and metabolic pathways, for pathogens and drugs, as outlined by the Metabolomics Society [Citation153]; these databases are viewed by millions of users on a daily basis, who consult, update and contribute data. The identification of communication systems would benefit structuring future artificial co-cultures. Some quorum sensing, allelopathic chemical and signaling molecules from various extracellular polymeric subclasses have been identified [Citation154,Citation155]. It is important to preserve the bio-molecular interactions within a database that is easily accessible. Many extracellular substances are of great interest to the industry. A compendium incorporating such information also improves on the understanding and provides a better framework in which co-culturing can be exploited.
A useful co-culture database would provide standardized culturing conditions or at least valuable metadata. This database should contain information on the microorganisms, relating to their growth dynamics, biomolecules released in axenic and in co-cultures, in addition to bioreactor conditions. The addition of an online simulator, such as HYSIS and UniSim in Chemical Engineering, would facilitate analysis, simulation and design of co-cultures and consortia in biomanufacturing.
Conclusions
Research for the creation of artificial co-cultures in biomanufacturing has its merits. As discussed in this review, benefits include minimization of contamination and enhanced co-production of similar products. Assembling and implementing co-cultures, derived naturally or artificially, is not straightforward. The ability to create very stable lichen-like systems in the laboratory may not be feasible for at least another decade. However, the first steps to take should be in the direction of understanding the trigger-response mechanisms in co-cultures in order to build a versatile engineering framework. With the appropriate tools and systematic approaches, such as the proposed database, the use of co-cultures can be developed and steered towards more complex and dynamic consortia, that can be used in biomanufacturing. In this regard, microalgae-based co-cultures offer promise, given their natural associations, versatility and ability to thrive with dissimilar species. The advantages of using them as the core on which to build the consortia rests on the fact that they are widely available, to produce an array of products with significant importance in the welfare of humans and animals. They offer environmentally sustainable biomanufacturing routes to be developed, given their ability to fix atmospheric carbon dioxide. In future, systematic construction of consortia with appropriate documentation and development should enable co-cultures to be effectively used in biomanufacturing.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Mcneil B, Giavasis I, Archer D, et al. Microbial production of food ingredients, enzymes and nutraceuticals. 1st ed. Mcneil B, Giavasis I, Archer D, et al., editors. Cambridge: Woodhead Publishing; 2013.
- Suvarna K, Lolas A, Hughes P, et al. Case studies of microbial contamination in biologic product manufacturing. Am Pharm Rev. 2011;50–57.
- Ryan J. Understanding and managing cell culture contamination. Corning Tech Bull. 2008;1–24. CLS-AN-020 REV 2.
- Yen H-W, Chen P-W, Chen L-J. The synergistic effects for the co-cultivation of oleaginous yeast-Rhodotorula glutinis and microalgae-Scenedesmus obliquus on the biomass and total lipids accumulation. Bioresour Technol. 2014;184:148–152.
- Dong Q, Zhao X. In situ carbon dioxide fixation in the process of natural astaxanthin production by a mixed culture of Haematococcus pluvialis and Phaffia rhodozyma. Catal Today. 2004;98:537–544.
- Delaux P-M, Radhakrishnan GV, Jayaraman D, et al. Algal ancestor of land plants was preadapted for symbiosis. Proc Natl Acad Sci. 2015;112:13390–13395.
- Taylor MW, Radax R, Steger D, et al. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev. 2007;71:295–347.
- Thiel V, Peckmann J, Richnow HH, et al. Molecular signals for anaerobic methane oxidation in Black Sea seep carbonates and a microbial mat. Mar Chem. 2001;73:97–112.
- Santos CC, Libeck Bda S, Schwan RF. Co-culture fermentation of peanut-soy milk for the development of a novel functional beverage. Int J Food Microbiol. 2014;186:32–41.
- Kouzuma a, Watanabe K. Microbial ecology pushes frontiers in biotechnology. Microbes Environ. 2014;29:1–3.
- Kim JY, Kim Y-G, Lee GM. CHO cells in biotechnology for production of recombinant proteins: current state and further potential. Appl Microbiol Biotechnol. 2012;93:917–930.
- Yang Z, Wang S, Halim A, et al. Engineered CHO cells for production of diverse, homogeneous glycoproteins. Nat Biotechnol. 2015;33:2014–2017.
- Johnson I. Human insulin from recombinant DNA technology. Science. 1983;219:632–637.
- Williams DC, Frank RMV, Muth WL, et al. Cytoplasmic inclusion bodies in Escherichia coli producing biosynthetic human insulin production. Science. 1982;215:687–688.
- Chen T, Wang Y. Optimized astaxanthin production in Chlorella zofingiensis under dark condition by response surface methodology. Food Sci Biotechnol. 2013;22:1343–1350.
- Graverholt OS, Eriksen NT. Heterotrophic high-cell-density fed-batch and continuous-flow cultures of Galdieria sulphuraria and production of phycocyanin. Appl Microbiol Biotechnol.. 2007;77:69–75.
- Prieto A, Canavate JP, Garcia-Gonzalez M. Assessment of carotenoid production by Dunaliella salina in different culture systems and operation regimes. J Biotechnol. 2011;151:180–185.
- Malvankar NS, Lovley DR. Microbial nanowires for bioenergy applications. Curr Opin Biotechnol. 2014;27:88–95.
- Lovley DR. Microbial fuel cells: novel microbial physiologies and engineering approaches. Curr Opin Biotechnol. 2006;17:327–332.
- Langer ES. Batch failure rates in biomanufacturing good training and investing in the right equipment upfront are among solutions. Genetic Eng Biotechnol News. 2008;28.
- Kazamia E, Riseley AS, Howe CJ, et al. An engineered community approach for industrial cultivation of microalgae. Ind Biotechnol (New Rochelle NY). 2014;10:184–190.
- Brenner K, You L, Arnold FH. Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 2008;26:483–489.
- Williams P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology (Reading, Engl). 2007;153:3923–3938.
- Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA. 2011;108:4578–4585.
- Cocolin L, Ercolini D. Zooming into food-associated microbial consortia: a “cultural” evolution. Curr Opin Food Sci. 2015;2:43–50.
- Singh BK, Millard P, Whiteley AS, et al. Unravelling rhizosphere–microbial interactions: opportunities and limitations. Trends Microbiol. 2004;12:386–393.
- Johansson JF, Paul LR, Finlay RD. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiol Ecol. 2004;48:1–13.
- Kent AD, Triplett EW. Microbial communities and their interactions in soil and rhizosphere ecosystems. Annu Rev Microbiol. 2002;56:211–236.
- Bayer EA, Lamed R, Himmel ME. The potential of cellulases and cellulosomes for cellulosic waste management. Curr Opin Biotechnol. 2007;18:237–245.
- Visick KL, Ruby EG. Vibrio fischeri and its host: it takes two to tango. Curr Opin Microbiol. 2006;9:632–638.
- Moissl-Eichinger C, Huber H. Archaeal symbionts and parasites. Curr Opin Microbiol. 2011;14:364–370.
- Santos CA, Reis A. Microalgal symbiosis in biotechnology. Appl Microbiol Biotechnol. 2014;98:5839–5846.
- Gebuhr C, Pohlon E, Schmidt AR, et al. Development of microalgae communities in the phytotelmata of allochthonous populations of Sarracenia purpurea (Sarraceniaceae). Plant Biol. 2006;8:849–860.
- Mendes B, Brantes L, Vermelho AB. Allelopathy as a potential strategy to improve microalgae cultivation. Biotechnol Biofuels. 2013;6:152.
- Benavente-Valdes JR, Aguilar C, Contreras-Esquivel JC, et al. Strategies to enhance the production of photosynthetic pigments and lipids in chlorophycae species. Biotechnol Rep. 2016;10:117–125.
- Natrah FMI, Bossier P, Sorgeloos P, et al. Significance of microalgal-bacterial interactions for aquaculture. Rev Aquacult. 2014;6:48–61.
- Subashchandrabose SR, Ramakrishnan B, Megharaj M, et al. Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol Adv. 2011;29:896–907.
- Bernstein HC, Carlson RP. Microbial consortia engineering for cellular factories: in vitro to in silico systems. Comput Struct Biotechnol J. 2012;3:1–8.
- Brenner K, Karig DK, Weiss R, et al. Engineered bidirectional communication mediates a consensus in a microbial biofilm consortium. Proc Natl Acad Sci USA. 2007;104:17300–17304.
- Hays SG, Patrick WG, Ziesack M, et al. Better together: engineering and application of microbial symbioses microbial consortia engineering for cellular factories: in vitro to in silico systems. Curr Opin Biotechnol. 2015;36:40–49.
- Pandhal J, Noirel J. Synthetic microbial ecosystems for biotechnology. Biotechnol Lett. 2014;36:1141–1151.
- Fussmann GF, Loreau M, Abrams PA. Eco-evolutionary dynamics of communities and ecosystems. Funct Ecol. 2007;21:465–477.
- Loeuille N, Leibold MA. Evolution in metacommunities: on the relative importance of species sorting and monopolization in structuring communities. Am Nat. 2008;171:788–799.
- Barranguet C, van Beusekom SAM, Veuger B, et al. Studying undisturbed autotrophic biofilms: still a technical challenge. Aquat Microb Ecol. 2004;34:1–9.
- Kavita A, Jha BKM. Extracellular polymeric substances from two biofilm forming Vibrio species: characterization and applications. Carbohydr Polym. 2013;94:882–888.
- Zhang B, Powers R. Analysis of bacterial biofilms using NMR-based metabolomics. Future Med Chem. 2012;4:1273–1306.
- Sekar VP, Nandakumar K, Nair KVK, et al. Early stages of biofilm succession in a lentic freshwater environment. Hydrobiologia. 2004;512:97–108.
- Beech IB, Sunner J. Biocorrosion: towards understanding interactions between biofilms and metals. Curr Opin Biotechnol. 2004;15:181–186.
- Dashti Y, Grkovic T, Abdelmohsen UR, et al. Production of induced secondary metabolites by a co-culture of sponge-associated actinomycetes, Actinokineospora sp. EG49 and Nocardiopsis sp. RV163. Mar Drugs. 2014;12:3046–3059.
- Volk RB, Furkert FH. Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiol Res. 2006;161:180–186.
- Ahmer BMM. Cell-to-cell signalling in Escherichia coli and Salmonella enterica. Mol Microbiol. 2004;52:933–945.
- González JE, Keshavan ND. Messing with bacterial quorum sensing. Microbiol Mol Biol Rev. 2006;70:859–875.
- Hooshangi S, Bentley WE. From unicellular properties to multicellular behavior: bacteria quorum sensing circuitry and applications. Curr Opin Biotechnol. 2008;19:550–555.
- March JC, Bentley WE. Quorum sensing and bacterial cross-talk in biotechnology. Curr Opin Biotechnol. 2004;15:495–502.
- Badri DV, Weir TL, van der Lelie D, et al. Rhizosphere chemical dialogues: plant-microbe interactions. Curr Opin Biotechnol. 2009;20:642–650.
- Shank EA, Kolter R. New developments in microbial interspecies signaling. Curr Opin Microbiol. 2009;12:205–214.
- Netzker T, Fischer J, Weber J, et al. Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front Microbiol. 2015;6:299.
- Rateb ME, Hallyburton I, Houssen WE, et al. Induction of diverse secondary metabolites in Aspergillus fumigatus by microbial co-culture. RSC Adv. 2013;3:14444–14450.
- Watanabe K. Microorganisms relevant to bioremediation. Curr Opin Biotechnol. 2001;12:237–241.
- Jones WR. Practical applications of marine bioremediation. Curr Opin Biotechnol. 1998;9:300–304.
- Atlas RM, Atlas MC. Biodegradation of oil and bioremediation of oil spills. Curr Opin Biotechnol. 1991;2:440–443.
- Díaz E, Jiménez JI, Nogales J. Aerobic degradation of aromatic compounds. Curr Opin Biotechnol. 2013;24:431–442.
- Ding S-Y, Xu Q, Crowley M, et al. A biophysical perspective on the cellulosome: new opportunities for biomass conversion. Curr Opin Biotechnol. 2008;19:218–227.
- Stenuit B, Agathos SN. Deciphering microbial community robustness through synthetic ecology and molecular systems synecology. Curr Opin Biotechnol. 2015;33:305–317.
- van Baarlen P, Kleerebezem M, Wells JM. Omics approaches to study host-microbiota interactions. Curr Opin Microbiol. 2013;16:270–277.
- Schofield MM, Sherman DH. Meta-omic characterization of prokaryotic gene clusters for natural product biosynthesis. Curr Opin Biotechnol. 2013;24:1151–1158.
- Sue T, Obolonkin V, Griffiths H, et al. An exometabolomics approach to monitoring microbial contamination in microalgal fermentation processes by using metabolic footprint analysis. Appl Environ Microbiol. 2011;77:7605–7610.
- Croft MT, Lawrence AD, Raux-Deery E, et al. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature. 2005;438:90–93.
- Angelis S, Noivak AC, Sydney EB, et al. Co-culture of microalgae, cyanobacteria, and macromycetes for exopolysaccharides production: process preliminary optimization and partial characterization. Appl Biochem Biotechnol. 2012;167:1092–1106.
- Goers L, Freemont P, Polizzi KM. Co-culture systems and technologies: taking synthetic biology to the next level. J R Soc Interface. 2014;0065:11.
- Markou G, Nerantzis E. Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv. 2013;31:1532–1542.
- Gebreslassie BH, Waymire R, You FQ. Sustainable design and synthesis of algae-based biorefinery for simultaneous hydrocarbon biofuel production and carbon sequestration. AICHE J. 2013;59:1599–1621.
- Trzcinski AP, Hernandez E, Webb C. A novel process for enhancing oil production in algae biorefineries through bioconversion of solid by-products. Bioresour Technol. 2012;116:295–301.
- DesRochers TM, Kuo IY, Kimmerling EP, et al. The effects of mycoplasma contamination upon the ability to form bioengineered 3D kidney cysts. PLoS One. 2015;10:1–11.
- Schmidtke A, Gaeke U, Weithoff G. A mechanistic basis for underyielding in phytoplankton communities. Ecology. 2010;91:212–221.
- Cheirsilp B, Suwannarat W, Niyomdecha R. Mixed culture of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for lipid production from industrial wastes and its use as biodiesel feedstock. N Biotechnol. 2011;28:362–368.
- Kitcha S, Cheirsilp B. Enhanced lipid production by co-cultivation and co-encapsulation of oleaginous yeast Trichosporonoides spathulata with microalgae in alginate gel beads. Appl Biochem Biotechnol. 2014;173:522–534.
- Su CH, Chien LJ, Gomes J, et al. Factors affecting lipid accumulation by Nannochloropsis oculata in a two-stage cultivation process. J Appl Phycol. 2011;23:903–908.
- Chi ZY, Liu Y, Frear C, et al. Study of a two-stage growth of DHA-producing marine algae Schizochytrium limacinum SR21 with shifting dissolved oxygen level. Appl Microbiol Biotechnol. 2009;81:1141–1148.
- Orphan VJ. Methods for unveiling cryptic microbial partnerships in nature. Curr Opin Microbiol. 2009;12:231–237.
- Minty JJ, Singer ME, Scholz SA, et al. Design and characterization of synthetic fungal-bacterial consortia for direct production of isobutanol from cellulosic biomass. Proc Natl Acad Sci USA. 2013;110:14592–14597.
- Oren a. Availability, uptake and turnover of glycerol in hypersaline environments. FEMS Microbiol Ecol. 1993;12:15–23.
- Tinzl-Malang SK, Rast P, Grattepanche F, et al. Exopolysaccharides from co-cultures of Weissella confusa 11GU-1 and Propionibacterium freudenreichii JS15 act synergistically on wheat dough and bread texture. Int J Food Microbiol. 2015;214:91–101.
- Zhang Z, Ji H, Gong G, et al. Synergistic effects of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for enhancement of biomass and lipid yields. Bioresour Technol. 2014;164:93–99.
- Buzzini P. Batch and fed-batch carotenoid production by Rhodotorula glutinis-Debaryomyces castellii co-cultures in corn syrup. J Appl Microbiol. 2001;90:843–847.
- Fukami T. Historical contingency in community assembly: integrating niches, species pools, and priority effects. Annu Rev Ecol Evol Syst. 2015;46:1–23.
- Chase JM. Community assembly: when should history matter? Oecologia. 2003;136:489–498.
- Paul C, Mausz MA, Pohnert G. A co-culturing/metabolomics approach to investigate chemically mediated interactions of planktonic organisms reveals influence of bacteria on diatom metabolism. Metabolomics. 2012;9:349–359.
- Wrede D, Taha M, Miranda AF, et al. Co-cultivation of fungal and microalgal cells as an efficient system for harvesting microalgal cells, lipid production and wastewater treatment. PLoS One. 2014;9:e113497.
- Smith MJ, Francis MB. Improving metabolite production in microbial co-cultures using a spatially constrained hydrogel. Biotechnol Bioeng. 2017;114:1195–1200.
- Ponomarova O, Patil KR. Metabolic interactions in microbial communities: untangling the Gordian knot. Curr Opin Microbiol. 2015;27:37–44.
- Dalmas FR, Astarita L, Defilippis L, et al. Growth inhibition of an Araucaria angustifolia (Coniferopsida) fungal seed pathogen, Neofusicoccum parvum, by soil streptomycetes. BMC Microbiol. 2013;13:168.
- Santos C, Caldeira M, Lopes da Silva T, et al. Enhanced lipidic algae biomass production using gas transfer from a fermentative Rhodosporidium toruloides culture to an autotrophic Chlorella protothecoides culture. Bioresour Technol. 2013;138:48–54.
- Byun CK, Hwang H, Choi WS, et al. Productive chemical interaction between a bacterial microcolony couple is enhanced by periodic relocation. J Am Chem Soc. 2013;135:2242–2247.
- Lobete MM, Fernandez EN, Van Impe JFM. Recent trends in non-invasive in situ techniques to monitor bacterial colonies in solid (model) food. Front Microbiol. 2015;6:148.
- Fukami T. Community assembly along a species pool gradient: implications for multiple-scale patterns of species diversity. Popul Ecol. 2004;46:137–147.
- Subashchandrabose SR, Ramakrishnan B, Megharaj M, et al. Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation. Environ Int. 2013;51:59–72.
- Christenson L, Sims R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol Adv. 2011;29:686–702.
- Machado IMP, Atsumi S. Cyanobacterial biofuel production. J Biotechnol. 2012;162:50–56.
- Mata TM, Martins AA, Caetano N. Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev. 2010;14:217–232.
- Frigon JC, Matteau-Lebrun F, Hamani Abdou R, et al. Screening microalgae strains for their productivity in methane following anaerobic digestion. Appl Energy. 2013;108:100–107.
- Parmar A, Singh NK, Pandey A, et al. Cyanobacteria and microalgae: a positive prospect for biofuels. Bioresour Technol. 2011;102:10163–10172.
- Posten C, Schaub G. Microalgae and terrestrial biomass as source for fuels-a process view. J Biotechnol. 2009;142:64–69.
- Chisti Y. Biodiesel from microalgae. Biotechnol Adv. 2007;25:294–306.
- Vigani M, Parisi C, Rodriguez-Cerezo E, et al. Food and feed products from micro-algae: market opportunities and challenges for the EU. Trends Food Sci Technol. 2015;42:81–92.
- Cuellar-Bermudez SP, Aguilar-Hernandez I, Cardenas-Chavez DL, et al. Extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microb Biotechnol. 2015;8:190–209.
- Borowitzka MA. High-value products from microalgae-their development and commercialisation. J Appl Phycol. 2013;25:743–756.
- Liu L, Pohnert G, Wei D. Extracellular metabolites from industrial microalgae and their biotechnological potential. Mar Drugs. 2016;14:191.
- Chen H, Jiang JG. Osmotic responses of Dunaliella to the changes of salinity. J Cell Physiol. 2009;219:251–258.
- Dragone G, Fernandes BD, Abreu AP, et al. Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl Energy. 2011;88:3331–3335.
- Ras M, Steyer JP, Bernard O. Temperature effect on microalgae: a crucial factor for outdoor production. Rev Environ Sci. Bio-Technol. 2013;12:153–164.
- Sayre R. Microalgae: The potential for carbon capture. Bioscience. 2010;60:722–727.
- Yao B, Xi BD, Hu CM, et al. A model and experimental study of phosphate uptake kinetics in algae: considering surface adsorption and P-stress. J Environ Sci. 2011;23:189–198.
- Elvira-Antonio N, Ruiz-Marin A, Canedo-Lopez Y. Effect of nitrogen content and CO2 consumption rate by adding sodium carbonate in the lipid content of Chlorella vulgaris and Neochloris oleoabundans. Int J Environ Prot. 2013;3:13–19.
- Feng DN, Chen ZA, Xue S, et al. Increased lipid production of the marine oleaginous microalgae Isochrysis zhangjiangensis (Chrysophyta) by nitrogen supplement. Bioresour Technol. 2011;102:6710–6716.
- Jiang Y, Yoshida T, Quigg A. Photosynthetic performance, lipid production and biomass composition in response to nitrogen limitation in marine microalgae. Plant Physiol Biochem. 2012;54:70–77.
- San Pedro A, Gonzalez-Lopez CV, Acien FG, et al. Marine microalgae selection and culture conditions optimization for biodiesel production. Bioresour Technol. 2013;134:353–361.
- Schlagermann P, Göttlicher G, Dillschneider R, et al. Composition of algal oil and its potential as biofuel. J Combust. 2012;2012:1–14.
- Campenni’ L, Nobre BP, Santos CA, et al. Carotenoid and lipid production by the autotrophic microalga Chlorella protothecoides under nutritional, salinity, and luminosity stress conditions. Appl Microbiol Biotechnol. 2013;97:1383–1393.
- Fu W, Guðmundsson L, Paglia G, et al. Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Appl Microbiol Biotechnol. 2013;97:2395–2403.
- Lamers PP, van de Laak CCW, Kaasenbrood PS, et al. Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol Bioeng. 2010;106:638–648.
- Phadwal K, Singh PK. Isolation and characterization of an indigenous isolate of Dunaliella sp. for beta-carotene and glycerol production from a hypersaline lake in India. J Basic Microbiol. 2003;43:423–429.
- Phadwal K, Singh P. Effect of nutrient depletion on beta-carotene and glycerol accumulation in two strains of Dunaliella sp. Bioresour Technol. 2003;90:55–58.
- Hard BC, Gilmour DJ. A mutant of Dunaliella parva CCAP 19/9 leaking large amounts of glycerol into the medium. J Appl Phycol. 1991;3:367–372.
- Tran D, Doan N, Louime C, et al. Growth, antioxidant capacity and total carotene of Dunaliella salina DCCBC15 in a low cost enriched natural seawater medium. World J Microbiol Biotechnol. 2014;30:317–322.
- Del Campo JA, García-González M, Guerrero MG. Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotechnol. 2007;74:1163–1174.
- Avila-Leon I, Matsudo M, Sato S, et al. Arthrospira platensis biomass with high protein content cultivated in continuous process using urea as nitrogen source. J Appl Microbiol. 2012;112:1086–1094.
- Liu J, Fan K, Jiang Y, et al. Production potential of Chlorella zofingienesis as a feedstock for biodiesel. Bioresour Technol. 2010;101:8658–8663.
- Sekar S, Chandramohan M. Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J Appl Phycol. 2007;20:113–136.
- Raja R, Hemaiswarya S, Kumar NA, et al. A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol. 2008;34:77–88.
- Spolaore P, Joannis-Cassan C, Duran E, et al. Commercial applications of microalgae. J Biosci Bioeng. 2006;101:87–96.
- Pignolet O, Jubeau S, Vaca-Garcia C, et al. Highly valuable microalgae: biochemical and topological aspects. J Ind Microbiol Biotechnol. 2013;40:781–796.
- Dayananda C, Kumudha A, Sarada R, et al. Isolation, characterization and outdoor cultivation of green microalgae Botryococcus sp. Sci Res Essays. 2012;5:2497–2505.
- de Morais MG, da Silva Vaz B, Etiele Greque de M, et al. Review article biologically active metabolites synthesized by microalgae. Biomed Res Int. 2015;2015:1–15.
- Blanco AM, Moreno J, Del Campo JA, et al. Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl Microbiol Biotechnol. 2007;73:1259–1266.
- Li J, Zhu D, Niu J, et al. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol Adv. 2011;29:568–574.
- Caswell M, Zilberman D. Algolculture: an Economical Analaysis of Algoculture. Corvallis (OR): Food and Agriculture Organization of the United Nations. 2000
- Kazamia E, Czesnick H, Nguyen TT, Van, et al. Mutualistic interactions between vitamin B12 -dependent algae and heterotrophic bacteria exhibit regulation. Environ Microbiol. 2012;14:1466–1476.
- Grant MAA, Kazamia E, Cicuta P, et al. Direct exchange of vitamin B12 is demonstrated by modelling the growth dynamics of algal–bacterial cocultures. ISME J. 2014;8(7):1-10.
- Xie B, Bishop S, Stessman D, et al. Chlamydomonas reinhardtii thermal tolerance enhancement mediated by a mutualistic interaction with vitamin B12-producing bacteria. ISME J. 2013;7:1544–1555.
- Helliwell KE, Scaife MA, Sasso S, et al. Unraveling vitamin B12-responsive gene regulation in algae. Plant Physiol. 2014;165:388–397.
- Praveen P, Loh K-C. Photosynthetic aeration in biological wastewater treatment using immobilized microalgae-bacteria symbiosis. Appl Microbiol Biotechnol. 2015;99:10345–10354.
- Cai S, Hu C, Du S. Comparisons of growth and biochemical composition between mixed culture of alga and yeast and monocultures. J Biosci Bioeng. 2007;104:391–397.
- Shu C-H, Tsai C-C, Chen K-Y, et al. Enhancing high quality oil accumulation and carbon dioxide fixation by a mixed culture of Chlorella sp. and Saccharomyces cerevisiae. J Taiwan Inst Chem Eng. 2013;44:936–942.
- Wang R, Tian Y, Xue S, et al. Enhanced microalgal biomass and lipid production via co-culture of Scenedesmus obliquus and Candida tropicalis in an autotrophic system. J Chem Technol Biotechnol. 2015;9:1387–1396.
- Narihiro T, Sekiguchi Y. Microbial communities in anaerobic digestion processes for waste and wastewater treatment: a microbiological update. Curr Opin Biotechnol. 2007;18:273–278.
- Beale D, Kouremenos K, Palombo E (editors). Microbial metabolomics. Switzerland: Springer International Publishing; 2016
- Frølund B, Palmgren R, Keiding K, et al. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996;30:1749–1758.
- Solomon KV, Haitjema CH, Thompson DA, et al. Extracting data from the muck: deriving biological insight from complex microbial communities and non-model organisms with next generation sequencing. Curr Opin Biotechnol. 2014;28:103–110.
- Ebada SS, Edrada RA, Lin W, et al. Methods for isolation, purification and structural elucidation of bioactive secondary metabolites from marine invertebrates. Nat Protoc. 2008;3:1820–1831.
- Kapoore RV, Vaidyanathan S. Towards quantitative mass spectrometry-based metabolomics in microbial and mammalian systems. Philos Trans A. 2016;374:1–14.
- Goulitquer S, Potin P, Tonon T. Mass spectrometry-based metabolomics to elucidate functions in marine organisms and ecosystems. Mar Drugs. 2012;10:849–889.
- Metabolomics Society [Internet]; [cited 2016 Nov 12]. Available from http://metabolomicssociety.org/resources/metabolomics-databases#home. 2014.
- Flemming H, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633.
- Xiao R, Zheng Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol Adv. 2016;34:1225–1244.
- Xue F, Miao J, Zhang X, et al. A new strategy for lipid production by mix cultivation of Spirulina platensis and Rhodotorula glutinis. Appl Biochem Biotechnol. 2010;160:498–503.
- Papone T, Kookkhunthod S, Leesing R. Microbial oil production by monoculture and mixed cultures of microalgae and oleaginous yeasts using sugarcane juice as substrate. World Acad Sci Eng Technol. 2012;64:1127–1131.
- Wang E-X, Ding M-Z, Ma Q, et al. Reorganization of a synthetic microbial consortium for one-step vitamin C fermentation. Microb Cell Fact. 2016;15:21.
- Ling J, Nip S, Cheok WL, et al. Lipid production by a mixed culture of oleaginous yeast and microalga from distillery and domestic mixed wastewater. Bioresour Technol. 2014;173:132–139.
- Ip P, Wong K, Chen F. Enhanced production of astaxanthin by the green microalga Chlorella zofingiensis in mixotrophic culture. Process Biochem. 2004;39:1761–1766.
- Del Campo JA, Rodríguez H, Moreno J, et al. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl Microbiol Biotechnol. 2004;64:848–854.
- Choi YE, Yun YS, Park JM. Evaluation of factors promoting astaxanthin production by a unicellular green alga, Haematococcus pluvialis, with fractional factorial design. Biotechnol Prog. 2002;18:1170–1175.
- Zhang BY, Geng YH, Li ZK, et al. Production of astaxanthin from Haematococcus in open pond by two-stage growth one-step process. Aquaculture. 2009;295:275–281.
- Harker M, Tsavalos AJ, Young AJ. Autotrophic growth and carotenoid production of Haematococcus pluvialis in a 30 liter air-lift photobioreactor. J Ferment Bioeng. 1996;82:113–118.
- Pisal D, Lele S. Carotenoid production from microalga, Dunaliella salina. Indian J Biotechnol. 2005;4:476–483.
- Gomez PI, Gonzalez MA. The effect of temperature and irradiance on the growth and carotenogenic capacity of seven strains of Dunaliella salina (Chlorophyta) cultivated under laboratory conditions. Biol Res. 2005;38:151–162.
- Rad FA, Aksoz N, Hejazi MA. Effect of salinity on cell growth and β -carotene production in Dunaliella sp. isolates from Urmia Lake in northwest of Iran. Afr J Biotechnol. 2011;10:2282–2289.
- Sathasivam R, Kermanee P, Roytrakul S, et al. Isolation and molecular id entification of β -carotene producing strains of Dunaliella salina and Dunaliella bardawil from salt soil samples by using species-specific primers and internal transcribed spacer (ITS) primers. Afr J Biotechnol. 2012;11:16677–16687.
- Mojaat M, Pruvost J, Foucault A, et al. Effect of organic carbon sources and Fe2+ ions on growth and β-carotene accumulation by Dunaliella salina. Biochem Eng J. 2008;39:177–184.
- Yoo C, Jun S-Y, Lee J-Y, et al. Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour Technol. 2010;101 Suppl:S71–S74.
- Garbayo I, Cuaresma M, Vilchez C, et al. Effect of abiotic stress on the production of lutein and beta-carotene by Chlamydomonas acidophila. Process Biochem. 2008;43:1158–1161.
- Sloth JK, Wiebe GW, Eriksen NT. Accumulation of phycocyanin in heterotrophic and mixotrophic cultures of the acidophilic red alga Galdieria sulphuraria. Enzyme Microb Technol. 2006;38:168–175.
